Abstract
α-Amanitin is the major causal constituent of deadly Amanita mushrooms that account for the majority of fatal mushroom poisonings worldwide. It is also an important biochemical tool for the study of its target, RNA polymerase II. The commercial supply of this bicyclic peptide comes directly from A. phalloides, the death cap mushroom, which is collected from its natural habitat. Isotopically labeled amanitin could be useful for clinical and forensic applications, but α-amanitin has not been chemically synthesized and A. phalloides cannot be cultured on artificial medium. Using Galerina marginata, an unrelated saprobic mushroom that grows and produces α-amanitin in culture, we describe a method for producing 15N-labeled α-amanitin using growth media containing 15N as sole nitrogen source. A key to success was preparing 15N-enriched yeast extract via a novel method designated “glass bead-assisted maturation.” In the presence of the labeled yeast extract and 15N-NH4Cl, α-amanitin was produced with >97% isotope enrichment. The labeled product was confirmed by HPLC, high-resolution mass spectrometry, and NMR.
1. Introduction
Amatoxins, including α-amanitin and β-amanitin, are hepatotoxic bicyclic peptides found in certain mushrooms, especially the genus Amanita (Wieland, 1986). Fatal poisonings occur every year throughout the world. The oral LD50 of α-amanitin is approximately 0.1 mg/kg, and a lethal dose can be obtained by eating a single large basidiocarp (fruiting body) (Wieland, 1986). α-Amanitin is also made by mushrooms in other genera, such as Galerina and Lepiota (Luo et al., 2012; Sgambelluri et al., 2014). The amatoxins and related phallotoxins are biosynthesized on ribosomes, unlike most other fungal cyclic peptides (Hallen et al., 2007; Luo et al. 2009; Luo et al., 2014).
Most cases of amanitin poisoning are accidental due to mis-identification of wild mushrooms. In these cases, a history of mushroom consumption and clinical evidence of liver malfunction are sufficiently strong indicators of amanitin poisoning to guide medical treatment. On the other hand, in some accidental or intentional poisonings the patient is not aware of having eaten mushrooms. Dogs and cats are also often victims of unobserved α-amanitin-related mushroom poisonings (Puschner et al., 2007). There is also some concern that fungal toxins such as α-amanitin have the potential to be used as bioweapons (Madsen, 2001; Russell and Paterson, 2006).
Stable-isotope labeled compounds have many applications in biochemical research, including metabolism and pharmacokinetics of drugs and other foreign compounds in animals and humans (Baillie, 1981; Haskins, 1982; Mutlib, 2008). One major use of stable-isotope labeled compounds is as internal standards to quantify metabolites in complex matrices such as blood, stomach contents, and urine. Stable-isotope labeled mycotoxins, such as fumonisin FB1 and deoxynivalenol, are widely used for the analysis of food products and animal feed (Hartl et al., 1999; Häubl et al., 2006; Spanjer et al., 2008; Malachová et al., 2014).
There are currently no available internal standards for any of the amatoxins (Johnson et al., 2010). To the best of our knowledge, α-amanitin has never been chemically synthesized, which thwarts this route to producing α-amanitin labeled with either radioactive or stable isotopes. Chemical radiolabeling with 125I led to a chemically distinct compound with reduced activity (Morris et al., 1978; Faulstich et al., 1981). In vivo labeling is hindered by the difficulty of culturing most species of Amanita. Even culturable species of Amanita grow very slowly (Zhang et al., 2005). Galerina marginata, on the other hand, is a saprotrophic mushroom that can be grown in culture (Enjalbert et al., 2004; Gulden et al., 2005). G. marginata contains α-amanitin in its fruiting bodies and the mycelium produces it in liquid culture (Benedict et al., 1966; Benedict and Brady, 1967; Muraoka and Shinozawa, 2000). We studied this fungus as a possible route for the production of heavy-isotope labeled α-amanitin that could be used as an internal standard for mass spectrometric analyses in forensic and clinical situations.
