Abstract
The objective of this study was to determine whether estimates of ultrasonic attenuation could detect changes in the cervix associated with medically induced cervical remodeling. Thirty-six full term pregnant women underwent two transvaginal ultrasonic examinations separated in time by 12 hours to determine cervical attenuation, cervical length and changes thereof. Ultrasonic attenuation and cervical length data were acquired from a z.one (Zonare Medical Systems, Mountain View, CA. USA) ultrasound system using a 5–9 MHz endovaginal probe. Cervical attenuation and cervical length significantly decreased in the 12 hours between the pre-cervical ripening time point and 12 hours later. The mean cervical attenuation was 1.1±0.4 dB/cm-MHz before cervical ripening agents were used and 0.8±0.4 dB/cm-MHz 12 hours later, p < 0.0001. The mean cervical length also decreased from 3.1±0.9 cm before the cervical ripening was administered to 2.0±1.1 cm 12 hours later, p < 0.0001. Cervical attenuation and cervical length detected changes in cervical remodeling 12 hours after cervical ripening administration.
Keywords: cervical ripening, cervical remodeling, ultrasonic attenuation, cervical length
Introduction
Globally, over 13 million infants are born preterm each year (Harris-Requejo 2010). Preterm birth is defined as birth before 37 completed weeks of pregnancy (Martin et al. 2009). Spontaneous preterm labor is a syndrome associated with multiple etiologies of which only a percentage may be associated with cervical insufficiency (Romero et al. 2006). Regardless of the etiology of preterm labor, the cervix must remodel to allow the passage of the fetus. The role of the cervix during pregnancy is to maintain the fetus within the uterine cavity until fetal maturity. The strength of the cervix is due to its anatomic properties (length, thickness) and tissue composition (collagen, water and extracellular matrix) (House and Socrate 2006).
Most of our understanding of cervical remodeling in pregnancy is from animal studies and very few human studies. Thus, we describe a widely accepted hypothesis of the process of cervical remodeling in human pregnancy based upon the available evidence (Danforth 1947; Leppert 1995; Owen and Iams 2003; Romero et al. 2006; Word et al. 2007; Feltovich et al. 2010). During pregnancy, total cervical collagen content increases to provide strength to the cervix (Harkness and Nightingale 1962; Goilichkowski et al. 1980; Kokenyesi and Woessner 1990; Eckman et al. 1991; Woessner and Kokenyesi 1991; Yu and Leppert 1991). Before the onset of labor contractions and over a period of weeks, the cervix remodels through a process of collagen disorganization (Weiss 2000; House and Socrate 2006; Vidaeff and Ramin 2006a; Vidaeff and Ramin 2006b) wherein water content and proteoglycans are increased, and collagen concentration is decreased (Danforth et al. 1960; Goilichkowski et al. 1980; Yu and Leppert 1991; Leppert et al. 2000; Feltovich et al. 2005; McFarlin et al. 2006), thus resulting in increased extensibility and reduced stiffness of the cervix. During this process, increased space results among the collagen fibrils (Leppert and Yu 1991; Leppert 1995; Leppert et al. 2000; Feltovich et al. 2005; Clark et al. 2006). The biostructure, biochemistry and biomechanics of the cervix change markedly from an unripe to a ripe cervix (Leppert 1995; Leppert et al. 2000; Lawn et al. 2010; Mahmoud et al. 2013).
Currently, the most powerful risk factors for preterm birth are assessed by patient history of a prior preterm birth and from a shortened length of the cervix at around 20 weeks of pregnancy. There is a approximately a 40% recurrence of preterm birth in women with a prior history of preterm birth (Iams and Berghella 2010). Although cervical length assessment has become one of the most widely used tools to identify women at high risk for preterm birth, it has low positive predictive value in low-risk women because the majority of individuals identified with a short cervix still deliver at term (Berghella et al. 2009; Romero et al. 2013). In women at low risk for preterm birth, the positive predictive value of cervical length to predict spontaneous preterm birth ranges from 20 to 52% (Romero et al. 2013). Generally, the shorter the cervix, the greater the probability of preterm birth (Iams and Berghella 2010; Campbell 2011; Romero et al. 2013). There has been considerable work by our group (McFarlin et al. 2006; Bigelow et al. 2008; McFarlin et al. 2010; Bigelow et al. 2011; Labyed and Bigelow 2011a; Labyed et al. 2011; Lau et al. 2013; Poellmann et al. 2013) and others (House and Socrate 2006; Feltovich et al. 2010; Feltovich et al. 2012; House et al. 2012; Feltovich and Hall 2013; Carlson et al. 2014) to develop noninvasive methods to detect and understand cervical microstructural tissue properties associated with cervical remodeling.
