Abstract
Background
The etiopathophysiology of schizophrenia has long been linked to stress and the influence of stress is important in all stages of the illness. Previous examinations of perceived stress and acute stress responses may not capture this longitudinal stress pathophysiology. We hypothesized that the cumulative negative effects of stress, indexed by allostatic load (AL), would be elevated in schizophrenia, and that the AL paradigm would be relevant to our understanding of pathophysiology in schizophrenia.
Methods
We assessed allostatic load in 30 patients with schizophrenia (SZ; mean age=33; 17 males) and 20 healthy controls (HC; mean age=35; 12 males) using 13cardiovascular, metabolic, neuroendocrine and immune biomarkers. Participants’ perceived stress over the past month; functional capacity and psychiatric symptoms were also measured.
Results
Controlling for age, SZ had significantly higher AL as compared to HC (p=0.007). Greater AL was present in both early course and chronic SZ, and was associated with reduced functional capacity (p=0.006) and more psychotic symptoms (p=0.048) in SZ. Current level of perceived stress was not significantly elevated in SZ or associated with AL in either group.
Conclusions
The higher AL found in SZ may reflect increased bodily “wear and tear”, possibly caused by more chronic stress exposure or maladaptive responses to stress over time, although additional research is required to differentiate these causes. The higher AL is similarly present in early and chronic SZ, suggesting primary maladaptive stress physiology rather than secondary effects from medications or chronic illness.
Keywords: allostasis, cardiovascular, psychosis, cortisol, stress response
1. Introduction
Schizophrenia is a severe mental illness characterized by hallucinations, delusions, negative symptoms, and often significant cognitive and affective impairment. The disorder generally has onset of psychotic symptoms during late adolescence to early adulthood with lifetime prevalence around 1%. There is considerable heterogeneity in the clinical presentation, course and treatment response (Tandon et al., 2009). Additionally, the neurobiology is varied across patients and may include reduced grey matter volume, alterations in white matter, abnormal neurophysiology, and a range of neurochemical and neuropathological alterations (Keshavan et al., 2008). One of the common findings is the link between stress and the pathophysiology of schizophrenia at all stages of the illness. Prenatal stress increases disease risk (van Os and Selten, 1998) and stressful events precede disease onset (Norman and Malla, 1993). Patients have greater affective reactivity to experimental stress exposure after illness onset (Cohen and Docherty, 2004) and stressful life events precipitate worsening of psychosis (Hultman et al., 1997, Docherty et al., 2009). In addition, both psychological and physiological stress reactivity is associated with psychotic episodes, quality of life, and symptom severity (Garner et al., 2011, Belvederi Murri et al., 2012, Brenner et al., 2011). It has been hypothesized that environmental exposures, including stressful events, induce psychological and/or physiological changes that result in a final common pathway in SZ of altered dopamine neurotransmission and/or cognitive biases (Howes and Kapur, 2009, Myin-Germeys and van Os, 2007, Selten and Cantor-Graae, 2005, Howes et al., 2004). Together, this evidence points to longitudinal associations between stress pathophysiology and schizophrenia. Capturing the longitudinal effect may be needed to guide research and treatment. Previous laboratory paradigms have attempted to link stress to schizophrenia by testing acute physiological stress responses to various behavioral and pharmacological paradigms (Jansen et al., 2000, Marcelis et al., 2004, Breier et al., 1988). Blunted, rather than elevated or prolonged, cortisol responses have been described following acute psychological stress challenges (Elman et al., 1998, Jansen et al., 2000, Jansen et al., 1998). An index of the chronic, cumulative effect of stress responses may be an important complement to the study of acute stress responses in examining the etiological contributions of stress to schizophrenia.
The biological concept of allostatic load incorporates several elements of stress pathophysiology in one comprehensive model. The term “allostasis” refers to how an organism accommodates to a stressor by adjusting homeostatic set points to maintain internal stability (McEwen, 2004). These adaptive mechanisms operate through primary mediators including hormones of the hypothalamic-pituitary-adrenal (HPA) axis, catecholamines, neurotransmitters, and immune responses (McEwen and Wingfield, 2003). The brain is the central mediator of these accommodations to stressors, thus they can be influenced by appraisal, learning, memory and coping (Sterling and Eyer, 1988, Ganzel et al., 2010, McEwen and Gianaros, 2010). While allostasis mechanisms are adaptive in the short-term, they can lead to an elevated allostatic state in which there is an imbalance of the primary mediators. The cumulative effect of an elevated allostatic state is termed allostatic load (AL), which refers to the wear and tear the body experiences after having to respond to and make accommodations in light of stress (McEwen, 2002). The core emotional regions of the brain are theorized to initiate allostatic accommodation, and Ganzel et al. (2010) have demonstrated that these neural systems are some of the first to display AL wear and tear. Furthermore, there are theorized to exist time periods of increased sensitivity to environmental experience, during which the stress response itself could be changed in qualitative or quantitative ways (Ganzel and Morris, 2011), a concept perhaps linking findings on prenatal stress, early adversity and childhood trauma in the etiology of schizophrenia.
The AL index is traditionally operationalized as the sum of dysregulated neuroendocrine, immune, metabolic and cardiovascular biomarkers (Seeman et al., 1997) reflecting a multisystemic view of the physiological toll that is placed on the body for adaptation. The AL model has mostly been utilized to predict worse health outcomes, including development of cardiovascular disease, declines in physical and cognitive functioning and greater mortality risk (Seeman et al., 2001, Seeman et al., 2004). Additionally, AL has been examined in relation to greater cumulative risk, including physical (i.e. crowding), psychosocial (i.e. maternal separation) and personal (i.e. poverty) risk exposures (Danese and McEwen, 2012, Evans and Kim, 2012, Evans, 2003). The first operationalization of AL was performed using the Mac Arthur Studies of Successful Aging (Seeman et al., 2001; Seeman et al., 2004). They quantified AL through a series of 10 biomarkers; systolic and diastolic blood pressure are indices of cardiovascular activity; waist-hip-ratio reflects levels of metabolism and adipose tissue deposition; serum high density lipoprotein (HDL) and total cholesterol are related to the development of atherosclerosis; glycosylated hemoglobin measures long-term glucose metabolism; serum dihydroepiandrosterone sulfate (DHEA-S) is a functional HPA axis antagonist; overnight urinary cortisol excretion is a measure of 12 hour HPA axis activity; and overnight urinary noradrenalin and adrenalin index 12 hour sympathetic nervous system activity (McEwen, 2000). Subsequent studies have employed additional biomarkers, including resting heart rate to index cardiovascular activity, BMI to index metabolism and adipose tissue deposition, and C-reactive protein for assessing overall immune status [see (Juster et al., 2010) for a review]. We therefore also included these measures especially as prior research has documented elevated pulse rates (Lake et al., 1980, Van Kammen and Kelley, 1991), BMI, and a pro-inflammatory state (Ostermann et al., 2013) in SZ. AL is most commonly quantified by summing the number of parameters for which persons fall into the “highest” risk quartile (Seeman et al., 1997).