2. Materials and Methods
2.1. 15N-labeled yeast extract
Dried baker’s yeast (Saccharomyces cerevisiae) was purchased at a local grocery store and grown on YPAD agar (1% yeast extract, 2% Bacto peptone, 2% dextrose, 2% Bacto agar, and 0.0075% L-adenine hemisulfate). For seed culture, a fresh colony of S. cerevisiae was transferred into 4 ml 15N-YNB medium, composed of (per liter), 30 g glucose, 1.2 g 15NH4Cl (Sigma-Aldrich #299251, 98% 15N), 1 g KH2PO4, 0.5 g MgSO4·7H2O, 0.1 g NaCl, 0.1 g CaCl2, 0.002 mg biotin, 0.4 mg calcium pantothenate, 0.002 mg folic acid, 2 mg inositol, 0.4 mg niacin, 0.2 mg p-aminobenzoic acid, 0.4 mg pyridoxine·HCl, 0.2 mg riboflavin, 0.4 mg thiamine·HCl, 0.5 mg boric acid, 0.04 mg copper sulfate, 0.1 mg sodium iodide, 0.2 mg ferric chloride, 0.4 mg magnesium sulfate, 0.2 mg sodium molybdate, and 0.4 mg zinc sulfate. The cultures were grown overnight with rotary shaking at 250 rpm and 30°C. The seed culture was then transferred to 50 ml 15N-YNB medium in 250-ml baffled flasks and incubated under the same shaking conditions overnight. The next day, the culture was transferred to 500 ml 15N-YNB medium in 2-L baffled flasks and incubated under the same shaking conditions for 24 h. The culture was centrifuged at 10,000 g for 10 min, the pellet re-suspended in a minimum amount of double de-ionized water (starting with 1 ml) with constant stirring until the entire pellet was re-suspended, and then transferred into a 50-ml conical screw cap tube (Sarstedt). Resuspension typically required 3 to 5 ml water per liter culture.
The suspension was then subjected to a procedure designated “glass-beads-assisted maturation”. This method is based on a commercial process used for making yeast extract. On the first of three days, the suspension was vortexed without glass beads for 2 min once per hour for 6 hr. On the second day, 1 ml of glass beads was added to every 3 ml of suspension followed by vortexing twice for 2 h each time, once in the morning and once five hours later. The glass beads (Sigma-Aldrich #G8772) were 425–600 µm in diameter and washed for one hour with 1 N HCl, rinsed thoroughly with deionized water, and autoclaved. The same vortexing treatment continued on the third day without adding additional glass beads. The suspension was centrifuged at 10,000 g for 5 min and the supernatant transferred to a clean tube. The pellet was then washed twice with an equal amount of water by vortexing for 30 min. The three pooled supernatants were lyophilized, resulting in 15N-labeled yeast extract in powder form. The yield was typically 0.8 to 1.4 g 15N-yeast extract per liter culture.
2.2 Culture of G. marginata in 15N-medium
Strain CBS 339.88 (obtained from the Centraalbureau voor Schimmelcultures, Utrecht, Netherlands) was maintained on standard potato dextrose agar (PDA) by transferring every two months. This particular strain is monokaryotic and makes α-amanitin on artificial media (Luo et al., 2012). The medium, designated 15N-HSV-5C (Muraoka and Shinozawa, 2000), contains, in one liter, 1 g 15N yeast extract, 5 g glucose, 0.2 g 15NH4Cl, 0.1 g KCl, 0.1 g CaSO4·2H2O, 1 mg thiamine·HCl, and 0.1 mg biotin, pH 5.2. 15N-HSV-5C (70 ml) in 250 ml flasks were used for liquid culture and inoculation was done by transferring 10 thin slices (8 mm × 8mm × 1mm) of the G. marginata PDA agar culture to each flask. Only the freshest edges of the colony were used. The slices were separated from each other with gentle swirling by hand. The flasks were then incubated at 110 rpm at room temperature for up to 40 d. Typically, G. marginata forms spherical mycelial balls up to 1.5 cm in diameter under these conditions and the color gradually changes from white to slightly brown (Fig. 1). A time course was done by collecting two mycelial balls from each flask every 3 days starting at day 20 with two biological replicates. In total, nine time points were obtained. The harvested mycelium was lyophilized to dryness and stored at −20 °C.
Fig. 1.
Liquid culture of G. marginata. A. Shake culture. B. Close-up of flasks showing mycelial balls.
