Abstract
Biological therapeutics targeting TNF, IL-1, and IL-6 are widely used for treatment of rheumatoid arthritis, inflammatory bowel disease, and a growing list of other syndromes, often with remarkable success. Now advances in neuroscience have collided with this therapeutic approach, perhaps rendering possible the development of nerve stimulators to inhibit cytokines. Action potentials transmitted in the vagus nerve culminate in the release of acetylcholine that blocks cytokine production by cells expressing acetylcholine receptors. The molecular mechanism of this cholinergic antiinflammatory pathway is attributable to signal transduction by the nicotinic alpha 7 acetylcholine receptor subunit, a regulator of the intracellular signals that control cytokine transcription and translation. Favorable preclinical data support the possibility that it may be possible to add nerve stimulators to the future therapeutic armamentarium, possibly replacing some drugs to inhibit cytokines.
Introduction
Among the leading causes of morbidity and mortality in Western societies are heart disease, cancer, stroke, diabetes, and sepsis. Recent advances in immunology reveal a significant pathogenic role for inflammation in the development and progression of these disorders. Inflammation accelerates deposition of atherosclerotic plaques leading to myocardial and cerebral infarction; mediates insulin resistance; stimulates tumor growth; and causes organ damage in lethal sepsis. Knowledge of these mechanisms has elevated the importance of understanding both the molecular basis of inflammation, and the regulatory systems that keep it in check during health.
Inflammation is induced by factors that are exogenous (e.g. pathogens and microbial products) and endogenous (e.g. HMGB1 released from injured cells) to the host (1; 2). These inducing agents interact with genome encoded pattern recognition receptors expressed on monocytes, macrophages, and other cells of the innate immune system (3). These receptor families, including the toll-like receptors (TLRs) and NOD-like receptors (NLRs), transduce intracellular signals leading to production and release of cytokines, eicosanoids, and other inflammatory molecules that directly mediate cellular responses causing inflammation(3). The cardinal signs of inflammation, including pain, erythema, edema, warmth, and loss of function, can be produced by exposing tissues to inflammatory cytokines. Non-resolving or persisting exposure to cytokine damages tissue, impairs organ function, and can be lethal. Biological therapeutics that specifically inhibit cytokine mediators of inflammation are widely used to treat arthritis, colitis, psoriasis, and a growing list of other disabling illnesses. Therefore, the dangers of uncontrolled inflammation are inherent to the molecular activity of cytokines themselves, and that maintenance of health requires tight control over the steps leading to production and release of cytokines.
Factors that trigger inflammation also enhance the activity of anti-inflammatory pathways, which function to counter-balance inflammation. This concurrent activation of pro- and anti-inflammatory mechanisms is analogous to other homeostatic systems, such as coagulation and fibrinolysis, which act in concert to coordinate hemostasis during hemorrhage. Anti-inflammatory pathways that exert critical roles in suppressing cytokine production include the hypothalamic-pituitary-adrenal axis, which culminates in glucocorticoid release; release of neutralizing soluble cytokine receptors; and production of anti-inflammatory cytokines (e.g. IL-10 and TGFβ). Interruption of these pathways (e.g. adrenalectomy), or failure of their function (genetic deficiency of IL-10), leads to excessive inflammation. This knowledge has enabled the development of novel therapies that suppress inflammation in humans by either directly targeting the activities of cytokines (e.g. anti-TNF antibodies), or by preventing cytokine release (glucocorticoids).
The inflammatory reflex suppresses inflammation
In the late 1990’s, while studying CNI-1493, an inhibitor of the p38 MAP kinase developed by one of us (KJT) as an anti-inflammatory molecule, we discovered an anti-inflammatory neural circuit (4; 5). Termed “the inflammatory reflex”, this neurological mechanism involves the vagus nerve, which can sense peripheral inflammation and transmit action potentials from the periphery to the brain stem (6). This in turn leads to the generation of action potentials in the descending vagus nerve that are relayed to the spleen, where pro-inflammatory cytokine production is inhibited (7). The molecular basis of this anti-inflammatory circuit, termed the cholinergic antiinflammatory pathway, includes the neurotransmitter acetylcholine interacting with the alpha-7 nicotinic acetylcholine receptor subunit expressed on monocytes, macrophages, and other cytokine producing cells(8). Signal transduction through this receptor inhibits cytokine release, suppresses inflammation, and confers protection against tissue damage in polymicrobial sepsis, arthritis, colitis, diabetes, atherosclerosis, ischemia-reperfusion injury, pancreatitis, myocardial ischemia, and hemorrhagic shock (5; 9; 10)(11; 12)(13)(14)(15) (16)(17–20).