Presently an in vivo, objective and noninvasive clinical method to determine tissue changes (increased collagen disorganization and water concentration) associated with cervical remodeling does not exist. Our research has sought to develop an ultrasonic method that could be correlated with changes associated with cervical remodeling, and potentially associated with preterm birth. We have translated promising basic science ultrasonic methods we used in an animal model (Bigelow et al. 2005; Bigelow and O’Brien 2006; McFarlin et al. 2006; Bigelow et al. 2008; Labyed and Bigelow 2011a; Labyed et al. 2011) for use in human pregnancy (McFarlin et al. 2006; Bigelow et al. 2008; McFarlin et al. 2010; Bigelow et al. 2011; Labyed and Bigelow 2011a; Labyed et al. 2011; Lau et al. 2013; Poellmann et al. 2013).
Estimates of ultrasonic attenuation have been used to evaluate the structure and function of tissue in health and disease (Insana et al. 1992; Wickline et al. 1992a; Wickline et al. 1992b; Insana 1995; O’Brien et al. 1995; Hall et al. 2000a; Hall et al. 2000b; Hall et al. 2000c; Baldwin et al. 2007). Ultrasonic attenuation has been found to be related to tissue stiffness, collagen content and organization and water concentration of tissues (Hall et al. 1997; Hall et al. 2000a; Hall et al. 2000b; Hall et al. 2000c; Baldwin et al. 2007), and also has been shown to be higher in collagen rich tissues such as a tendon (O’Brien 1977) and rat cervix (Bigelow et al. 2008) compared to low collagen content tissues, such as fat (O’Brien 1977). In pregnancy, as the collagen-rich cervix remodels to prepare for labor and birth, the cervix transforms from a rigid structure to a soft, extensible structure (Leppert 1995; House and Socrate 2006). We hypothesized that by estimating changes in cervical ultrasonic attenuation we could detect and monitor these dynamic changes in tissue microstructure and function.
Our prior animal model work demonstrated that the decreased collagen concentration associated with cervical remodeling affected ultrasonic attenuation (McFarlin et al. 2006; Bigelow et al. 2008). We found that estimates of cervical attenuation were significantly correlated with gestational age in the rat cervix as ripening occurred (McFarlin et al. 2006; Bigelow et al. 2008; Bigelow et al. 2011). In a small methodology development study of the human pregnant cervix, we were able to acquire and analyze backscattered radio frequency (RF) ultrasonic signals to estimate attenuation as the cervix remodeled (McFarlin et al. 2010). There was a statistically significant relationship between decreasing attenuation and interval to delivery (McFarlin et al. 2010). Women who delivered preterm had lower cervical attenuation values than those who delivered full term. The long-term goal of our program of research is to use ultrasonic attenuation to identify women at risk for preterm birth. Ultrasonic attenuation is a feature that could be incorporated into clinical ultrasound systems.
During pregnancy when labor must be medically induced, mechanical and pharmacological agents such as prostaglandins and cervical balloons can accelerate the process of cervical remodeling before inducing labor contractions (Boulvain et al. 2008). Prostaglandins have produced marked cervical remodeling changes in times as small as five to twelve hours (Leppert 1995; Calder et al. 2008). As a proof-of-concept study, our objective herein was to determine whether cervical attenuation was sensitive to cervical remodeling associated with medically induced cervical remodeling, a known process where cervix tissue remodels in a short time frame of 12 hours rather than over many weeks during pregnancy and spontaneous labor. Our hypothesis was that a significant decrease in cervical attenuation occurs after a ripening agent was administered.
Materials and Methods
Thirty-six pregnant women who were admitted for induction of labor were recruited for the study. Women were eligible to be included in the study if they were: ≥ 18 years of age; able to read, write and understand English; going to receive a prostaglandin cervical ripening agent for induction of labor; and agreed to twice undergo transvaginal ultrasonic examinations during labor induction. We chose to study women receiving prostaglandin cervical ripening agents for induction of labor, as marked change in cervical remodeling is known to occur within 12 hours (Facchinetti et al. 2012). Women were excluded from participating in the study if they had an anomalous fetus or were too ill to give informed consent. Women were recruited once they were admitted to the Labor and Delivery unit. None of the women underwent induction of labor for the purposes of this research. The research was approved by the Human Subjects Review Boards of the University of Illinois at Chicago and Rush University Medical Center. An informed consent statement was signed by each woman participating in the study.