2.3. Toxin extraction
Lyophilized fungal mycelium (0.02 g for the time course experiments, or 0.5 g for large scale extractions) was ground in liquid nitrogen in a ceramic mortar and pestle. Extraction solution, composed of methanol:water:0.01 M HCl (5:4:1) (Enjalbert et al., 1992), was added at 5 ml/0.1 g biomass. The suspensions were ground for another 5 min and then transferred to 1.5-ml microcentrifuge tubes (for the time course) or 50-ml conical screw-cap tubes (Sarstedt) (for the large scale extractions) and incubated at room temperature overnight. The next day, the suspensions were centrifuged at 10,200 × g for 3 min and the supernatants were collected, lyophilized, and re-suspended in 120 µl water (for the time course) or 3 ml water (for the large scale extraction). The extracts were then boiled for 5 min in 1.5-ml microcentrifuge tubes sealed with clamps and centrifuged at 10,200 × g for 5 min. The supernatants were filtered through a 0.22 µm filter (Millipore Millex-GV for the time course experiments or Millex-GS for large scale extractions) and stored at −20°C.
2.4. Toxin analyses and purification
The HPLC system was an Agilent Model 1200 equipped with a multiwavelength UV detector set to 280, 295, and 305 nm and an Agilent quadrupole 6120 mass spectrometer. Elution solution A was 0.02 M aqueous ammonium acetate:acetonitrile (90:10, v/v), adjusted to pH 5 with glacial acetic acid, and solution B was 0.02 M aqueous ammonium acetate:acetonitrile (76:24, v/v), also adjusted to pH 5. The flow rate and gradient profile were as described by Enjalbert et al. (1992).
For the time course experiments, separations were performed on a Higgins Proto 300 analytical C18 column (150 × 4.6 mm, 5 µm particle size, Higgins Analytical, Mountain View, CA). For large-scale toxin purification, a two-step chromatography procedure was used. The first separation was performed on a semi-preparative C18 column (25 cm × 10 mm, 5 µm, Supelcosil LC-18 Semi-prep, Supelco, Bellefonte, PA). The flow rate was 2 ml/min with a step-wise gradient profile of 100% A for 3 min, 43% A for 7 min, and 0% A for 9 min. Loading was 1 ml of the toxin extract. Fractions were collected and 10 µl of each sample submitted to high-resolution MS analysis on a Waters Xevo G2-S QToF system with all 14N replaced by 15N in the parameters setting. Fractions containing 15N-labeled α-amanitin were pooled, lyophilized, and then re-dissolved in 100 µl H2O. A second separation was performed on the Higgins C18 column. The flow rate was 1 ml/min with a gradient of 100% solution A to 100% solution B in 15 min. The fractions containing amanitin were collected, dried under vacuum (SpeedVac, Thermo Scientific), and re-dissolved in 100 µl H2O. A third HPLC analysis was used to measure the purity of the resulting toxin. Purified toxin (100 µl) was run under the same conditions as described in the second separation. Purity of the toxin was calculated by integration of the corresponding peak area at wavelength 280 nm. The α-amanitin was quantitated by comparison to standard α-amanitin (Sigma-Aldrich #A2263).
2.5. NMR of 15N-labeled α-amanitin
15N-labeled α-amanitin was dissolved in 90% H2O/10% D2O to a final concentration of 1.8 mM. 4,4-Dimethyl-4-silapentane-1-sulfonic acid (DSS) (10 µM) (Sigma-Aldrich) was included as the chemical shift reference. A 2-D 1H-15N-HSQC spectrum was recorded at 25 °C with 2048 data points in F2, 256 increments in F1, 16 scans per increment, on a Bruker Avance 900 MHz spectrometer using a TCI cryoprobe. Spectral windows were 16 ppm in F2 and 32 ppm in F1. 15N shifts were referenced indirectly (Wishart et al., 1995).
3. Results
3.1. Fungal culture and toxin production
In preliminary studies, G. marginata was found to grow poorly in the absence of yeast extract. We tested a variety of alternate nitrogen sources (yeast nitrogen base, NH4Cl, amino acids, and (NH4)2SO4), but none of them were satisfactory. Commercial 14N yeast extract was found to result in poorly labeled amanitin, i.e., the enrichment with 15N was only ~50%. A commercial source being unavailable, we prepared 15N-yeast extract in-house.