In psychiatry, AL has been investigated in bipolar disorder (Vieta et al., 2013, Kapczinski et al., 2008), depression (McEwen, 2004), PTSD (McFarlane, 2010) and their comorbid physical health conditions (McIntyre et al., 2007, Bizik et al., 2013). The AL paradigm is proposed as a relevant model for explaining the course of these illnesses, reflecting predispositions and then effects of repeated stress on the brain across biological mechanisms that contribute to psychiatric and somatic outcomes. AL could be instrumental to our understanding of how early life stress alters the stress response system as well as how cumulative stress associated with psychiatric illnesses corresponds to bodily changes evidenced by disease progression and comorbidity (Grande et al., 2012).
AL in SZ has recently been identified as an area as in urgent need of investigation (Misiak et al., 2014). In schizophrenia it is important to determine if cumulative stress effects arise from greater exposure to stress or maladaptive physiological responses to stress. The literature on frequency of stressful life events in retrospectively collected samples is mixed, with some studies reporting a greater frequency of events (Bebbington, P., Bowen, J., Ramana, R., 1997) in SZ and others failing to replicate this result (Dohrenwend et al., 1995). A prospectively studied cohort of recent onset SZ reported less frequent stressful life events as compared to controls yet SZ also reported less feelings of control over the stressful events that they did experience (Horan et al., 2005). In the current study we assessed individual’s perceived level of stress over the past month to investigate how the amount of recently experienced stress may be related to AL.
To our knowledge, this is the first investigation of the comprehensive AL index in schizophrenia. We hypothesized that: 1) patients with schizophrenia would have significantly higher allostatic load score as compared to controls, and 2) greater allostatic load score would be positively correlated with impaired functional capacity. We were also interested in testing whether AL was associated with perceived stress level, which may help to determine (or exclude) if elevated AL in schizophrenia could be due to increased stress exposure. This study will be the first to report on the combined AL index in SZ and its association with symptoms, duration of illness and functional measures.
2. Material and methods
2.1 Sample
Fifty individuals participated in the study and provided data for all 13 AL biomarkers (29 males, aged 18–57, mean age=33±12). Schizophrenia patients (n=30) were recruited from outpatient clinics of the Maryland Psychiatric Research Center and neighboring outpatient clinics. Healthy controls (n=20), who were frequency matched by age, sex and race to the patients, were recruited through media advertisements and random digit dialing. The Structured Clinical Interview for DSM-IV (First et al., 1995) was utilized to obtain DSM-IV diagnoses in all 50 individuals, which were based on consensus agreement from two psychiatrists. Controls had no current DSM-IV Axis I diagnoses and no family history of psychosis in the prior two generations. Twenty-seven patients were taking one or more antipsychotic medications and three were not on antipsychotic medications for two weeks or more. Of the 27 patients on antipsychotics, four were taking first generation antipsychotics, 22 taking second generation, and one was taking both first and second generation antipsychotics. Major medical and neurological illnesses, history of head injury with cognitive sequelae, mental retardation, substance dependence within the past 6 months, or current substance abuse (except nicotine) were exclusionary. An additional criterion on menstrual cycles was implemented for females to reduce variances from cyclic hormonal effects: females were tested within the first 10 days of their menstrual cycle if they experienced regular cycles. Participants gave written informed consent as approved by the University of Maryland IRB.
2.2 Biomarker Assessment
We collected 13 biological measures for allostatic load calculation: overnight 12-hour urine cortisol, epinephrine, and norepinephrine; fasting blood DHEA, fasting HDL and total cholesterol; BMI, waist-hip ratio; resting blood pressure and resting heart rate; glycosylated hemoglobin A1C; and C-reactive protein. These 13 biomarkers include the original 10 from the MacArthur Studies, as well as resting heart rate, BMI, and C-reactive protein, as these were all commonly included in AL conceptualizations based on review of AL studies (Juster et al., 2010) and could be practically measured. We used a reliable, discreet ambulatory 12-hour urine collection routine that was well accepted by all participants. Participants were sent home with a 24-hour plastic urine collection container and a cooler. They were asked to discard their first urination at 2000h, and to collect all subsequent urinations, ending at 0800h the next morning. Participants kept a record of the time the collection began and ended, kept the container on ice, and returned the sample to our lab in the morning. For blood biomarkers, participants were asked to remain fasting since midnight, only having water and medication prior to the blood draw. Height and weight were measured with participants wearing light clothing and no shoes. Waist circumference was measured above light clothing at the narrowest point between ribs and the iliac crest. Hip circumference was measured above light clothing at the maximum diameter of the buttocks. Blood pressure was measured three times after a participant was rested for 10 minutes or more, with the average of the final two measures used as the value. All measures including sample collections were done between 0900h-1100h across participants. Blood and urine samples were sent to a CLIA certified commercial laboratory for analysis. We also assayed the urine for creatine level to adjust the three biomarker values obtained from the overnight urine. We also tested the blood to determine estradiol and progesterone levels, as clinical research has shown that schizophrenia symptoms worsen during low-estrogen phases of the menstrual cycle, and estrogen has been hypothesized to play a protective role in the illness (Grigoriadis and Seeman, 2002).