Thirty-six women agreed to participate in the study and underwent a transvaginal ultrasonic examination with a standard 5–9 MHz endovaginal transducer to estimate ultrasonic attenuation and cervical length before cervical ripening agents were administered and then scanned again 12 hours after cervical ripening agents were administered. Immediately after each cervical scan, and without changing any scanner settings, a reference scan was recorded in a well-characterized, tissue-mimicking reference phantom with known ultrasonic properties (sound speed, attenuation coefficient, backscatter coefficient) that were comparable to average human tissue.
Cervical length was measured according to the standard method developed by Iams (Iams et al. 1996). The length of the cervix was measured with the transvaginal ultrasound probe placed in the anterior fornix of the vagina while the woman’s bladder was empty. Without placing pressure on the cervix, a sagittal image of the cervix was obtained where the internal and external os were present. The cervix was measured three times along the line made by the interface of the mucosal surfaces. The shortest cervical length was recorded.
The steps for processing the data are summarized in Figure 1. In-phase Quadrature (IQ) data were obtained from z.one ultrasound system (Zonare Medical Systems, Mountain View, CA) and converted to RF data (bandwidth: 3.4–7.1 MHz). The IQ data contained scans of the cervix and reference phantom. A Gammex® tissue-mimicking phantom (Gammex®, Middleton, WI) with a known attenuation of 0.5 dB/cm-MHz was used as a reference phantom.
Figure 1. Step by step process for estimating cervical attenuation coefficient.
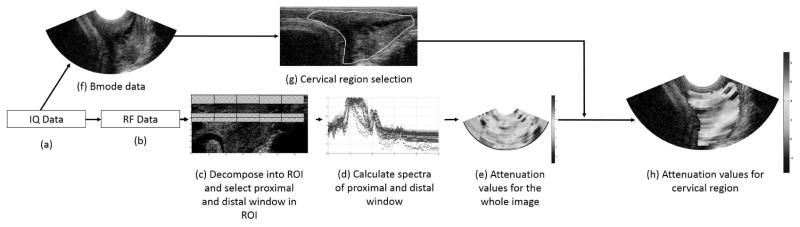
(a) IQ data obtained from machine, (b) IQ data converted to RF data, (c) RF data decomposed into regions of interest (ROIs) based on the pulse length and number of echoes. Spectral difference algorithm used; hence proximal and distal windows for each ROI are identified, (d) Spectrograms of proximal and distal windows are calculated, (e) Estimate the attenuation coefficient for the entire image, (f) IQ data converted to B-mode image, (g) User selects the cervical region from the B-mode image, (h) Attenuation coefficients estimated for the selected cervix region.
The RF echo data from the cervix were windowed into smaller regions of interest (sub-ROIs), and the attenuation was estimated for each sub-ROI to represent a spatial map of the attenuation throughout the closed portion of the cervix. Early in our methodology (McFarlin et al. 2010), we selected the most homogeneous appearing area of the cervix. However, we were concerned about sub-ROI selection bias and reproducibility of the measure. Thus, our approach with this and follow-up studies has been to map the entire closed portion of the cervix and use a mean attenuation value from all of the sub-ROIs as the cervical attenuation.
The spectral log difference method was used to estimate the attenuation by calculating the slope of the straight line that fits the log ratio (difference between log spectra) of the two power spectra (cervix and reference phantom spectra) from the proximal and the distal segments of the sub-ROIs (Labyed and Bigelow 2011b). The spectral log difference method is one that is least susceptible to tissue heterogeneity as was observed in our previous studies (Bigelow et al. 2011; Labyed and Bigelow 2011b). Our criterion for selecting the size of the sub-ROIs was to have enough tissue to make attenuation estimates with the least error, and at the same time minimize the heterogeneity of the tissue included in the sub-ROI. Figure 2 displays an attenuation image (a spatial map of the sub-ROIs) of the cervix that shows consistency with known tissue layers of the human cervix (Leppert 1995). For example, the image shows the attenuation of the cervical mucus layer to be essentially zero (Aisemberg et al. 2013), which is consistent with the known attenuation of water (Shung et al. 1992), while the tissue layers in the cervix have an attenuation range from 0 to 2.5 dB/cm-MHz.
Figure 2. Scan of the cervix displaying tissue architecture via attenuation mapping.