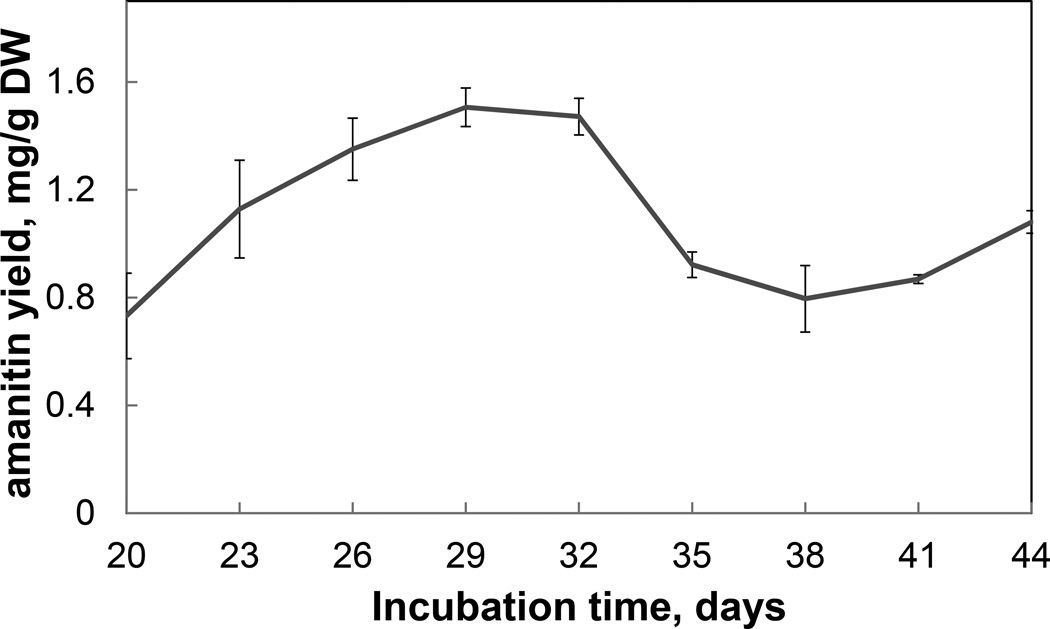
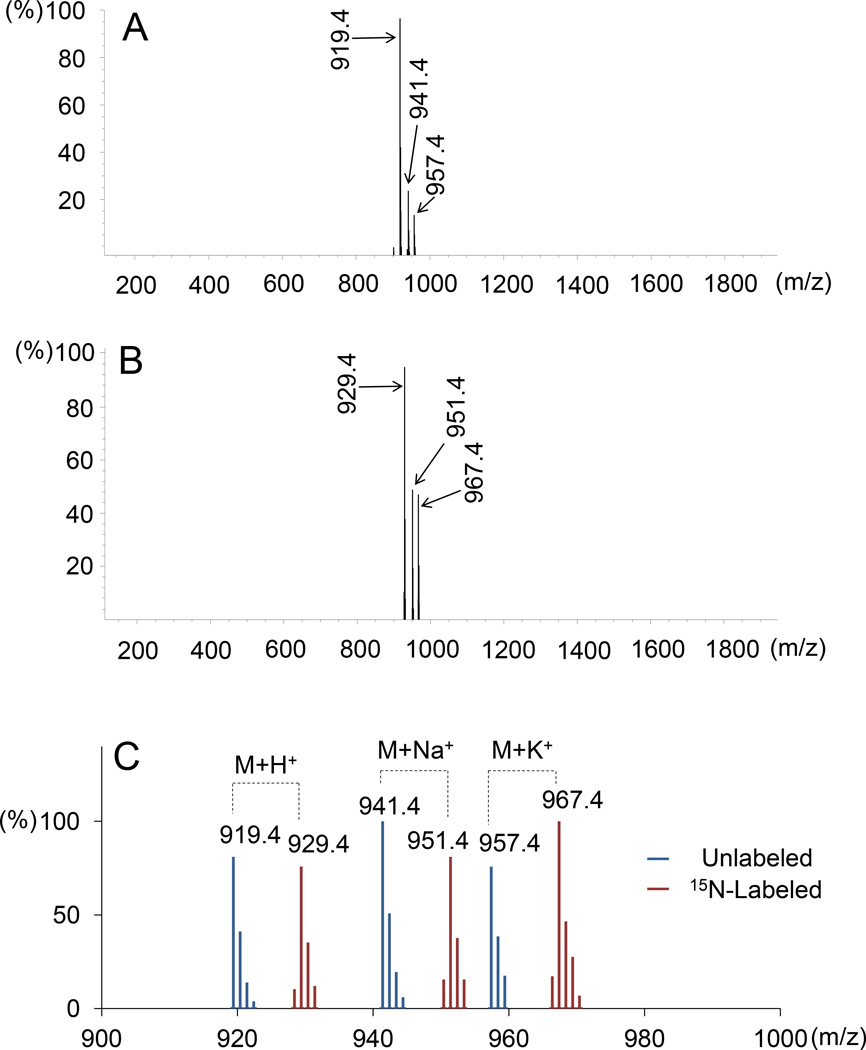
Using 15N yeast extract and 15NH4Cl as the sole sources of N, toxin production by G. marginata grown in shake culture peaked at about day 29 at 1.6 mg/g dry weight (Fig. 2). For large scale purification, the mycelial mass was harvested at day 29 or 30. α-Amanitin extracted from lyophilized mycelium stored for 6 months at −20°C showed no signs of degradation. Toxin production was monitored by HPLC reverse-phase separation and UV detection. A fully 15N-labeled form of α-amanitin (native mass 918.4) would have a mass of 928.4 due to the ten N atoms. Growing in liquid 15N-HSV-5C, G. marginata still produced a major peak of absorbance at 280 nm at the same retention time as native α-amanitin, but the signal at m/z 919.4 [M+H+] was no longer present. Instead, a new mass of 929.4 [M+H+] became the most significant, indicative of a compound labeled at all 10 N atoms with 15N.