To date, the inflammatory reflex is the best- studied anti-inflammatory neural circuit, but undoubtedly, as expertise in this field continues to improve, it is likely that other pathways will be elucidated (21). These findings now raise some fundamental questions about understanding clinical disease pathogenesis. Pre-eminent among these is whether it is possible to measure activity within the inflammatory reflex in humans, in order to understand or predict the risk of uncontrolled inflammation. Here we review available data addressing this question, and focus on the potentially informative data regarding measurements of vagus nerve signaling. We discuss the concept that measuring changes in vagus nerve activity may provide useful information about real-time activity of the inflammatory reflex in patients with inflammatory diseases. We also examine the evidence that measuring underlying vagus nerve activity may have clinical utility for predicting damage from ongoing inflammation, and review the potential implications of utilizing this diagnostic information to then guide therapeutic modulation of immune responses.
The cholinergic anti-inflammatory pathway
The “cholinergic anti-inflammatory pathway” is the descending, or motor arc of the inflammatory reflex(6). It is comprised of vagus nerve signals leading to acetylcholine-dependent interaction with the alpha 7 nicotinic acetylcholine receptor subunit (α7nAChR) on monocytes and macrophages, resulting in reduced cytokine production (8; 22). The cholinergic anti-inflammatory pathway can be activated experimentally by electrical or mechanical vagus nerve stimulation, or through administration of α7 agonists, to inhibit inflammatory cytokine production, prevent tissue injury, and improve survival in multiple experimental models of systemic inflammation and sepsis (4; 5; 9–14; 16–18). Mononuclear cells express muscarinic and nicotinic acetylcholine receptors on their cell surface, and experimental evidence suggests that α7 is required for the regulation of cytokine release by acetylcholine(8). This pathway is unique in the context of vagus nerve signaling, because in contrast to classical parasympathetic nervous system signaling through muscarinic receptors on target organs, this circuit requires signaling through a specific nicotinic receptor subunit.
Under basal conditions, the cholinergic anti-inflammatory pathway exerts a tonic, inhibitory influence on innate immune responses to infection and tissue injury. Interrupting this pathway, by either severing the vagus nerves, or by knocking out the α7 gene (CHRNA7), produces an inflammatory phenotype characterized by exaggerated responses to bacterial products and injury(5; 8). For example, electrical stimulation of the cervical vagus nerve in wild-type mice reduces pro-inflammatory cytokine production, but α7nAChR-knockout animals are resistant to these effects and produce higher levels of cytokines despite vagus nerve stimulation. Even in the absence of vagus nerve stimulation, α7nAChR-knockout mice generate a significantly elevated pro-inflammatory cytokine response following challenge with endotoxin, giving direct evidence that the α7 receptor is necessary to maintain the tonic inhibitory influence of the cholinergic anti-inflammatory pathway. Signal transduction mechanisms involving theα7nAChR are an area of active study by a number of groups, and more data is needed to fully understand this mechanism. There is general agreement that the cytokine suppressing signals from α7nAChR are not dependent upon activation of ion channels, the principal mechanism by which α7nAChR mediates signaling in neurons. Rather, the current signal transduction model indicates that receptor ligand interaction activates JAK-STAT dependent inhibition of the nuclear translocation of nuclear factor (NF)-κB, resulting in decreased transcription of cytokine genes (22–24).