2.3 Clinical Assessments
Clinical symptoms were assessed by the 20 item anchored Brief Psychiatric Rating Scale (BPRS; (Woerner et al., 1988), with analyses examining the Total and Positive Symptom scales. Disease onset was conceptualized as the first date at which any item from the BPRS Positive Symptom scale (conceptual disorganization, suspiciousness, hallucinatory behavior, unusual thought content and disorientation) reached a Moderate level, per patient self-report. The UCSD Performance-Based Skills Assessment-2 (UPSA-2; Mausbach et al., 2008) was utilized as a proxy measure of real world functional capacity. The UPSA-2 is a well-validated tool in SZ that measures functional capacity in six areas: medication management, household chores, communication, finance, transportation, and recreational activities (Green et al., 2011). Previously we have shown that a maladaptive acute stress response was associated with reduced functional capacity using this measure (Nugent et al., 2014). The Perceived Stress Scale (PSS) was used to determine participant’s self-perception of experienced stress over the past month (Cohen et al., 1983); this scale includes 10 items, six of which are negatively stated (e.g., unable to control things, felt difficulties were piling up) and four positively worded items (e.g., felt confident in handling problems, been able to control irritations). While the original author of the scale advised that the scale should be interpreted as a whole, subsequent studies have shown support for a two factor model reflecting “Stress” and perceived self-efficacy, termed “Coping” here (Golden-Kreutz et al., 2004, Örücü and Demir, 2009). Internal consistency for the clinical scales was acceptable for the BPRS Total score, with Cronbach’s alpha (α=0.76); BPRS Positive Symptoms (α=0.74); PSS Total (α=0.87); PSS Stress (α=0.84); PSS Coping (α=0.80); and UPSA-2 (α=0.70).
2.4 Statistical analyses
Consistent with previous calculations of the allostatic load summary index (Seeman et al., 2001), we identified the 75th percentile of each of the 13 biomarkers (the 25th percentile for HDL and DHEA), based upon the healthy control distribution. Controls and SZ who had biomarker values greater to or equal to the 75th percentile (or less than or equal to the 25th percentile for HDL and DHEA) scored a value of one for that biomarker. Individuals currently taking medications to alter blood pressure (1 HC, 2 SZ), cholesterol (2 SZ) or blood sugar levels (2 SZ) were automatically given a score of one for each of the corresponding biomarkers, regardless of their tested value. The AL score represents the sum across all 13 biomarkers, thus could range from zero to 13. Group differences in sociodemographic and clinical characteristics were examined by chi-square, t or Fisher’s exact tests, and then sociodemographic predictors of AL were examined by linear regression separately in each group. Age and marital status were significantly associated with AL, thus were included as covariates in analyses comparing SZ and controls. As smoking status was unbalanced between groups, it was included as a covariate in analyses comparing SZ and controls. Group mean differences in AL, adjusting for age, smoking and marital status, were estimated by ANCOVA. Separate linear regressions examined the association between AL and BPRS total symptoms, BPRS Positive Symptoms, UPSA-2 functional capacity score, PSS Total, PSS Stress and PSS Coping. To explore individual biomarkers, thirteen separate chi-square models tested the difference in proportions of each diagnostic group that was labeled high risk for each biomarker. Internal consistency of the clinical scales was calculated using Cronbach’s alpha. As sample sizes were small, we have reported the effect sizes (r2 or Cohen’s w).
3. Results
Sociodemographic characteristics of the study sample are presented in Table 1. Schizophrenia patients and controls were frequency-matched on age, sex, and race. A significantly greater proportion of controls were currently working or going to school. SZ had significantly reduced functional capacity, as measured by the UPSA-2, compared to controls.
Table 1.
Sociodemographic and Clinical Characteristics
| Characteristic | HC (n=20) | SZ (n=30) | X2 or t value | p-value |
|---|---|---|---|---|
| Male, % | 60% | 57% | 0.055 | 0.815 |
| Age, mean (SD) | 35.7 (12.8) | 32.6 (12.1) | 0.885 | 0.381 |
| Race (% White, % Black, % Other) | 55:35:5 | 50:47:3 | a | 0.532 |
| Education (%<HS: % HS Graduate: % Some College or more) | 5:15:80 | 20:10:70 | a | 0.292 |
| Marital Status (%Single: %Married: % Divorced or Widowed) | 65:15:20 | 93:7:0 | a | 0.016 |
| Smoker, %1 | 25% | 40% | 1.203 | 0.273 |
| BPRS Psychiatric Symptoms (range 21–54), mean (SD) | -- | 31.1 (8.4) | -- | -- |
| UPSA-2 Functional Capacity (range 69–119), mean (SD) | 107.2 (7.5) | 95.6 (12.7) | 3.591 | <0.001 |
| Perceived Stress Scale Total (range 2–25), mean (SD) | 13.3 (4.4) | 14.5 (7.5) | 0.614 | 0.543 |
| Perceived Stress Scale (Stress Subscale, range 1–18), mean (SD) | 9.1 (3.6) | 9.8 (5.0) | 0.511 | 0.612 |
| Perceived Stress Scale (Coping Subscale, range 0–10), mean (SD) | 4.2 (1.9) | 4.7 (3.2) | 0.608 | 0.547 |
Fisher’s Exact Test.
BPRS = Brief Psychiatric Rating Scale, UPSA-2 = UCSD Performance-Based Skills Assessment-2.
3.1 Recent stress
SZ and controls did not differ in their overall self-perception of stress over the past month, as measured by the Perceived Stress Scale. SZ and controls were also not significantly different on the two subscales, reflecting similar mean scores for the items related both to perceptions of the uncontrollability, unpredictability and inability to cope with life circumstances as well as similar levels of perceived success in coping with stress.