Fig. 2.
Time course of 15N-labeled α-amanitin accumulation in G. marginata grown in shake culture. Each data point is the average of two biological replicates. Error bars indicate the range.
3.2. Characterization of 15N-labeled α-amanitin
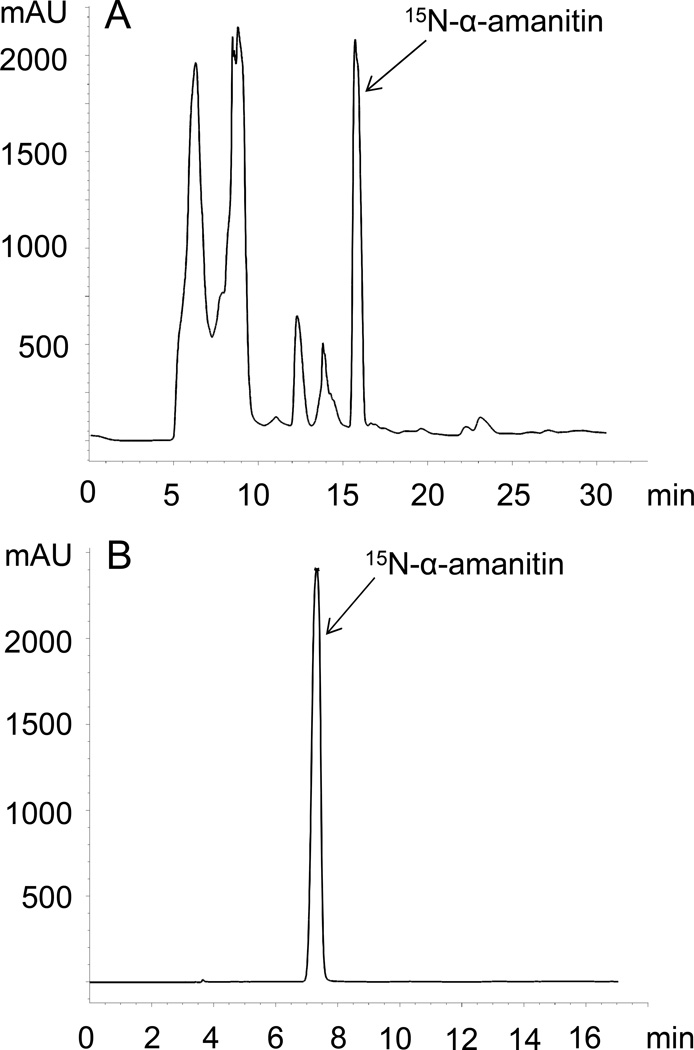
Based on integration of the α-amanitin OD280 peak at the last stage of purification (Fig. 3), the total amount of 15N-labeled α-amanitin obtained was 8 mg from G. marginata harvested from approximately 6 l of culture. The chemical purity judged by integration at OD280 was estimated to be 99.8%. Based on the relative peak height of the m/z signal one mass unit smaller than the fully labeled compound (i.e., an M+H+ signal of 928.4), the isotope enrichment was calculated to be >97%. The MS spectra of native and 15N-labeled α-amanitins are shown in Fig. 4.
