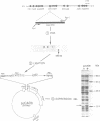
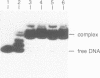
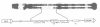Abstract
Growth of Escherichia coli on acetate requires operation of the anaplerotic sequence known as the glyoxylate bypass. In this pathway three different enzymes are activated: malate synthase, isocitrate lyase and isocitrate dehydrogenase kinase/phosphatase which are encoded by genes aceB, aceA and aceK, respectively. These three genes are clustered, in that order, in the same acetate (ace) operon whose expression is under the transcriptional control of the iclR gene located downstream from aceK. We have cloned the iclR gene in the pKK233-2 vector which allows optimization of both transcription and translation initiation. The IclR repressor has been overproduced, then purified to homogeneity in a one-step procedure by cation exchange chromatography after ammonium sulfate fractionation. Its specific interaction with the operator/promoter region of the ace operon has been analyzed by gel retardation and DNase I footprinting experiments. The IclR repressor has been shown to recognize a 35 bp palindromic sequence which largely overlaps the -35 recognition site of RNA polymerase. Moreover, the formation of the complex between IclR and the operator/promoter region has been found to be impaired by phosphoenol pyruvate but insensitive to acetate, acetyl-CoA, pyruvate, and oxaloacetate. These results are discussed in terms of primary regulation of the expression of the ace operon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Higgins C. F., Ames G. F. Physical map of the Salmonella typhimurium histidine transport operon: correlation with the genetic map. J Bacteriol. 1981 Aug;147(2):401–409. doi: 10.1128/jb.147.2.401-409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseggio N., Davies W. D., Davidson B. E. Identification of the promoter, operator, and 5' and 3' ends of the mRNA of the Escherichia coli K-12 gene aroG. J Bacteriol. 1990 May;172(5):2547–2557. doi: 10.1128/jb.172.5.2547-2557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick A. C., Holms W. H., Nimmo H. G. The phosphorylation of Escherichia coli isocitrate dehydrogenase in intact cells. Biochem J. 1984 Sep 15;222(3):797–804. doi: 10.1042/bj2220797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byrne C., Stokes H. W., Ward K. A. Nucleotide sequence of the aceB gene encoding malate synthase A in Escherichia coli. Nucleic Acids Res. 1988 Oct 11;16(19):9342–9342. doi: 10.1093/nar/16.19.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T., Klumpp D. J., LaPorte D. C. Glyoxylate bypass operon of Escherichia coli: cloning and determination of the functional map. J Bacteriol. 1988 Jan;170(1):386–392. doi: 10.1128/jb.170.1.386-392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortay J. C., Bleicher F., Duclos B., Cenatiempo Y., Gautier C., Prato J. L., Cozzone A. J. Utilization of acetate in Escherichia coli: structural organization and differential expression of the ace operon. Biochimie. 1989 Sep-Oct;71(9-10):1043–1049. doi: 10.1016/0300-9084(89)90109-0. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinier A., Nègre D., Cortay J. C., Marcandier S., Maloy S. R., Cozzone A. J. Sequence analysis of the iclR gene encoding the repressor of the acetate operon in Salmonella typhimurium. Nucleic Acids Res. 1990 Jun 25;18(12):3656–3656. doi: 10.1093/nar/18.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Chung T. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem. 1985 Dec 5;260(28):15291–15297. [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982 Dec 2;300(5891):458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- Lakshmi T. M., Helling R. B. Acetate metabolism in Escherichia coli. Can J Microbiol. 1978 Feb;24(2):149–153. doi: 10.1139/m78-027. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Borthwick A. C., el-Mansi E. M., Holms W. H., MacKintosh C., Nimmo G. A. Regulation of the enzymes at the branchpoint between the citric acid cycle and the glyoxylate bypass in Escherichia coli. Biochem Soc Symp. 1987;54:93–101. [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W., Egan P. A., Maloy S. R. Kinetics of the utilization of medium and long chain fatty acids by mutant of Escherichia coli defective in the fadL gene. J Biol Chem. 1979 Sep 25;254(18):9130–9134. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Hughes K. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: dominance studies with strains merodiploid in gene fadR. J Bacteriol. 1980 Aug;143(2):726–730. doi: 10.1128/jb.143.2.726-730.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnarborg A., Klumpp D., Chung T., LaPorte D. C. Regulation of the glyoxylate bypass operon: cloning and characterization of iclR. J Bacteriol. 1990 May;172(5):2642–2649. doi: 10.1128/jb.172.5.2642-2649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]