3.2 Allostatic load index
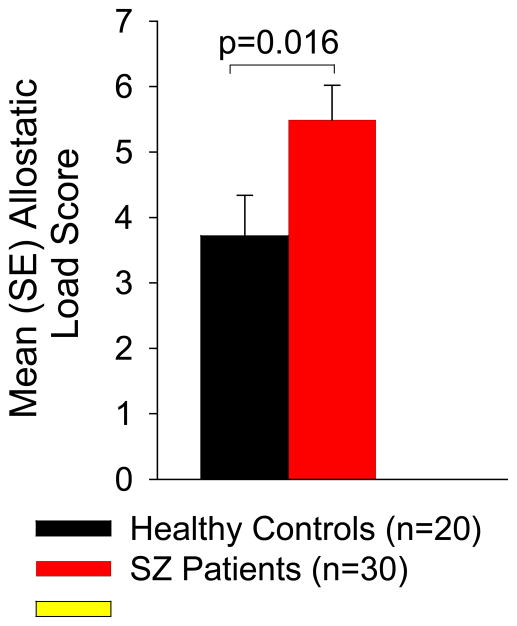
Group mean(SD) and effect size of group difference for each AL parameter are presented in Table 2. AL did not differ by sex in SZ or controls; mean(SD) AL in SZ males=4.71(2.78) vs. SZ females=5.85(2.34), t= −1.191, p=0.244, r2=0.048; mean(SD) AL in control males=3.67(2.64) vs. control females=3.5(2.07), t=0.150, p=0.882, r2=0.001. AL did not differ by race in SZ (F=0.41, p=0.668, r2=0.029) or controls (F=0.10, p=0.906, r2=0.011). AL did not significantly differ by education level in SZ (F=1.91, p=0.153, r2=0.180) or controls (F=0.48, p=0.701, r2=0.082), although there appeared a larger effect of education level on AL in SZ. Due to the lack of significant group differences (Table 1) of these variables on AL, these variables were not used as covariates in subsequent group comparisons. However, greater age was significantly associated with higher AL in SZ (β=0.095, p=0.016, r2=0.191) and in controls (β =0.120, p=0.002, r2=0.416). Smoking was not significantly associated with AL in SZ (t= −0.792, p=0.435, r2=0.022), or controls (t=0.0, p=1, r2<0.001), although there was a non-significantly larger percentage of the SZ group who smoked (40%) vs. 20% of controls who smoked (X2=1.20, p=0.273). AL did not differ by marital status in SZ (F=2.32, p=0.139, r2=0.079), yet married controls (n=3) had significantly greater AL as compared to never married controls (t= −3.47, p=0.004, r2=0.354). Controlling for age, smoking and marital status, SZ had significantly greater AL as compared to controls (β =1.77, p=0.016; r2=0.090; Figure 1). Additional analysis on estradiol and progesterone levels, which were not part of the allostatic load calculation, were not significantly associated with AL score in either group (all p>0.080).
Table 2.
Individual and Elevated Values for Each of the 13 Allostatic Load Biomarkers, by Diagnostic Group
| Biomarker | HC | SZ | Elevated Value (scored a 1 for calculation of AL) |
|---|---|---|---|
| Cardiovascular | |||
| Resting SBP (mmHg) | 115.23±3.27 | 114.64±2.81 | >= 123.50 |
| Resting DBP (mmHg) | 74.08±2.43 | 73.32±2.08 | >= 81.00 |
| Resting heart rate (beats per minute) | 72.30±3.91 | 80.90±3.35 | >= 77.00 |
| Metabolic - lipids | |||
| BMI (kg/m2) | 27.84±1.66 | 29.39±1.42 | >= 29.77 |
| Waist-Hip Ratio | 0.89±0.02 | 0.90±0.02 | >= 0.93 |
| HDL Cholesterol (mg/dL) | 58.92±5.02 | 54.54±4.30 | <= 42.00 |
| Total Cholesterol (mg/dL) | 164.89±10.50 | 173.50±8.99 | >= 216.00 |
| Metabolic - glucose metabolism | |||
| Glycosylated hemoglobin (HbA1c; DCCT %) | 5.484±0.13 | 5.61±0.11 | >= 5.60 |
| Inflammation | |||
| CRP (mg/L) | 1.33±1.29 | 4.05±1.10 | >= 1.00 |
| Sympathetic Nervous System | |||
| Urine Epinephrine (ug/g creatine) | 4.42±1.00 | 5.80±0.84 | >= 4.25 |
| Urine Norepinephrine (ug/g creatine) | 22.45±5.13 | 34.84±4.35 | >= 24.79 |
| HPA Axis | |||
| Urine Cortisol (ug/g creatine) | 12.28±2.59 | 12.94±2.05 | >= 16.53 |
| Blood DHEA (ug/dL) | 244.17±39.95 | 232.99±34.23 | <= 163.80 |
| Allostatic Load | 3.72±0.62 | 5.49±0.53 | -- |
All values in the first two columns represent mean (SE) for each diagnostic group, adjusting for age, smoking and marital status. Individual biomarkers contributed to the allostatic load score as detailed in the third column.
Figure 1.
Allostatic Load in Controls and Schizophrenia Patients. Schizophrenia patients display significantly higher mean allostatic load, adjusted for age, smoking and marital status, as compared to healthy controls.
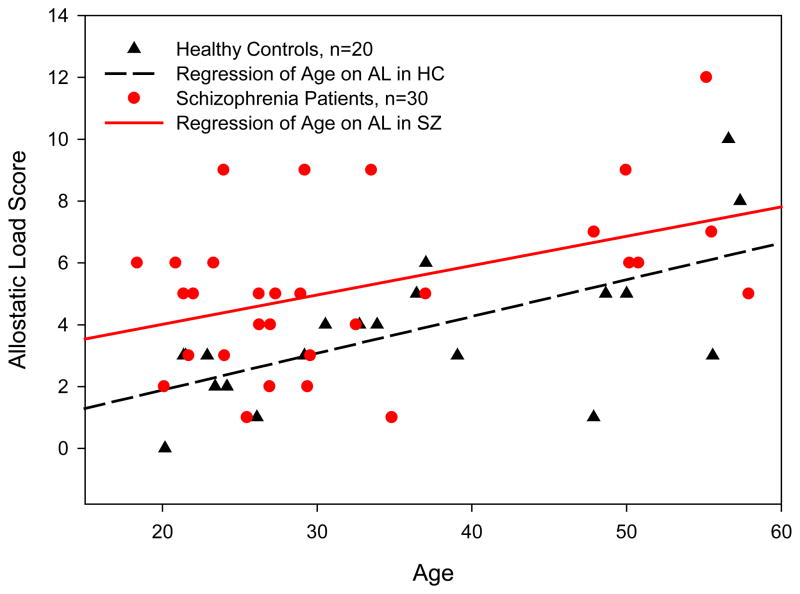
To examine duration of illness effects, we compared SZ with less than five years duration of illness (n=13) to age matched controls, and adjusting for age, smoking and marital status, SZ had elevated AL [mean(SD)=4.40(0.47) vs. 2.53(0.51), F=7.72, p=0.013, r2=0.251]. We then also compared SZ with five years or more duration of illness (n=17) to age matched controls: again controlling for age, smoking and marital status, SZ had higher AL [mean(SD)=6.31(0.88) vs. 3.75(1.17), F=4.05, p=0.056, r2=0.115]. This exploratory analysis suggests that higher AL is already present early in the SZ disease course, and SZ may not experience substantial further increases in AL over time beyond normal age related effects. This finding is displayed in Figure 2, which shows similar slopes of association between age and AL in SZ and HC, however there is an elevation in AL level already present in SZ at the younger ages.