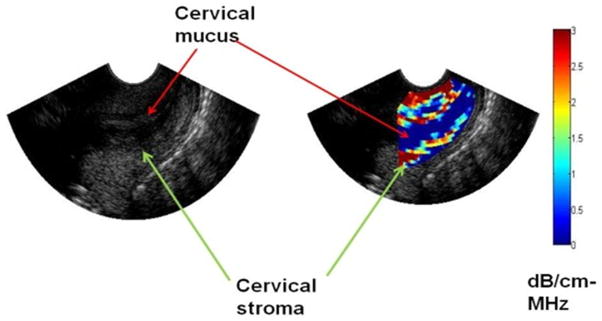
The colored pixels represent attenuation values the stroma of the cervix and the blue pixels in the cervical canal (mucus which is almost totally water) have an attenuation value close to 0, consistent with the attenuation of water.
We evaluated the inter-rater reliability of attenuation mapping of the cervix by having 50% of the scans mapped and reprocessed independently by a second examiner and found an inter-rater reliability of r = 0.97, p < 0.001. All data were entered into an electronic database and analyzed with IBM SPSS 19.0 (Armonk, NY) statistical software. Analysis included descriptive statistics, paired two-tailed t tests, Pearson correlation and ANOVA. α < 0.05 was considered significant.
Results
The thirty-six women participating in the study were at full term pregnancy (≥39 weeks of gestation). Table 1 displays maternal characteristics of study participants. Elective induction of labor, and post-dates pregnancy were the most common indications listed for labor induction. While the majority of women in the study delivered vaginally, 16 (44%) delivered by cesarean section. There was no significant difference in attenuation and cervical length at each time point among the women delivering vaginally or by cesarean (Table 2). The main indication for cesarean section was arrest of labor.
Table 1.
Characteristics of the sample.
| Variable | All Participants N = 36 |
|---|---|
| Maternal age years (m, SD) | 29.4 (6.7) |
|
| |
| Race/Ethnicity (number) | |
| Black | 13 |
| White | 11 |
| Latina | 3 |
| Asian | 9 |
|
| |
| Gravida (m, SD) | 2.2 (1.5) |
|
| |
| Living Children (m, SD) | 0.7 (1.2) |
|
| |
| Gestational age, weeks. (m, SD) | 39.6 (1.3) |
Table 2.
Cervical attenuation and cervical length at both time points and mode of delivery.
| Cervical Variable | NSVD, Mean (SD) | Cesarean, Mean (SD) | F statistic | p |
|---|---|---|---|---|
| Cervical length initial (cm) | 2.9 (0.9) | 3.0 (0.8) | 0.04 | 0.83 |
| Cervical length @ 12 hours (cm) | 2.1 (1.0) | 0.89 | 0.77 | |
| Attenuation initial (dB/cm-MHz) | 1.08 (45) | 1.20 (0.39) | 0.80 | 0.37 |
| Attenuation @ 12 hours (dB/cm-MHz) | 0.88 (0.33) | 0.76 (0.32) | 1.15 | 0.29 |
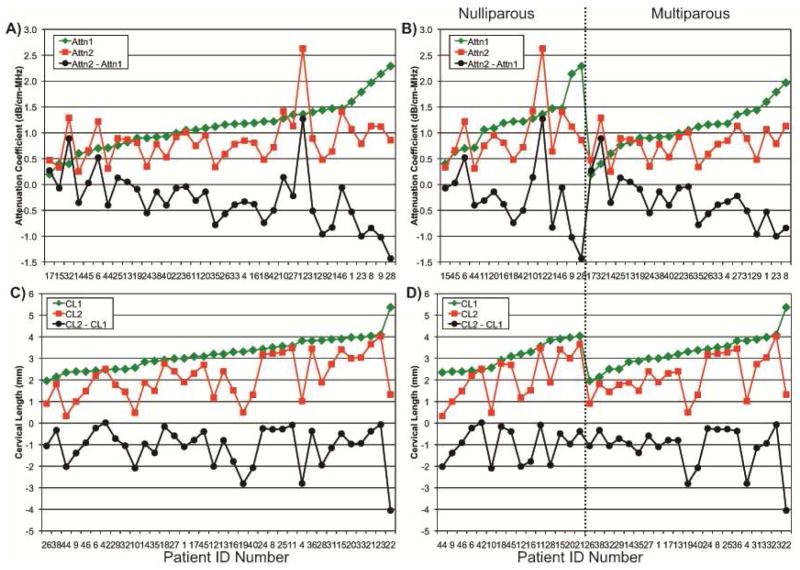
Figure 3 displays individual attenuation (Fig. 3A) and cervical length (Fig. 3C) outcomes. The women who had the highest attenuation at the pre-cervical ripening time point were those who also had the greatest decrease in attenuation at the second scan 12 hours later. Initial attenuation values pre- and post-cervical ripening were similar among nulliparous and multiparous women (Fig. 3B). Cervical attenuation significantly decreased (p<0.0001) in the 12 hours between the pre-cervical ripening attenuation time point and 12 hours later. The mean cervical attenuation of these full term women was 1.1±0.4 dB/cm-MHz before cervical ripening agents were used and 0.8±0.4 dB/cm-MHz 12 hours later. Thus, by using ultrasonic attenuation, we were able to detect changes in cervical remodeling in a short period of time, suggesting that ultrasonic attenuation is sensitive to tissue property changes.