Organs of the reticuloendothelial system, including the lungs, liver, and spleen, contain innate immune cells that mediate the immediate, early response to pathogens and injury. During endotoxemia, an experimental model of Gram-negative bacterial shock produced by administration of lipopolysaccharide, the spleen is the major organ source of systemic TNF that accumulates in blood(7; 25). TNF production in spleen accounts for >90% of the total TNF burden that reaches the circulation, so it is perhaps not surprising that splenectomy significantly reduces circulating TNF levels in endotoxin-challenged mice.
Vagus nerve stimulation fails to inhibit systemic TNF production in splenectomized mice or in mice following interruption of either the common celiac branch of the abdominal vagus nerve, or the splenic nerve, indicating that cholinergic anti-inflammatory pathway control of TNF culminates in spleen(7; 26; 27). As expected by the observation that vagus nerve stimulation suppresses serum TNF, vagus nerve stimulation significantly reduces TNF synthesis in spleen, an effect that requires α7nAChR. Moreover, administration of selective α7nAChR agonists to splenectomized mice fails to reduce cytokine levels; rather, this exacerbates pro-inflammatory cytokine production and increases lethality (7; 13; 25). These findings indicate that the spleen is a critical physiological interface between cholinergic anti-inflammatory signaling and regulation of systemic immune responses.
In addition to regulating cytokine release, the cholinergic anti-inflammatory pathway modulates the expression of activation markers on circulating leukocytes that transit the spleen. Vagus nerve stimulation down-regulates neutrophil activation by attenuating expression of CD11b, a surface molecule required for cell adhesion and chemotaxis (28). The mechanism of CD11b suppression requires F-actin polymerization, the rate-limiting step for CD11b surface expression. Using a carrageenan air pouch model to study recruitment of neutrophils to sites of local soft tissue inflammation, stimulating the vagus nerve significantly inhibits neutrophil recruitment(28; 29). An intact spleen is required for this response, because vagus nerve stimulation of splenectomized animals fails to inhibit neutrophil recruitment to the air pouch(25; 28). In the absence of exogenous vagus nerve stimulation, removing the spleen also interferes with endogenous neutrophil trafficking. These results indicate that vagus nerve signals to spleen control the activity of circulating immune cells, regulating the ability of these cells to respond to inflammatory stimuli, and migrate to local zones of ongoing inflammation, even when these regions are not innervated directly by the vagus nerve. Together these findings point to a specific, centralized neural pathway innervating the spleen that is positioned to both suppress inflammatory cytokine production and down-regulate the activity of circulating inflammatory cells.
It is interesting to consider the clinical experience with splenectomized patients. Even with the availability of effective vaccines, splenectomized patients are at increased risk for potentially lethal bacterial infections from the syndrome of overwhelming post-splenectomy sepsis. The pathogenesis of this syndrome is debated, but it has been attributed to inadequate immune responsiveness, particularly to encapsulated bacteria (30). Based on these new insights provided by the cholinergic anti-inflammatory pathway, it is intriguing to consider whether patients dying of post-splenectomy complications develop uncontrolled, and ultimately lethal, cytokine responses due to the absence of a functional inflammatory reflex.
Central activation of the cholinergic anti-inflammatory pathway
The original concept of the inflammatory reflex followed experimental observations that administration of extremely low quantities of CNI-1493 into the brain inhibited systemic TNF production during endotoxemia(31). This effect was not attributable to either leakage of the drug from the brain into the periphery, or to stimulation of the hypothalamic-pituitary-adrenal axis. Surprisingly at the time, the systemic cytokine inhibitory actions of intracerebral CNI-1493 were dependent upon the vagus nerve, because vagotomy abolished the TNF-suppressing effects of intracerebral administration(9). This was ultimately explained by the fact that CNI-1493 is a weak agonist of the M1 class of muscarinic receptors, which stimulate activity within descending forebrain cholinergic neural pathways that mediate increased efferent vagus nerve activity(32; 33). Intracerebral M1 muscarinic receptors had been implicated in the control of visceral functions by the vagus nerve, including glycogen synthesis in the liver, pancreatic exocrine secretion, and cardiovascular reflexes(34; 35). Administration of selective muscarinic receptor agonists into the brain stimulates vagus nerve mediated suppression of cytokine production during endotoxemia(32; 33). These effects are directly attributable to intracerebral signals, because peripheral administration of muscarinic receptor agonists that cannot cross the blood-brain barrier fails to inhibit cytokine release. There is now widespread interest in studying the anti-inflammatory effects of clinically approved, centrally acting acetylcholinesterase inhibitors, which increase brain acetylcholine levels and enhance M1 signaling. Preclinical studies indicate that these agents increase the activity of the cholinergic anti-inflammatory pathway, and suppress inflammation in the periphery. It may be possible to exploit this approach in clinical trials of treating inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and psoriasis, because these diseases can be controlled by inhibiting cytokine activity.