Figure 2.
Allostatic Load Score by Age in Schizophrenia Patients and Healthy Controls. Significantly elevated allosatic load, as compared to healthy controls, is present in young adult schizophrenia patients. Increases in allostatic load associated with age are similar in schizophrenia patients and healthy controls.
3.3 Individual biomarkers within the allostatic load calculation
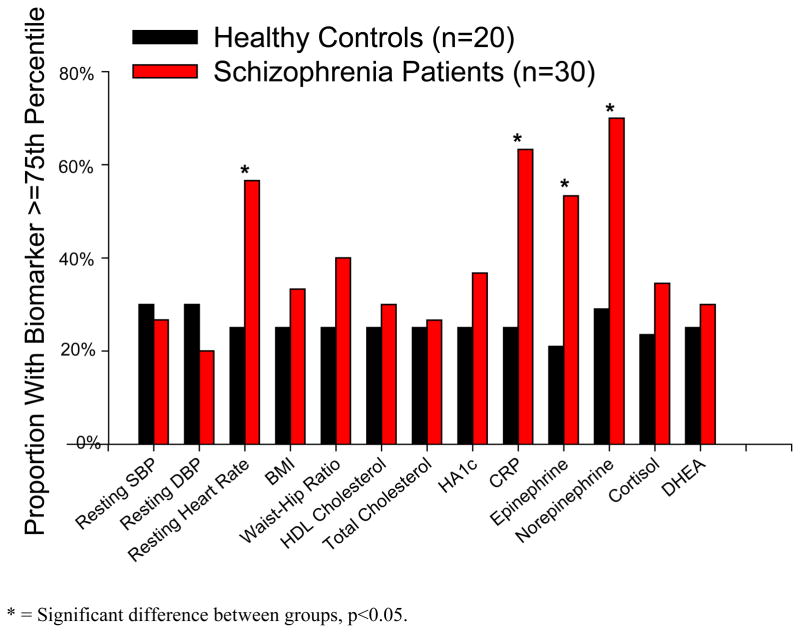
Exploring the individual biomarkers comprising the AL index, a greater proportion of the SZ group had resting heart rate (X2=4.88, p=0.027, Cohen’s effect size w=0.31), C-reactive protein (X2=7.06, p=0.008, w=0.38), urine epinephrine (X2=5.02, p=0.025, w=0.32), and urine norepinephrine (X2=6.94, p=0.008, w=0.38) values above the 75th percentile cutpoint (Figure 3). However, the AL construct was not designed to test these individual measures and when corrected for 13 comparisons (Bonferroni p<0.0038) these differences in individual biomarkers are not significant between groups.
Figure 3.
Exploration of Individual Components Contributing to Allostatic Load Score. For each biomarker, the 75th percentile of the control sample was used as a cutpoint for determining elevated risk. A significantly greater proportion of the schizophrenia patients had resting heart rate, urine epinephrine, and urine norepinephrine values above the 75th percentile cutpoint (all p<0.027).
3.4 Clinical correlates of allostatic load
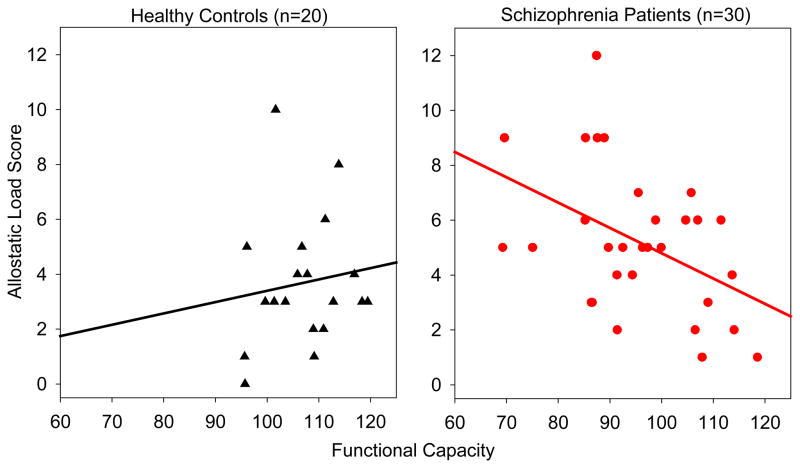
Greater AL score was significantly associated with worse functional capacity (β=-2.17, p=0.013, r2=0.200) in SZ, but not in controls (β=0.19, p=0.795, r2=0.004) as measured by the UPSA-2. There was a significant interaction of the difference in the slopes of these associations between groups (p=0.041; Figure 4). AL was not significantly associated with total self-reported stress over the past month in SZ (β=0.962, p=0.090, r2=0.084) or controls (β= −0.69, p=0.123, r2=0.161). After correcting the significance threshold for six comparisons, the perceived level of stress subscale was not significantly associated with AL in SZ (β=0.774, p=0.111, r2=0.122) or controls (β= −0.715, p=0.044, r2=0.260). In addition, perceived success in coping with stress was also not significantly associated with AL in SZ (β=0.188, p=0.550, r2=0.018) or controls (β=0.025, p=0.899, r2=0.001). Overall psychiatric symptoms, as measured by the BPRS total score, were not significantly associated with AL in SZ (β=0.712, p=0.237, r2=0.050) or controls (β=−0.515, p=0.183, r2=0.096). In SZ, greater AL was associated with greater positive symptoms (β=0.582, p=0.044, r2=0.137), as measured by the BPRS Positive Symptoms subscale score.