Fig. 3.
HPLC chromatograms of the (A) first and (B) third separation of 15N-labeled α-amanitin. The y-axis is OD280 (mAU).
Fig. 4.
Mass spectrometric analysis of (A) native and (B) 15N-labeled α-amanitin. A. [M+H+] = 919.4, [M+Na+] = 941.4, and [M+K+] = 957.4; B. [M+H+] = 929.4, [M+Na+] = 951.4, and [M+K+] = 967.4. (C) Overlaid spectra of 14N-amanitin and 15N-amanitin, shown in expanded scale.
α-Amanitin has a formula of C39H54N10O14S with a monoisotopic mass of 918.3541. The calculated mass of the [M+H+] ion was 919.3620. The high resolution mass spectrum of purified 15N-labeled α-amanitin showed a mass of 929.3330, matching the formula C39H5515N10O14S (calculated mass 929.3323) with an error of 0.8 ppm.
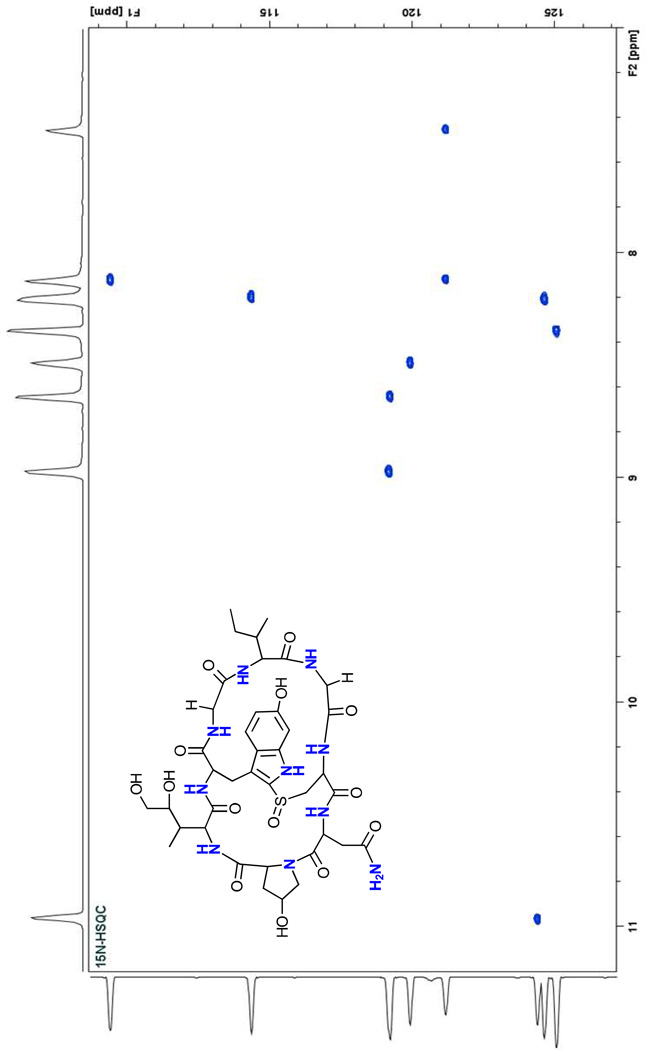
The two-dimensional 1H-15N HSQC spectrum of the labeled product contained ten cross-peaks corresponding to the ten 15N nuclei in α-amanitin (Fig. 5). Three resonances are readily assignable: A cross-peak with a 1H shift of 10.96 ppm can be assigned to the tryptophan indole NH and two other resonances at 7.45 and 8.12 ppm to the amide NH2 of the side-chain of asparagine. All ten signals were present at approximately the same intensity, suggesting equal incorporation of the 15N label at each position.
Fig. 5.
1H-15N HSQC spectrum of 15N-labeled α-amanitin. The spectrum shows ten signals corresponding to the ten N atoms in α-amanitin.