Clinical implications of the inflammatory reflex
Neural circuits function reflexively to maintain physiological stability in visceral organs. Each reflex is comprised of a sensory or afferent arc that detects environmental and chemical changes. This information is relayed to neural centers in the central nervous system, which integrate the input and relay neural signals to an output or motor nucleus. Efferent signals are then relayed by motor neurons traveling to the innervated organ to produce a response that maintains an “appropriate” or healthy level of function. The net physiological effect of this reflexive behavior is a system output that varies according to a set point function curve. For example, consider the control of heart rate: increases in heart rate activate a reflex circuit that leads to increased activity in the vagus nerve, which slows heart rate and restores homeostasis. A major unanswered question in clinical immunology is whether it is possible to record neural activity in the vagus nerve as a surrogate marker of activity in the inflammatory reflex, to determine the sensitivity of the innate immune system to inflammation.
Measuring vagus nerve activity: Heart rate variability
A widely used approach to measuring vagus nerve activity in humans is based on cardiac physiology. Heart rate is controlled by action potentials transmitted via the vagus nerve to the sinoatrial node of the heart, where vagus nerve-dependent acetylcholine release essentially “prolongs” the time to the next heartbeat, thus slowing the pulse. Measuring the time between individual hearts beats, as can be accomplished with software that captures the distance between R waves on the EKG tracing, provides information about the instantaneous heart rate. These data are then plotted as a function of time to provide analysis of heart rate variability (HRV), or the dynamic variation of heart rate under control of the sympathetic and parasympathetic nervous input (36). Heart rate variability represents the time differences between successive heartbeats (also known as the beat-to-beat interval), and is synonymous with RR variability, referring to the R waves on the electrocardiogram corresponding to ventricular depolarization.
Analysis of the time differences between successive heartbeats to assess heart rate variability can be accomplished with reference to time (time domain analysis) or frequency (frequency domain analysis). The former is based on the normal-to-normal (NN) interval, or the time difference between successive QRS complexes (RR interval) resulting from sinus node depolarization on a standard, continuous electrocardiogram (EKG). Statistical analysis of measurements of the NN intervals, or those derived from the differences between NN intervals, yields various measures of inter-beat variability. Examples include the standard deviation of the NN interval (SDNN), i.e. the square root of variance, the standard deviation of the average NN interval calculated over short periods (SDANN), and the square root of the mean squared differences of successive NN intervals greater than 50 ms (RMSSD).
Frequency domain analysis, which is more widely used to analyze heart rate variability, utilizes spectral methods to interpret the RR tachogram. Power spectral density (PSD) analysis provides information of how power (variability) distributes as a function of frequency. A mathematical algorithm, Fast Fourier Transform (FFT), generates spectral (frequency) components that are labeled ultra-low frequency (ULF), very low frequency (VLF), low frequency (LF), and high frequency (HF) power components. Power components can be expressed in absolute values (ms2) or in normalized units (n.u.), which are used to represent the relative contribution of each power component to the total variance (power) in the recording.
Extensive physiological and pharmacological studies have examined the neural contributions to the frequency components of heart rate variability. For example, administration of acetylcholine antagonists or vagotomy down modulates the HF power component, and electrical vagus nerve stimulation increases HF power (37). These results indicate that the HF power component reflects efferent vagus nerve activity to the sinoatrial node. The interpretation of LF power is less clear, but most agree that the LF component is a measure of sympathetic activity or a combination of sympathetic and parasympathetic activity (37). The ratio of low-to-high frequency spectral power (LF/HF) has been proposed as an index of sympathetic to parasympathetic balance of heart rate fluctuation. A consensus has not been achieved concerning the physiologic correlates of VLF and ultra low frequency power (ULF).