Figure 4.
Allostatic Load and Functional Capacity by Diagnostic Group. Association of allostatic load and functional capacity, as measured by the UPSA-2. Greater allostatic load was significantly associated with reduced functional capacity in schizophrenia patients, but not in controls.
3.5 Current medications in relation to allostatic load
Some psychiatric medications are known to influence several components of AL. Therefore, we carefully examined the potential confounds from current medications on AL. AL was not significantly associated with CPZ equivalent dosage in SZ (β=0.0002, p=0.766). The three nominally significant component measures, resting heart rate (β=0.0008, p=0.854), urine epinephrine (β=0.0006, p=0.557), and urine norepinephrine (β=0.006, p=0.293) were all not significantly associated with CPZ equivalent dosage. Thirteen SZ taking an antidepressant medication had insignificantly higher AL score (t=1.341, p=0.191) compared to SZ not on an antidepressant. Some commonly used psychiatric medications, including benztropine and clozapine, are anticholinergic and can increase resting heart rate. Removing the two patients on benztropine, AL in the SZ group remained significantly elevated as compared to HC (β=1.93, p=0.007). Finally, five SZ were taking clozapine; their heart rate did not differ significantly from those patients not on clozapine (mean (SE) =83.8(7.7) vs. 78.2(3.1), p=0.475).
4. Discussion
Patients with schizophrenia exhibit significantly higher allostatic load as compared to healthy controls, without necessarily experiencing a higher recent stress level per self-report of stress in the past month. Greater AL score was significantly associated with reduced functional capacity, a validated measure of real-world functioning for SZ (Mausbach et al., 2008, Patterson et al., 2001). Higher AL is thought to reflect the increased bodily “wear and tear”, which may be due to either a regularly higher stress level or maladaptation to stress over time (McEwen, 2000). However, separating the two aspects could be difficult. We tried to partially address the issue by measuring recent stress perception and found that at least in the recent one month, there was no higher stress perception in SZ patients compared with controls, and perceived stress level was not related to the extent of AL in either SZ or HC. This is consistent with theory of AL because the recent experience of stress should not substantially impact a long term measure such as AL. More comprehensive measures of stressful life events, lifetime socioeconomic status, ongoing social and financial stressors, and experiences of stigma in patients with SZ could be more useful in determining the role of perceived stress in AL.
An important consideration is that high AL was present early on in the disease course of schizophrenia and persisted. Age-related increases in AL were similar in both SZ and HC. These results do contrast the staging model of AL proposed in Bipolar disorder, for which most patients display more intact biological and psychiatric functioning before disease onset, yet develop increasing dysfunction and comorbidity as the number and duration of mood episodes increases (Kapczinski et al., 2010, Berk et al., 2011). Rather, our finding suggests that high AL in schizophrenia may be due to maladaptive stress pathophysiology existing at the time of disease onset or developing very early in the course of the disease (McEwen, 2002) rather than due to cumulative effects from chronic psychosis or medication exposure. However, longitudinal studies simultaneously measuring AL, perceived stress and clinical status at multiple time-points may be required for affirmative interpretation. This longitudinal type of study would also provide insight into whether higher AL in the patients may be associated with poor diet, less physical activity, smoking and increased drug use and other lifestyle factors. Maladaptive behaviors could also reflect compensatory actions to stressors (i.e. overeating may reduce negative affect (Dallman et al., 2003)). Psychiatric medications can create a “pharmacological AL” by inducing adverse affects that prompt patients to remediate the biological system(s) using unhealthy behaviors that may lead to metabolic syndromes such as higher cholesterol and BMI in patients (Juster et al., 2011). While we believe that these AL differences do not solely result from differing lifestyle behaviors a proper assessment of health behaviors is necessary to determine their contribution to AL in SZ. Larger samples assessed longitudinally will be necessary to determine the role that psychiatric medications play in mediating and/or moderating stress effects.
Functional capacity assessed by the UPSA-2 is a well validated measure for SZ functioning and is closely associated with cognitive functioning (Patterson et al., 2001). Therefore, the association between AL and UPSA-2 could be due to a lack of cognitive resources needed to both function successfully in everyday life and to respond adaptively to stress. Alternatively, reduced ability to function could induce a more chronic level of stress in certain patients, driven by the consequences of unemployment, poor social support, and limited recreational activities. Although the directionality of a causal pathway between functional capacity and stress response cannot be affirmed using the current cross-sectional study, the finding of greater cumulative stress associated with reduced functional capacity in SZ is still clinically important; if our findings can be replicated, it may have implications for how to improve clinical intervention. For example, enhancing coping skills and even pharmacological approaches aimed at fostering adaptive responses to acute stressors and reducing chronic stress burden could be emphasized to improve functional outcomes.
Our finding of higher AL corresponding to more severe psychotic symptoms is also interesting. We did not find antipsychotic medication type or dosage to be significantly related to AL. Perhaps individuals with a more severe form of illness, or whose symptoms do not respond to medication may experience greater cumulative stress burden. Symptoms such as paranoia and hallucinations can be inherently stressful (Garner et al., 2011, Walder et al., 2000, McGorry et al., 1991), and can contribute to increased chronic stress burden in a secondary manner through impaired social support and increased exposure to social stigma. As discussed earlier, SZ experience significantly higher AL early in the course of the illness. This suggests that their stress response system is functionally abnormal either at or prior to disease onset. This elevated AL finding even in patients of younger ages highlights the potential usefulness of targeting adaptive stress mechanisms in treatment to foster beneficial outcomes and reducing morbidity.
Persons with SZ have a reduced life expectancy of 20 years on average (Brown et al., 2010, McGrath et al., 2008, Saha et al., 2007, Brown, 1997) and cardiovascular disease is the leading cause of their death (Brown et al., 2000). AL has been successfully applied to predict worse health outcomes in other health conditions including greater mortality risk and declines in physical and cognitive functioning conditions (Seeman et al., 2001, Seeman et al., 2004). Elevated resting heart rate and plasma C-reactive protein have been associated with incident cardiovascular disease, CVD mortality and shorter life expectancies (Cooney et al., 2010, Levine, 1997, Emerging Risk Factors Collaboration, 2010, Koenig et al., 1999). While increases in norepinephrine in acute stress situations are adaptive, elevated baseline norepinephrine has been found to increase risk for death in patients with cardiac (Cohn et al., 1984) and renal disease (Zoccali et al., 2002). These factors happen to be the leading elements that, together with several other less significant factors in the AL index, contribute to the elevated AL found in schizophrenia. These results are consistent with prior reports of elevated pulse and altered autonomic functioning in SZ (Van Kammen and Kelley, 1991, Ostermann et al., 2013, Chung et al., 2013; Schisterman et al., 2009).