4. Discussion
To the best of our knowledge, this is the first production of labeled α-amanitin. A major impediment in development of a successful in vivo protocol using G. marginata was the need of this fungus for yeast extract to grow and produce amanitin. Commercial (14N) yeast extract caused excessive isotope dilution, and therefore we developed a novel method to make 15N-labeled yeast extract called “glass bead-assisted maturation”. Using this, we were able to produce 15N-amanitin with better than 98% labeling of its ten N atoms. The product is sufficient for use as an internal standard in mass spectrometry-based applications.
Unlike most Amanita species, G. marginata is a saprotrophic fungus and therefore can be more readily cultured than the obligately ectomycorrhizal amanitin-producing species of Amanita (Benedict et al., 1966; Benedict et al., 1967; Zhang et al., 2005; Luo et al., 2012; Luo et al., 2014). In comparison, the other reported case of amanitin production in culture used the fungus Amanita exitialis, which produces α- and β-amanitin on agar medium. The process takes up to 50 days and the amanitin levels reach ~10% of the concentration found in basidiocarps (Zhang et al., 2005). To the best of our knowledge, A. exitialis has not been used to produce labeled amanitins. G. marginata grows faster and reaches peak production of α-amanitin around 30 d. The toxin concentration found in G. marginata culture was calculated to be two times that found in the culture of A. exitialis. In our lab, consistent toxin production has been observed using the same strain of G. marginata for over eight years, suggesting that its biosynthetic pathway is stable.
Highlights.
Efficient enrichment required growing Galerina with 15N-ammonium chloride plus 15N-labeled yeast extract
Enrichment of >97% was obtained in all 10 N atoms of α-amanitin
The labeled material will be useful for forensic and clinical applications
Acknowledgements
This research was supported by contract 200-2014-M-58133 from the Centers for Disease Control, Atlanta, GA. Additional support came from grant 1R01-GM088274 from the U.S. National Institutes of Health General Medical Sciences. We thank the Michigan State University (MSU) Mass Spectrometry Core Facility for high-resolution MS, and the MSU Max T. Rogers NMR facility for NMR analysis. We thank Yair Shachar-Hill, Department of Plant Biology, MSU, for advice on in vivo isotope labeling, and Melissa Carter, CDC, for valuable discussions about the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hong Luo, Email: hongluo@msu.edu.
Jonathan D. Walton, Email: walton@msu.edu.
References
- 1.Baillie TA. The use of stable isotopes in pharmacological research. Pharmacol. Rev. 1981;33:81–132. [PubMed] [Google Scholar]
- 2.Benedict RG, Tyler VE, Brady LR, Weber LJ. Fermentative production of Amanita toxins by a strain of Galerina marginata. J. Bacteriol. 1966;91:1380–1381. doi: 10.1128/jb.91.3.1380-1381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict RG, Brady LR. Further studies on fermentative production of toxic cyclopeptides by Galerina marginata (Fr Kühn) Lloydia. 1967;30:372–378. [Google Scholar]
- 4.Enjalbert F, Gallion C, Jehl F, Monteil H, Faulstich H. Simultaneous assay for amatoxins and phallotoxins in Amanita phalloides Fr by high-performance liquid chromatography. J. Chromatogr. 1992;598:227–236. doi: 10.1016/0021-9673(92)85052-u. [DOI] [PubMed] [Google Scholar]
- 5.Enjalbert F, Cassanas G, Rapior S, Renault C, Chaumont JP. Amatoxins in wood-rotting Galerina marginata. Mycologia. 2004;96:720–729. doi: 10.1080/15572536.2005.11832920. [DOI] [PubMed] [Google Scholar]
- 6.Faulstich H, Trischmann H, Wieland T, Wulf E. Ether derivatives of α-amanitin. Introduction of spacer moieties, lipophilic residues, and radioactive labels. Biochemistry. 1981;20:6498–6504. doi: 10.1021/bi00525a031. [DOI] [PubMed] [Google Scholar]
- 7.Gulden G, Stensrud Ø, Shalchian-Tabrizi K, Kauserud H. Galerina Earle: a polyphyletic genus in the consortium of dark-spored agarics. Mycologia. 2005;97:823–837. doi: 10.3852/mycologia.97.4.823. [DOI] [PubMed] [Google Scholar]