Measures of heart rate variability have been strongly correlated to morbidity and mortality from diverse diseases. Early clinical findings, first observed more than 50 years ago, revealed that variability in RR intervals predict the onset of fetal distress before any measurable changes in absolute heart rate(38–40). There is now extensive experience using heart rate variability measures in diverse disease syndromes, and these data indicate that decreased vagus nerve activity is associated with increased morbidity and mortality. These correlations include increased morbidity and mortality following cardiac surgery or myocardial infarction, increased mortality from sepsis, and progression or disease severity in autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, and sarcoidosis(41–49). Prior to knowledge of the inflammatory reflex, it was thought that decreased vagus nerve activity in these cases resulted from neural damage associated with the underlying diseases. It is now possible to consider an alternative explanation, that decreased vagus nerve activity, and the associated loss of the tonic inhibitory influence of the cholinergic anti-inflammatory pathway on innate immune responses and cytokine release, may enable significantly enhanced cytokine responses to stimuli that would have been otherwise harmless in the presence of a functioning neural circuit.
Planned and ongoing clinical studies in patients with cytokine mediated diseases, including rheumatoid arthritis, inflammatory bowel disease, sepsis, psoriasis, and depression are providing new insights into measures of vagus nerve activity as a direct correlate to cholinergic anti-inflammatory pathway activity. We recently assessed RR interval variability in rheumatoid arthritis and observed that vagus nerve activity was significantly decreased in patients as compared to healthy controls(50; 51) Moreover, serum levels of HMGB1, a cytokine that has been implicated in the pathogenesis of rheumatoid arthritis and other inflammatory syndromes, are significantly related to RR interval variability. There was no significant relationship between disease severity and vagus nerve activity, which is consistent with the hypothesis that impaired vagus nerve activity is not the result of advancing disease. Vagus nerve activity was predictive of the innate immune response to endotoxin, and administration of endotoxin to healthy human subjects revealed a significant correlation of basal high-frequency variability to the magnitude of TNF release (52). Together these results support the hypothesis that diminished vagus nerve signals, which normally provide an inhibitory influence on cytokine production, contribute to enhanced or unregulated production of TNF and other inflammatory mediators.
Depressed vagus nerve activity has been implicated in exaggerated inflammation in peripheral organs following brain death, and as expected, heart rate variability decreases significantly following brain death in rats (53). Interestingly, before harvesting donor organs, vagus nerve stimulation significantly decreases cytokine concentrations in serum and reduces the expression of pro-inflammatory cytokines, E-selectin, IL-1β, and ITGA6. Moreover, assessment of renal function reveals significant improvements in recipients of grafts from donors that had been subjected to vagus nerve stimulation as compared to unstimulated donor grafts (53). These results agree with a direct, contributory role of impaired vagus nerve signaling in excessive cytokine release during ischemia before organ harvesting.
Numerous studies have investigated the relationship between depression, systemic cytokine production, and heart rate variability. Depression is associated with abnormalities in innate and adaptive immune function, including increased production of pro-inflammatory cytokines, decreased production of anti-inflammatory cytokines, and increased expression of surface markers associated with immune cell activation(54–56). It is plausible that over-expression of cytokines in the brain may influence depressive behavior, because cognitive impairment, behavioral dysfunction, and sickness syndrome effects are mediated by cytokines, including TNF. Current data are unable to determine whether the onset of a major depressive episode precedes the development of a dysfunctional immune response, or vice versa. Patients with major depressive disorder also exhibit decreased heart rate variability, and the severity of the impairment correlates with the clinical severity of depression(57). Moreover, implantable vagus nerve stimulators are used in patients with treatment-resistant depression, with improvements observed in a significant percentage of patients(58–60). It is now interesting to consider whether these observations regarding the relationship between depression and maladaptive immune responses result from impaired vagus nerve regulation of cytokine release. It should be possible to design clinical studies to address the question of whether increasing vagus nerve activity using a nerve stimulator corrects the dysregulated cytokine response, and lowers the exposure of the brain to cytokines that disrupt behavior. End point selection of these studies will be is critically important, and should include assays of stimulated cytokine release (e.g. whole blood endotoxin stimulated cytokine release), because basal cytokine levels are not well correlated to disease severity (61).
