Abstract
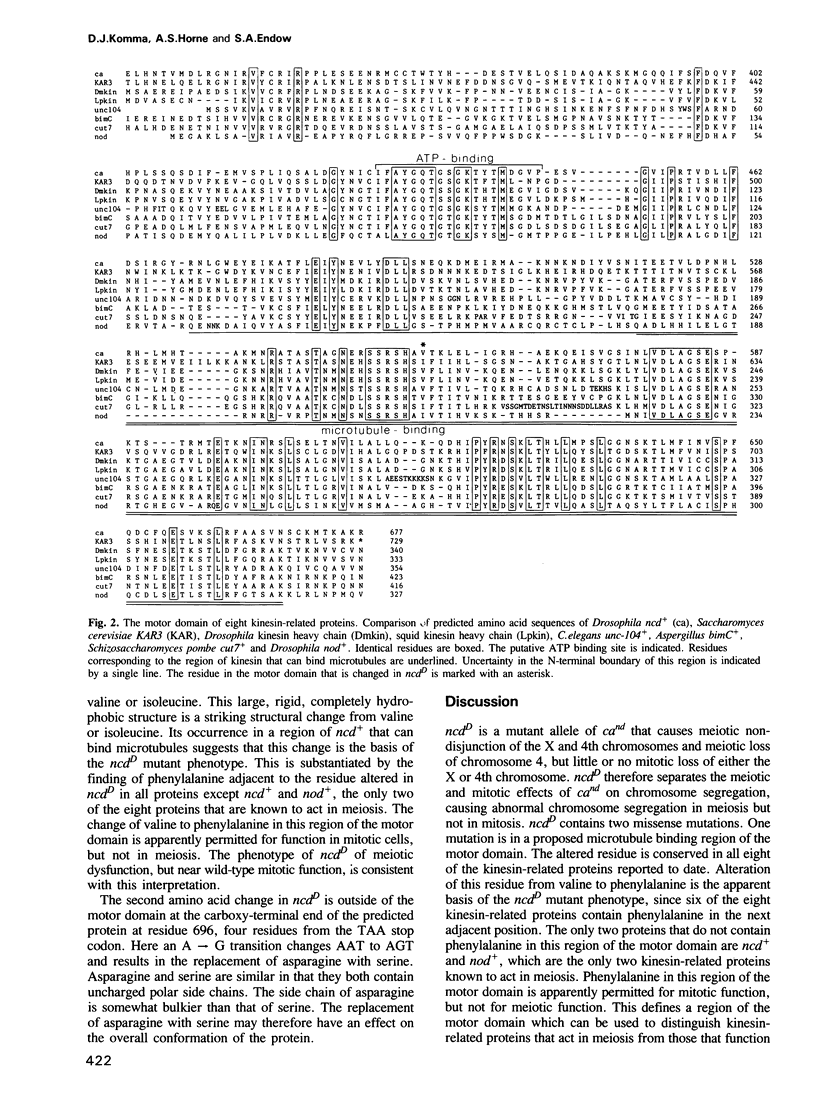
The claret (ca) locus in Drosophila encodes a kinesin-related motor molecule that is required for proper distribution of chromosomes in meiosis in females and in the early mitotic divisions of the embryo. Here we demonstrate that a mutant allele of claret non-disjunctional (ca(nd)), non-claret disjunctional Dominant (ncdD), causes abnormalities in meiotic chromosome segregation, but is near wild-type with respect to early mitotic chromosome segregation. DNA sequence analysis of this mutant allele reveals two missense mutations compared with the predicted wild-type protein. One mutation lies in a proposed microtubule binding region of the motor domain and affects an amino acid residue that is conserved in all kinesin-related proteins reported to date. This region of the motor domain can be used to distinguish meiotic and mitotic motor function, defining an amino acid sequence criterion for classifying motors according to function. ncdD's mutant meiotic effect, but near wild-type mitotic effect, suggests that interactions of the ca motor protein with spindle microtubules differ in meiosis and mitosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