4.1 Limitations
The cross-sectional design and sample size of the current study are the main limitations that prohibited thorough investigation of the role of pre-existing somatic health conditions, the mediating and/or moderating effects of pharmacological AL, and the time course of higher AL biomarkers in relation to disease onset and changes in functional capacity. Furthermore, we did not assess physical activity and dietary intake, or more comprehensive measures of stress throughout the lifetime such as stressful life events and socio-economic status. In addition, while we observed similar elevations in AL across varying durations of illness, our small sample spanned a large age range for which we may have been underpowered to investigate specific age related effects.
5. Conclusions
Our findings from this first investigation of AL in schizophrenia support the research and potential clinical value in applying AL to assess the role of chronic physiological stress in disease progression and somatic morbidity. As an index of the systemic sequelae of cumulative stress exposure, AL will be helpful not only in research on the medical comorbidities of schizophrenia, but also as a measure to complement and integrate other lines of inquiry into the pathophysiology of the illness. Inflammation, oxidative stress, and metabolic disturbances are all considered potential biological pathways contributing to the course of schizophrenia. Current theoretical frameworks that attempt to integrate these pathophysiological mechanisms with environmental and genetic risk factors for schizophrenia include stress-diathesis (Walker and Diforio, 1997), behavioral sensitization (van Winkel et al., 2008), and accelerated aging (Kirkpatrick et al., 2008) models. These models are non-mutually exclusive and all consider the critical substrate of the physiological consequences of stress exposure to be neurobiological changes. Thus, AL may be a useful measure to examine in conjunction with neuroimaging modalities to explore links between the peripheral and central consequences of chronic stress exposure and maladaptive stress responses in schizophrenia.
Highlights.
Higher allostatic load found in schizophrenia patients compared to controls
Higher allostatic load present early in the course of illness
Greater allostatic load associated with reduced functional capacity in patients
Current perceived stress is not associated with allostatic load
Acknowledgments
We would like to thank our study participants and the staff at the Maryland Psychiatric Research Center. Particularly, we appreciate the help of Judy Liu, Beshaun Davis, and Kavita Thangavelu with the collection of participant data. This work was supported by a NARSAD Young Investigator Award and National Institute of Health grants R01MH085646, R01DA027680, P50MH103222, and T32MH067533.
Role of the Funding Source
This work was supported by a NARSAD Young Investigator Award and National Institute of Health grants R01MH085646, R01DA027680, P50MH103222, and T32MH067533. These funding sources did not have a role in the study design or interpretation of the data.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Conflict of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
Contributors
K. Nugent: Designed study, collected and analyzed data, wrote first draft of manuscript, edited manuscript in response to reviewers’ comments, and responded to reviewer’s comments.
J. Chiappelli: Assisted with study design and edited manuscript draft.
L. Rowland: Assisted with study design and edited manuscript draft.
L. E. Hong: Designed study, edited manuscript draft, and responded to reviewers’ comments.
All authors have approved the final draft of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2012;37:1600–1613. doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Bowen J, Ramana R. Life events and psychotic disorders. In: Miller TW, editor. Clinical Disorders and Stressful Life Events. International Universities Press; Madison, CT: 1997. [Google Scholar]
- Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic–pituitary–adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza A, Dean O, Giorlando F, Maes M, Yücel M, Gama C, Dodd S, Dean B. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience & biobehavioral reviews. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bizik G, Picard M, Nijjar R, Tourjman V, McEwen BS, Lupien SJ, Juster RP. Allostatic load as a tool for monitoring physiological dysregulations and comorbidities in patients with severe mental illnesses. Harv Rev Psychiatry. 2013;21:296–313. doi: 10.1097/HRP.0000000000000012. [DOI] [PubMed] [Google Scholar]
- Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic–pituitary–adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38:603–611. doi: 10.1016/j.psyneuen.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D. Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Res. 1988;25:187–194. doi: 10.1016/0165-1781(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Brenner K, St-Hilaire A, Liu A, Laplante DP, King S. Cortisol response and coping style predict quality of life in schizophrenia. Schizophr Res. 2011;128:23–29. doi: 10.1016/j.schres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Brown S. Excess mortality of schizophrenia. A meta-analysis. The British Journal of Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. The British journal of psychiatry. 2010;196:116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Barraclough B, Inskip H. Causes of the excess mortality of schizophrenia. The British Journal of Psychiatry. 2000;177:212. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- Chung M, Yang AC, Lin Y, Lin C, Chang F, Shen S, Ouyang W, Loh E, Chiu H. Association of altered cardiac autonomic function with psychopathology and metabolic profiles in schizophrenia. Psychiatry Res. 2013;210:710–715. doi: 10.1016/j.psychres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Docherty NM. Affective reactivity of speech and emotional experience in patients with schizophrenia. Schizophr Res. 2004;69:7–14. doi: 10.1016/S0920-9964(03)00069-0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619.e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Docherty NM, St-Hilaire A, Aakre JM, Seghers JP. Life events and high-trait reactivity together predict psychotic symptom increases in schizophrenia. Schizophr Bull. 2009;35:638–645. doi: 10.1093/schbul/sbn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. Life events and other possible psychosocial risk factors for episodes of schizophrenia and major depression: a case-control study. In: Mazure C, editor. Does stress cause psychiatric illness? American Psychiatric Press; Washington, D.C: 1995. pp. 43–65. [Google Scholar]
- Elman I, Adler CM, Malhotra AK, Bir C, Pickar D, Breier A. Effect of acute metabolic stress on pituitary-adrenal axis activation in patients with schizophrenia. Am J Psychiatry. 1998;155:979–981. doi: 10.1176/ajp.155.7.979. [DOI] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. The Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39:924. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: the mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23:979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV-patient edition (SCID-P) New York: Biometric Research; 1995. [Google Scholar]