The role of inflammation in the development and progression of atherosclerosis is another area of significant interest, and a number of recent studies have explored the potential relationship between the inflammatory reflex and atherogenic risk (62–65). For example, C-reactive protein (CRP), implicated as an independent risk factor for cardiovascular mortality and morbidity, was measured in 678 healthy subjects. CRP levels were significantly inversely related with vagus nerve activity, as assessed by heart rate variability, thus providing clinical evidence that vagus nerve activity may modulate systemic inflammatory responses in cardiovascular disease(36). Another large study of CRP and IL-6 levels was conducted in 682 outpatients with coronary heart disease, and vagus nerve activity was inversely correlated with CRP and IL-6 levels(66). The relationship between circulating inflammatory markers has also been studied in healthy university students, (20.56 +/− 0.82 years), and those subjects in the highest tertile of hs-CRP had significantly decreased vagus nerve activity(67). Moreover, vagus nerve activity was inversely correlated with hs-CRP, with the lowest hs-CRP levels observed in the most physically active subjects, who also had the highest levels of vagus nerve activity. Thus, loss of the inflammation suppressing activity of vagus nerve signals may contribute to overproduction of CRP, which in turn is controlled by cytokines, including IL-1 and IL-6.
Sloan and colleagues recently assessed whether aerobic exercise training can modulate vagus nerve activity and whole blood production of TNF(68). In a study of 61 healthy sedentary subjects (age 20–45 yr), those receiving the highest intensity aerobic training had significant reductions in TNF production. These data suggest that in healthy young adults, a 12-wk high-intensity aerobic training program sufficient to increase VO2 max can inhibit cytokine release from blood monocytes. A larger study is presently underway to assess the role of vagus nerve activity in conferring protection against inflammatory cytokine release in humans(68). It has been proposed that a major cardio-protective benefit of exercise is derived from enhanced vagus nerve activity which inhibits inflammatory risk and atherogenesis.
There is a need to determine whether augmenting vagus nerve activity in patients who are deficient in this activity will reduce cytokine production and the levels of other inflammatory factors, including CRP and IL-6. This has not been reported in patients with autoimmune or other active inflammatory diseases, but a study of 183 healthy adults (mean age = 45) revealed that higher vagus nerve activity is significantly associated with lower production of TNF and IL-6 in endotoxin- stimulated whole blood assays(69). This association was independent of demographic and health characteristics, including age, gender, race, years of education, smoking, hypertension, and white blood cell count. These authors concluded that vagus nerve activity is inversely related to the activity of inflammatory mediators, which has potential implications for studying mechanisms linking psychosocial factors to risk for inflammatory diseases.
Are the cardiac and immune regulatory vagus nerve pathways linked?
As clinical studies of vagus nerve- mediated inflammatory responses expand, it may be possible to dissociate the neural pathways that regulate immunity from those that regulate other vagus nerve functions. In animal studies of direct stimulation of the vagus nerve it is possible to activate the cholinergic anti-inflammatory pathway by delivering an electrical charge that is below the threshold required to significantly change heart rate(13). Thus, the neural tracts descending in the vagus nerve to modulate immune responses function at a lower firing threshold than the cardio-inhibitory fibers. It is likely that there are anatomic and physiologic differences that underlie these responses. For example, cardio-inhibitory vagus nerve fibers in mammals are B and C fiber types, which require significantly higher stimulation intensities to fire, as compared to myelinated A-type fibers, which do not participate in heart rate regulation. These “lower threshold” A signaling fibers may convey the anti-inflammatory signal of the cholinergic anti-inflammatory pathway to peripheral immune cells(13).