Figure 3. Thirty-six subjects each scanned twice and approximately 12-hours apart, for which ultrasonic attenuation (Attn) and cervical length (CL) were determined.
Attn1 and CL1 denote before a cervical ripening agent was administered, Attn2 and CL2 denote 12 hours after the administration of a cervical ripening agent, and Attn2 – Attn1 and CL2 - CL1 denote the changes between the two attenuation and cervical length outcomes. Fig. 3A and 3C are ordered by increasing Attn1 and CL1. Fig. 3B and 3D segmented by parity into nulliparous and multiparous, and then for each, into their respective ordering by increasing Attn1 and CL1.
As expected, mean cervical length also decreased from 3.1±0.9 cm before the cervical ripening was administered to 2.0 ±1.1 cm 12 hours later, p < 0.0001. Pre-cervical ripening, cervical length ranged from 2.0 to 5.5 cm (Fig. 3C). Initial cervical lengths pre- and post-cervical ripening were similar among nulliparous and multiparous women (Fig. 3D). There was no correlation between cervical attenuation and cervical length, suggesting that these measures may be independently detecting structures in the cervix.
Discussion
In this study, we were able to detect changes in ultrasonic attenuation of the cervix at 12 hours after a cervical ripening agent was administered. Our previous work found a relationship between cervical tissue properties and attenuation (McFarlin et al. 2006; McFarlin et al. 2010; Labyed et al. 2011). Interestingly, ultrasonic attenuation was not correlated with cervical length, thus suggesting that cervical attenuation and cervical length are likely independent measures because each may be sensitive to different structures and/or spatial scales of the cervix. One can think about spatial scales between cervical attenuation and cervical length. Ultrasonic attenuation is a measure of cervical tissue at a scale of around the ultrasonic wavelength, that is, about 300 μm and is affected by tissue hydration and density. For example, the attenuation of water and cervical mucus is about 0 dB/cm-MHz (see Figure 2). On the other hand, ultrasonic cervical length is a measure at a larger scale, around a couple of centimeters (about 100 times greater than the ultrasonic wavelength). It is not surprising that the attenuation coefficient ranges from 0 to 2.5 dB/cm-MHz; cervical tissue is heterogeneous in nature (Feltovich et al. 2010; Lau et al. 2013). Thus, to address the heterogeneity issue we averaged all of the attenuation values within the selected portion of the cervix.
Both cervical length and attenuation decreased significantly from the initial scan to the scan 12 hours after a cervical ripening agent was administered. We hypothesized that there would be a significant decrease in cervical attenuation after the cervix was exposed to a ripening agent. We did not power the study or was it our intention in this study to use this technology to predict mode of delivery (cesarean or vaginal birth). Cervical remodeling is only one factor when determining whether a woman will deliver vaginally or by cesarean. Factors such as the size and position of the fetus, size of the maternal pelvis, strength of uterine contractions, and fetal tolerance of labor also need to be clinically considered.
Our previous animal study results indicated: a decrease in attenuation with gestational age of rat pregnancy; marked cervical collagen content and water increase in pregnancy; and increased collagen disorganization during the process of cervical remodeling. In human pregnancy (McFarlin et al. 2006; Bigelow et al. 2011), ultrasonic attenuation has been related to the number of weeks to delivery and risk of preterm birth in a pilot study (McFarlin et al. 2010). The attenuation values in this study are similar to attenuation values during pregnancy in our previous study (McFarlin et al. 2010). Thus, ultrasonic attenuation may have the potential to be an additional objective measure of cervical tissue remodeling. Although macro structural changes of the cervix can be quantified with cervical length (Iams et al. 1996; Berghella et al. 2009; Iams and Berghella 2010; Romero et al. 2013) and cervical consistency index (Parra-Saavedra et al. 2011), there is currently no method to objectively estimate cervical tissue property changes noninvasively.