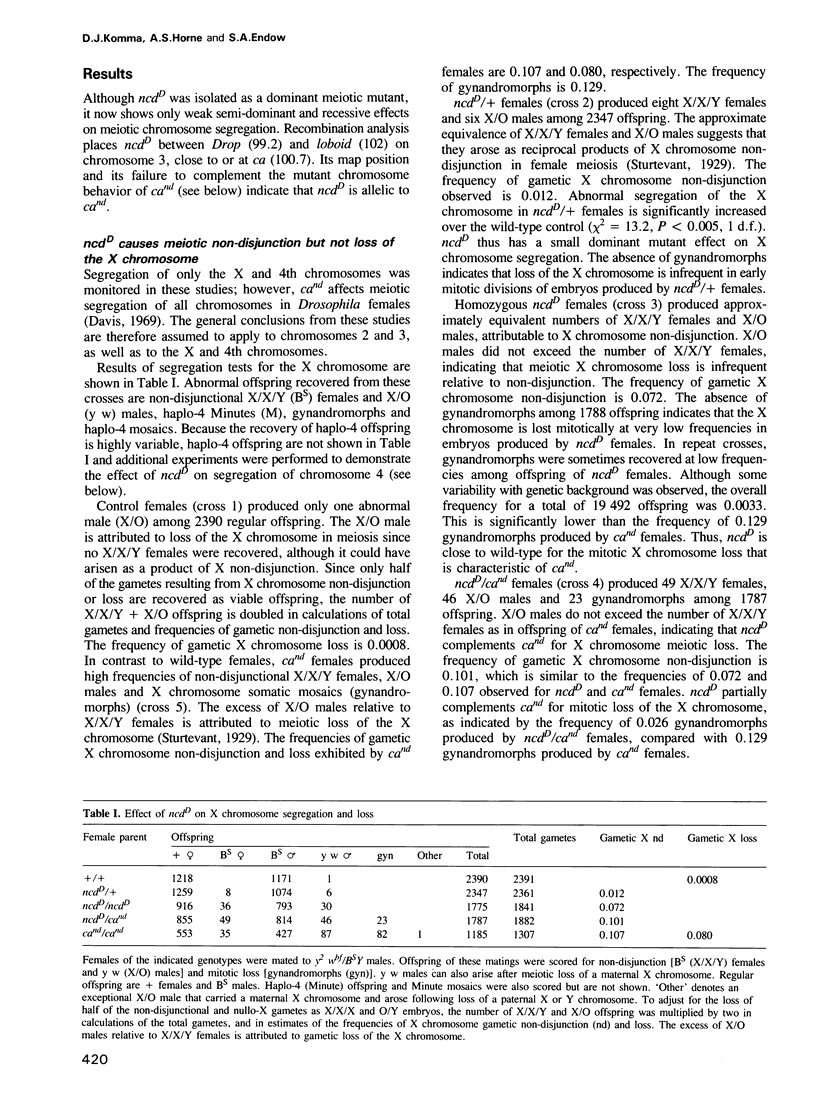
- Davis D. G. Chromosome Behavior under the Influence of Claret-Nondisjunctional in DROSOPHILA MELANOGASTER. Genetics. 1969 Mar;61(3):577–594. doi: 10.1093/genetics/61.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
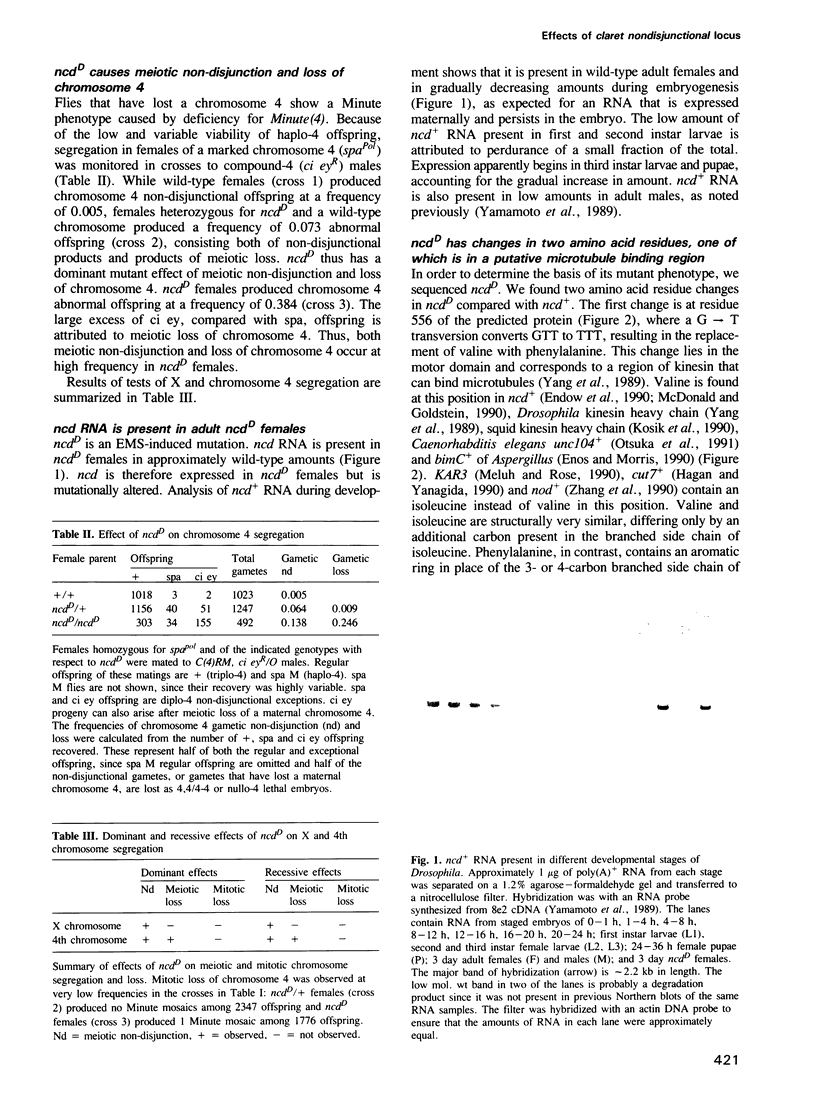
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990 Oct 11;347(6293):563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Kimble M., Church K. Meiosis and early cleavage in Drosophila melanogaster eggs: effects of the claret-non-disjunctional mutation. J Cell Sci. 1983 Jul;62:301–318. doi: 10.1242/jcs.62.1.301. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Schnapp B., Inouye H., Neve R. L. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990 Feb 25;265(6):3278–3283. [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- McDonald H. B., Stewart R. J., Goldstein L. S. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990 Dec 21;63(6):1159–1165. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- Meluh P. B., Rose M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990 Mar 23;60(6):1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Evans L., Schulze E., Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986 May 23;45(4):515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald H. Cytologic Studies on the Abnormal Development of the Eggs of the Claret Mutant Type of Drosophila Simulans. Genetics. 1936 May;21(3):264–281. doi: 10.1093/genetics/21.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Salmon E. D., Endow S. A. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990 Oct 25;347(6295):780–782. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Weber D. K., Johnson T., Sakaguchi A. Y. Supercoil sequencing using unpurified templates produced by rapid boiling. Biotechniques. 1988 Oct;6(9):839, 841-3. [PubMed] [Google Scholar]
- Yamamoto A. H., Komma D. J., Shaffer C. D., Pirrotta V., Endow S. A. The claret locus in Drosophila encodes products required for eyecolor and for meiotic chromosome segregation. EMBO J. 1989 Dec 1;8(12):3543–3552. doi: 10.1002/j.1460-2075.1989.tb08526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Zhang P., Knowles B. A., Goldstein L. S., Hawley R. S. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell. 1990 Sep 21;62(6):1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]