- Ganzel BL, Morris PA. Allostasis and the developing human brain: Explicit consideration of implicit models. Dev Psychopathol. 2011;23:955–974. doi: 10.1017/S0954579411000447. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, Yun Y, McGorry PD. Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psychiatr Res. 2011;45:249–255. doi: 10.1016/j.jpsychires.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Golden-Kreutz DM, Browne MW, Frierson GM, Andersen BL. Assessing stress in cancer patients: a second-order factor analysis model for the Perceived Stress Scale. Assessment. 2004;11:216–223. doi: 10.1177/1073191104267398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande I, Magalhães PV, Kunz M, Vieta E, Kapczinski F. Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav. 2012;106:46–50. doi: 10.1016/j.physbeh.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Green MF, Schooler NR, Kern RS, Frese FJ, Granberry W, Harvey PD, Karson CN, Peters N, Stewart M, Seidman LJ. Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry. 2011;168:400–407. doi: 10.1176/appi.ajp.2010.10030414. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Seeman MV. The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Canadian journal of psychiatry. 2002;47:437–442. doi: 10.1177/070674370204700504. [DOI] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75:363–374. doi: 10.1016/j.schres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. The International Journal of Neuropsychopharmacology. 2004;7:S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Wieselgren I, Öhman A. Relationships between social support, social coping and life events in the relapse of schizophrenic patients. Scand J Psychol. 1997;38:3–13. doi: 10.1111/1467-9450.00002. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berl ) 2000;149:319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Jansen L, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden Jeroen A, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr Res. 1998;33:87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Juster R, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, Marin M, Wan N, Sekerovic Z, Lord C. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev Psychopathol. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Juster R, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dal-Pizzol F, Teixeira A, Magalhaes P, Kauer-Sant’Anna M, Klamt F, de Bittencourt Pasquali M, Quevedo J, Gama C, Post R. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol Psychiatry. 2010;15:784–786. doi: 10.1038/mp.2009.112. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, Kauer-Sant’Anna M, Grassi-Oliveira R, Post RM. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neuroscience & Biobehavioral Reviews. 2008;32:675–692. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: What we know in 2008 Part 3: Neurobiology. Schizophr Res. 2008;106:89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr. Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Lake CR, Sternberg DE, van Kammen DP, Ballenger JC, Ziegler MG, Post RM, Kopin IJ, Bunney WE. Schizophrenia: elevated cerebrospinal fluid norepinephrine. Science. 1980;207:331–333. doi: 10.1126/science.7350667. [DOI] [PubMed] [Google Scholar]
- Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30:1104–1106. doi: 10.1016/s0735-1097(97)00246-5. [DOI] [PubMed] [Google Scholar]
- Marcelis M, Cavalier E, Gielen J, Delespaul P, Van Os J. Abnormal response to metabolic stress in schizophrenia: marker of vulnerability or acquired sensitization? Psychol. Med. 2004;34:1103–1111. doi: 10.1017/s0033291703001715. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Bowie CR, Harvey PD, Twamley EW, Goldman SR, Jeste DV, Patterson TL. Usefulness of the UCSD Performance-Based Skills Assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. J Psychiatr Res. 2008;42:320–327. doi: 10.1016/j.jpsychires.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McFarlane A. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9:3–10. doi: 10.1002/j.2051-5545.2010.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Chanen A, McCarthy E, Van Riel R, McKenzie D, Singh BS. Posttraumatic Stress Disorder Following Recent-Onset Psychosis An Unrecognized Postpsychotic Syndrome. J Nerv Ment Dis. 1991;179:253–258. doi: 10.1097/00005053-199105000-00002. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Beyer JL, Woldeyohannes HO, Law CW, Miranda A, Konarski JZ, Kennedy SH. Medical comorbidity in bipolar disorder: re-prioritizing unmet needs. Curr Opin Psychiatry. 2007;20:406–416. doi: 10.1097/YCO.0b013e3281938102. [DOI] [PubMed] [Google Scholar]
- Misiak B, Frydecka D, Zawadzki M, Krefft M, Kiejna A. Refining and integrating schizophrenia pathophysiology–relevance of the allostatic load concept. Neuroscience & Biobehavioral Reviews. 2014;45:183–201. doi: 10.1016/j.neubiorev.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK. Stressful life events and schizophrenia. I: A review of the research. The British Journal of Psychiatry. 1993;162:161. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- Nugent KL, Chiappelli J, Rowland LM, Daughters SB, Hong LE. Distress intolerance and clinical functioning in persons with schizophrenia. Psychiatry Res. 2014;220:31–36. doi: 10.1016/j.psychres.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Örücü MÇ, Demir A. Psychometric evaluation of perceived stress scale for Turkish university students. Stress Health. 2009;25:103–109. [Google Scholar]
- Ostermann S, Herbsleb M, Schulz S, Donath L, Berger S, Eisentrager D, Siebert T, Muller HJ, Puta C, Voss A, Gabriel HW, Koch K, Bar KJ. Exercise reveals the interrelation of physical fitness, inflammatory response, psychopathology, and autonomic function in patients with schizophrenia. Schizophr Bull. 2013;39:1139–1149. doi: 10.1093/schbul/sbs085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang M, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005;187:101–102. doi: 10.1192/bjp.187.2.101. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition, and Health. John Wiley & Sons; Chichester, UK: 1988. pp. 629–649. [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, just the facts 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Kelley M. Dopamine and norepinephrine activity in schizophrenia: An intergrative perspective. Schizophr Res. 1991;4:173–191. doi: 10.1016/0920-9964(91)90032-m. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. The British Journal of Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Popovic D, Rosa A, Solé B, Grande I, Frey B, Martinez-Aran A, Sanchez-Moreno J, Balanzá-Martínez V, Tabarés-Seisdedos R. The clinical implications of cognitive impairment and allostatic load in bipolar disorder. European Psychiatry. 2013;28:21–29. doi: 10.1016/j.eurpsy.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Walker EF, Lewine RJ. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol Psychiatry. 2000;48:1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–684. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24:112–117. [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]