Acknowledgments
Funding for this project was supported in part by the University of Illinois at Chicago Center for Clinical and Translational Science, NIH, grant UL1TR000050 and NIH/NICHD grant R21HD062790 to BLM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank the women who participated in this study as well as the Obstetric residents and nurses at Rush University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PloS one. 2013;8:e56161. doi: 10.1371/journal.pone.0056161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SL, Yang M, Marutyan KR, Wallace KD, Holland MR, Miller JG. Ultrasonic detection of the anisotropy of protein cross linking in myocardium at diagnostic frequencies. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2007;54:1360–9. doi: 10.1109/tuffc.2007.396. [DOI] [PubMed] [Google Scholar]
- Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane database of systematic reviews. 2009:CD007235. doi: 10.1002/14651858.CD007235.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow TA, Labyed Y, McFarlin BL, Sen-Gupta E, O’Brien WDJ. Comparison of algorithms for estimating ultrasound attenuation when predicting cervical remodeling in a rat model. Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging. 2011:883–6. [Google Scholar]
- Bigelow TA, McFarlin BL, O’Brien WD, Jr, Oelze ML. In vivo ultrasonic attenuation slope estimates for detecting cervical ripening in rats: Preliminary results. J Acoust Soc Am. 2008;123:1794–800. doi: 10.1121/1.2832317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow TA, O’Brien WD., Jr Impact of local attenuation approximations when estimating correlation length from backscattered ultrasound echoes. J Acoust Soc Am. 2006;120:546–53. doi: 10.1121/1.2208456. [DOI] [PubMed] [Google Scholar]
- Bigelow TA, Oelze ML, O’Brien WD., Jr Estimation of total attenuation and scatterer size from backscattered ultrasound waveforms. J Acoust Soc Am. 2005;117:1431–9. doi: 10.1121/1.1858192. [DOI] [PubMed] [Google Scholar]
- Boulvain M, Kelly A, Irion O. Intracervical prostaglandins for induction of labour. Cochrane database of systematic reviews. 2008:CD006971. doi: 10.1002/14651858.CD006971. [DOI] [PubMed] [Google Scholar]
- Calder AA, Loughney AD, Weir CJ, Barber JW. Induction of labour in nulliparous and multiparous women: a UK, multicentre, open-label study of intravaginal misoprostol in comparison with dinoprostone. BJOG. 2008;115:1279–88. doi: 10.1111/j.1471-0528.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Munoz del Rio A, Hall TJ. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet Gynecol. 2014;43:452–8. doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. American journal of obstetrics and gynecology. 2006;194:1391–8. doi: 10.1016/j.ajog.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Danforth DN. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. American journal of obstetrics and gynecology. 1947;53:541–57. doi: 10.1016/0002-9378(47)90273-1. [DOI] [PubMed] [Google Scholar]
- Danforth DN, Buckingham JC, Roddick JW., Jr Connective tissue changes incident to cervical effacement. Am J Obstet and Gynecol. 1960;80:939–45. doi: 10.1016/0002-9378(60)90472-5. [DOI] [PubMed] [Google Scholar]
- Eckman G, Almstrom H, Gramstrom L, Malmstrom A, Norman M, Woessner JF., Jr . Connective tissue in human cervical ripening. In: Leppert PC, Woessner JF Jr, editors. The Extracellular Matrix of the Uterus, Cervix and Fetal Membranes: Synthesis, degradation and hormonal regulation. Ithica, N.Y: Perinatology Press; 1991. pp. 87–104. [Google Scholar]
- Facchinetti F, Fontanesi F, Del Giovane C. Pre-induction of labour: comparing dinoprostone vaginal insert to repeated prostaglandin administration: a systematic review and meta-analysis. J Mat Fetal Neo Med. 2012;25:1965–9. doi: 10.3109/14767058.2012.668584. [DOI] [PubMed] [Google Scholar]
- Feltovich H, Hall TJ. Quantitative imaging of the cervix: setting the bar. Ultrasound Obstet Gynecol. 2013;41:121–8. doi: 10.1002/uog.12383. [DOI] [PubMed] [Google Scholar]
- Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol. 2012;207:345–54. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltovich H, Ji H, Janowski JW, Delance NC, Moran CC, Chien EK. Effects of selective and nonselective PGE2 receptor agonists on cervical tensile strength and collagen organization and microstructure in the pregnant rat at term. Am J Obstet Gynecol. 2005;192:753–60. doi: 10.1016/j.ajog.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Feltovich H, Nam K, Hall TJ. Quantitative ultrasound assessment of cervical microstructure. Ultrason Imaging. 2010;32:131–42. doi: 10.1177/016173461003200302. [DOI] [PubMed] [Google Scholar]
- Goilichkowski AM, King SR, Mascaro K. Pregnancy related changes in rat cervix gycosaminogylcans. Biochem J. 1980;192:1–8. doi: 10.1042/bj1920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS, Dent CL, Scott MJ, Wickline SA. High-frequency ultrasound detection of the temporal evolution of protein cross linking in myocardial tissue. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2000a;47:1051–8. doi: 10.1109/58.852089. [DOI] [PubMed] [Google Scholar]
- Hall CS, Nguyen CT, Scott MJ, Lanza GM, Wickline SA. Delineation of the extracellular determinants of ultrasonic scattering from elastic arteries. Ultrasound Med Biol. 2000b;26:613–20. doi: 10.1016/s0301-5629(99)00165-9. [DOI] [PubMed] [Google Scholar]
- Hall CS, Scott MJ, Lanza GM, Miller JG, Wickline SA. The extracellular matrix is an important source of ultrasound backscatter from myocardium. J Acoust Soc Am. 2000c;107:612–9. doi: 10.1121/1.428327. [DOI] [PubMed] [Google Scholar]
- Hall CS, Verdonk ED, Wickline SA, Perez JE, Miller JG. Anisotropy of the apparent frequency dependence of backscatter in formalin fixed human myocardium. J Acoust Soc Am. 1997;101:563–8. doi: 10.1121/1.418119. [DOI] [PubMed] [Google Scholar]
- Harkness RD, Nightingale MA. The extensibility of the cervix uteri of the rat at different times of pregnancy. J Physiol. 1962;160:214–20. doi: 10.1113/jphysiol.1962.sp006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Requejo J. The global impact of preterm birth. In: Breghella V, editor. Preterm birth: prevention and management. Oxford, UK: Wiley-Blackwell; 2010. pp. 1–7. [Google Scholar]
- House M, Feltovich H, Hall TJ, Stack T, Patel A, Socrate S. Three-dimensional, extended field-of-view ultrasound method for estimating large strain mechanical properties of the cervix during pregnancy. Ultrason Imaging. 2012;34:1–14. doi: 10.1177/016173461203400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet Gynecol. 2006;28:745–9. doi: 10.1002/uog.3850. [DOI] [PubMed] [Google Scholar]
- Iams JD, Berghella V. Care for women with prior preterm birth. Am J Obstet Gynecol. 2010;203:89–100. doi: 10.1016/j.ajog.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. NEJM. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- Insana MF. Modeling acoustic backscatter from kidney microstructure using an anisotropic correlation function. J Acoust Soc Am. 1995;97:649–55. doi: 10.1121/1.412287. [DOI] [PubMed] [Google Scholar]
- Insana MF, Wood JG, Hall TJ. Identifying acoustic scattering sources in normal renal parenchyma in vivo by varying arterial and ureteral pressures. Ultrasound Med Biol. 1992;18:587–99. doi: 10.1016/0301-5629(92)90073-j. [DOI] [PubMed] [Google Scholar]
- Kokenyesi R, Woessner JF., Jr Relationship between dilatation of the rat uterine cervix and a small dermatan sulfate proteoglycan. Biol Reprod. 1990;42:87–97. doi: 10.1095/biolreprod42.1.87. [DOI] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA. A theoretical comparison of attenuation measurement techniques from backscattered ultrasound echoes. J Acoust Soc Am T. 2011a;129:2316–24. doi: 10.1121/1.3559677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA. A theoretical comparison of attenuation measurement techniques from backscattered ultrasound echoes. J Acoust Soc Am. 2011b;129:2316–24. doi: 10.1121/1.3559677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA, McFarlin BL. Estimate of the attenuation coefficient using a clinical array transducer for the detection of cervical ripening in human pregnancy. Ultrasonics. 2011;51:34–9. doi: 10.1016/j.ultras.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau TY, Sangha HK, Chien EK, McFarlin BL, Wagoner Johnson AJ, Toussaint KC., Jr Application of Fourier transform-second-harmonic generation imaging to the rat cervix. J Micro. 2013;251:77–83. doi: 10.1111/jmi.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, Group GR. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC pregnancy and childbirth. 2010;10 (Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–79. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Kokenyesi R, Klemenich CA, Fisher J. Further evidence of a decorin-collagen interaction in the disruption of cervical collagen fibers during rat gestation. Am J Obstet Gynecol. 2000;182:805–11. doi: 10.1016/s0002-9378(00)70329-2. [DOI] [PubMed] [Google Scholar]