- 8.Häubl G, Berthiller F, Krska R, Schuhmacher R. Suitability of a fully 13C isotope labeled internal standard for the determination of the mycotoxin deoxynivalenol by LC-MS/MS without clean up. Anal. Bioanal. Chem. 2006;384:692–696. doi: 10.1007/s00216-005-0218-z. [DOI] [PubMed] [Google Scholar]
- 9.Hallen HE, Watling R, Adams GC. Taxonomy and toxicity of Conocybe lactea and related species. Mycol. Res. 2003;107:969–979. doi: 10.1017/s0953756203008190. [DOI] [PubMed] [Google Scholar]
- 10.Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA. 2007;104:19097–19101. doi: 10.1073/pnas.0707340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartl M, Herderich M, Humpf H-U. Rapid determination of fumonisin FB1 in corn products by high-performance liquid chromatography-electrospray mass spectrometry. Eur. Food Res. Technol. 1999;209:348–351. [Google Scholar]
- 12.Haskins NJ. The application of stable isotopes in biomedical research. Biomed. Mass Spectrom. 1982;9:269–277. doi: 10.1002/bms.1200090702. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RC, Kalb SR, Barr JR. Toxin analysis using mass spectrometry. In: Budowle B, Schutzer SE, Breeze RG, Keim PS, Morse SA, editors. Microbial Forensics. 2nd ed. Amsterdam: Elsevier; 2010. pp. 405–421. [Google Scholar]
- 14.Luo H, Hallen-Adams HE, Walton JD. Processing of the phalloidin proprotein by prolyl oligopeptidase from the mushroom Conocybe albipes. J. Biol. Chem. 2009;284:18070–18077. doi: 10.1074/jbc.M109.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo H, Hallen-Adams HE, Scott-Craig JS, Walton JD. Ribosomal biosynthesis of α-amanitin in Galerina marginata. Fung. Genet. Biol. 2012;49:123–129. doi: 10.1016/j.fgb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo H, Hong S-Y, Sgambelluri RM, Angelos E, Li X, Walton JD. Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in α-amanitin biosynthesis. Chem. Biol. 2014;21:1610–1617. doi: 10.1016/j.chembiol.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen JM. Toxins as weapons of mass destruction. Clin. Lab Med. 2001;21:593–605. [PubMed] [Google Scholar]
- 18.Malachová A, Sulyok M, Beltrán E, Berthiller F, Krska R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. 2014;A 1362:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Morris PW, Venton DL, Kelley KM. Biochemistry of the amatoxins: preparation and characterization of a stably iodinated alpha-amanitin. Biochemistry. 1978;17:690–698. doi: 10.1021/bi00597a021. [DOI] [PubMed] [Google Scholar]
- 20.Muraoka S, Shinozawa T. Effective production of amanitins by two-step cultivation of the basidiomycete, Galerina fasciculata FG-060. J. Biosci. Bioeng. 2000;89:73–76. doi: 10.1016/s1389-1723(00)88053-6. [DOI] [PubMed] [Google Scholar]
- 21.Mutlib AE. Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. Chem. Res. 2008;21:1672–1689. doi: 10.1021/tx800139z. [DOI] [PubMed] [Google Scholar]
- 22.Puschner B, Rose HH, Filigenzi MS. Diagnosis of Amanita toxicosis in a dog with acute hepatic necrosis. J. Vet. Diagn. Invest. 2007;19:312–317. doi: 10.1177/104063870701900317. [DOI] [PubMed] [Google Scholar]
- 23.Russell R, Paterson M. Fungi and fungal toxins as weapons. Mycol. Res. 2006;110:1003–1010. doi: 10.1016/j.mycres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Sgambelluri RM, Epis S, Sassera D, Luo H, Angelos ER, Walton JD. Profiling of amatoxins and phallotoxins in the genus Lepiota by liquid chromatography combined with UV absorbance and mass spectrometry. Toxins. 2014;6:2336–2347. doi: 10.3390/toxins6082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spanjer MC, Rensen PM, Scholten JM. LC-MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. 2008;25:472–489. doi: 10.1080/02652030701552964. [DOI] [PubMed] [Google Scholar]
- 26.Wieland T. Peptides of poisonous Amanita mushrooms. New York: Springer; 1986. [Google Scholar]
- 27.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. 1H, 13C, and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Chen ZH, Hu JS, Wei BY, Zhang ZG, Hu WQ. Production and characterization of amanitin toxins from a pure culture of Amanita exitialis. FEMS Microbiol. Lett. 2005;2:223–228. doi: 10.1016/j.femsle.2005.08.049. [DOI] [PubMed] [Google Scholar]