The relationship between activation thresholds of the cholinergic anti-inflammatory pathway and regulation of heart rate variability is an area of intensive study, and the available clinical evidence indicates that when vagus nerve activity is deficient, inflammation is excessive. There are theoretical and practical advantages to developing devices that can selectively activate the cholinergic anti-inflammatory pathway without stimulating cardiac fibers. It may be possible to draw correlative analysis from measurements of heart rate variability to identify individuals with reduced vagus nerve signaling who are susceptible to tissue damage from inflammation. Heart rate variability could serve as a biomarker to identify patients that may benefit from pharmacological or electrical stimulation of the cholinergic anti-inflammatory pathway. As Holter monitors are used to track changes in heart rhythm, heart rate variability monitors may one day provide indices of diminished or enhanced vagus anti-inflammatory activity. During therapy for inflammation, it may be possible to measure the physiologic level of exogenous cholinergic stimulation delivered to each patient, and to modulate the delivery of therapy by altering voltage, pulse, and time in order to tailor the treatment to the individual, based on changes in heart rate variability. Autoimmune diseases are characterized by waxing and waning clinical episodes, and HRV measurements may one day be used to predict impending relapse by revealing declining activity in the cholinergic anti-inflammatory pathway, thus signaling the need for additional treatment to enhance the neural network.
It is also theoretically possible that monitoring heart rate variability and vagus nerve activity may prove to be useful as a long-term measure of inflammation in chronic diseases. Similar to tracking hemoglobin A1c levels in patients with diabetes, or daily blood pressure monitoring in patients with hypertension, heart rate variability monitoring could theoretically be developed to monitor the activity of inflammatory risk in these and other cytokine-mediated diseases. Non-invasive methods to determine heart rate variability are available, and it will be of interest to assess the usefulness of portable monitoring devices at home that interface with central monitoring stations to provide online analysis of changes in the inflammatory reflex. Correction of chronic, maladaptive levels of inflammation using nerve stimulators might prevent the progression of debilitating and deadly diseases, potentially replacing the need for some biological therapeutics.
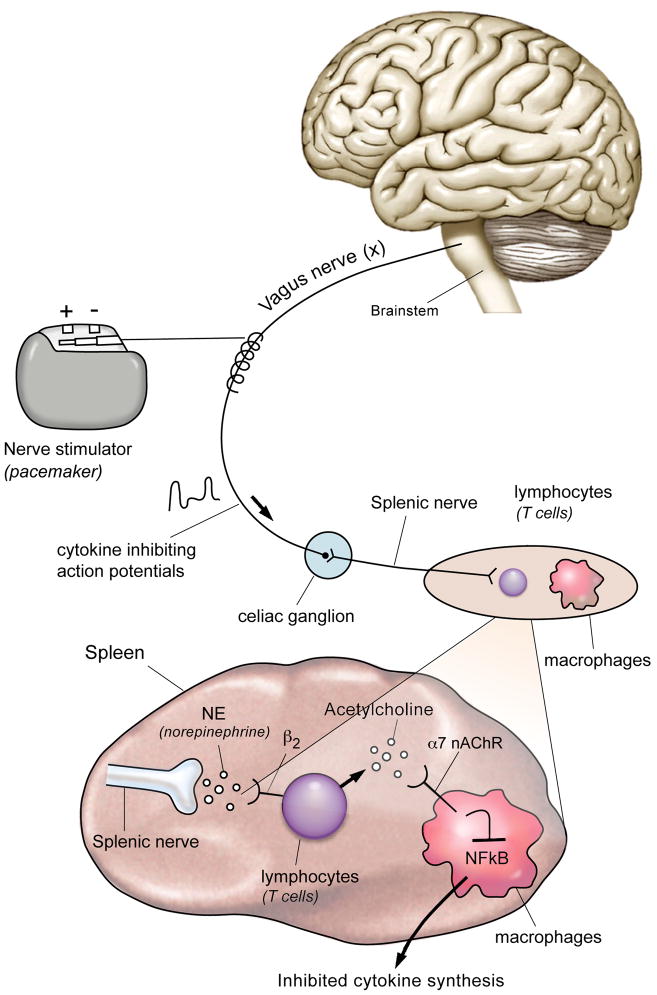
Figure 1.