- Mahmoud H, Wagoner Johnson A, Chien EK, Poellmann MJ, McFarlin B. System-level biomechanical approach for the evaluation of term and preterm pregnancy maintenance. J Biomech Egin. 2013;135:021009. doi: 10.1115/1.4023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a bit too early: recent trends in late preterm births. NCHS data brief. 2009:1–8. [PubMed] [Google Scholar]
- McFarlin BL, Bigelow TA, Laybed Y, O’Brien WD, Oelze ML, Abramowicz JS. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol. 2010;36:218–25. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin BL, O’Brien WDJ, Oelze ML, Zachary JF, White-Traut RC. Quantitative ultrasound assessment of the rat cervix. J Ultrasound Med. 2006;25:1031–40. doi: 10.7863/jum.2006.25.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WD., Jr Relationship between Collagen and Ultrasonic Attenuation and Velocity in Tissue. Proc Ultrasonics Intl. 1977;77:194–205. [Google Scholar]
- O’Brien WD, Jr, Sagar KB, Waritier DC, Rhyne TL. Acoustic propagation properties of normal, stunned, and infarcted myocarium. Morphological and biochemical determinents. Circulation. 1995;81:154–60. doi: 10.1161/01.cir.91.1.154. [DOI] [PubMed] [Google Scholar]
- Owen J, Iams JD. What we have learned about cervical ultrasound. Semin Perinat. 2003;27:194–203. doi: 10.1016/s0146-0005(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol. 38:44–51. doi: 10.1002/uog.9010. [DOI] [PubMed] [Google Scholar]
- Poellmann MJ, Chien EK, McFarlin BL, Wagoner Johnson AJ. Mechanical and structural changes of the rat cervix in late-stage pregnancy. J Mech Behav Biomed Mat. 2013;17:66–75. doi: 10.1016/j.jmbbm.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shung KK, Smith MB, Tsui B. Principles of Medical Imaging. San Diego: Academic Press; 1992. [Google Scholar]
- Vidaeff AC, Ramin SM. From concept to practice: the recent history of preterm delivery prevention. Part I: cervical competence. Am J Perinat. 2006a;23:3–13. doi: 10.1055/s-2005-923437. [DOI] [PubMed] [Google Scholar]
- Vidaeff AC, Ramin SM. From concept to practice: the recent history of preterm delivery prevention. Part II: Subclinical infection and hormonal effects. Am J Perinat. 2006b;23:75–84. doi: 10.1055/s-2006-931803. [DOI] [PubMed] [Google Scholar]
- Weiss G. Endocrinology of parturition. J Clin Endocrinol Metab. 2000;85:4421–5. doi: 10.1210/jcem.85.12.7074. [DOI] [PubMed] [Google Scholar]
- Wickline SA, Verdonk ED, Sobel BE, Miller JG. Identification of human myocardial infarction in vitro based on the frequency dependence of ultrasonic backscatter. J Acoust Soc Am. 1992a;91:3018–25. doi: 10.1121/1.402936. [DOI] [PubMed] [Google Scholar]
- Wickline SA, Verdonk ED, Wong AK, Shepard RK, Miller JG. Structural remodeling of human myocardial tissue after infarction. Quantification with ultrasonic backscatter. Circulation. 1992b;85:259–68. doi: 10.1161/01.cir.85.1.259. [DOI] [PubMed] [Google Scholar]
- Woessner JF, Jr, Kokenyesi R. Relationship between dilatation of the rat uterine cervix and a small dermatan sulfate proteoglycan. In: Leppert PC, Woessner JF Jr, editors. The Extracellular Matrix of the Uterus, Cervix and Fetal Membranes: Synthesis, degradation and hormonal regulation. Ithica, N. Y: Perinatology Press; 1991. pp. 97–112. [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- Yu SY, Leppert PC. The collagenous tissues of the cervix during pregnancy and delivery. In: Leppert PC, Woessner JF Jr, editors. The extracellular Matrix of the Uterus, Cervix, and Fetal Membranes: Synthesis, degradation and hormonal regulation. Ithaca, N.Y: Perinatology Press; 1991. pp. 68–76. [Google Scholar]