Action potentials transiting the vagus nerve synapse in the celiac ganglion, the origin of the splenic nerve. The splenic nerve controls lymphocytes in the spleen, which can produce acetylcholine that interacts with α7 nicotinic acetylcholine receptors expressed on cytokine producing macrophages. Intracellular signal transduction through this receptor inhibits the activity of NFκB to suppress cytokine production. Nerve stimulators can provide an identical signal to initiate the antiinflammatory pathway, an approach that reverse signs and symptoms in preclinical disease models of arthritis, inflammatory bowel disease, ischemia-reperfusion injury, heart failure, pancreatitis, sepsis, and other syndromes.
Acknowledgments
Supported in part by grants from the NIH (NIGMS) to KJT.
Footnotes
Conflict of interest statement
No conflict of interest was declared
Reference List
- 1.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A Critical Cysteine is Required for HMGB1 Binding to TLR4 and Activation of Macrophage Cytokine Release. Proc Natl Acad Sci USA. 2010;26:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 7.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 9.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 11.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der PT. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 12.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der PT. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 13.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 14.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Maanen MA, Lebre MC, van der PT, Larosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 16.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 17.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani A, Ghiaroni V, Passaniti M, Leone S, Bazzani C, Caputi AP, Squadrito F, Bertolini A. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 19.Ottani A, Giuliani D, Galantucci M, Spaccapelo L, Novellino E, Grieco P, Jochem J, Guarini S. Melanocortins counteract inflammatory and apoptotic responses to prolonged myocardial ischemia/reperfusion through a vagus nerve-mediated mechanism. Eur J Pharmacol. 2010;637:124–130. doi: 10.1016/j.ejphar.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Xie H, Wen T, Liu H, Zhu W, Chen X. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J Rheumatol. 2010;37:766–775. doi: 10.3899/jrheum.090663. [DOI] [PubMed] [Google Scholar]
- 21.Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol. 2010;11:561–564. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Hudson LQ, Lin X, Patel N, Johnson SM, Chavan S, Goldstein R, Czura CJ, Miller EJ, Al Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF Release by Choline Requires Alpha7 Subunit Nicotinic Acetylcholine Receptor-Mediated Signaling. Mol Med. 2008 doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 25.Huston JM, Wang H, Ochani M, Ochani K, Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ, Yang H. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J Immunol. 2008;181:3535–3539. doi: 10.4049/jimmunol.181.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, Yeboah MM, Chatterjee PK, Tracey KJ, Metz CN. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183:552–559. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection--an update. Crit Care Med. 1999;27:836–842. doi: 10.1097/00003246-199904000-00050. [DOI] [PubMed] [Google Scholar]
- 31.Tracey KJ. Suppression of TNF and other proinflammatory cytokines by the tetravalent guanylhydrazone CNI-1493. Prog Clin Biol Res. 1998;397:335–343. [PubMed] [Google Scholar]
- 32.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Wu X, Zhu J, Yan J, Owyang C. Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathways in the rat. J Physiol. 2003;552:571–587. doi: 10.1113/jphysiol.2003.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimazu T, Matsushita H, Ishikawa K. Cholinergic stimulation of the rat hypothalamus: effects of liver glycogen synthesis. Science. 1976;194:535–536. doi: 10.1126/science.9692. [DOI] [PubMed] [Google Scholar]
- 36.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 38.Gaziano EP, Freeman DW. Analysis of heart rate patterns preceding fetal death. Obstet Gynecol. 1977;50:578–582. [PubMed] [Google Scholar]
- 39.Sampson MB, Mudaliar NA, Lele AS. Fetal heart rate variability as an indicator of fetal status. Postgrad Med. 1980;67:207–5. doi: 10.1080/00325481.1980.11715459. [DOI] [PubMed] [Google Scholar]
- 40.Lauersen NH, Wilson KH, Bilek A, Rao VS, Kurkulos M. A new modality in nonstress testing: evaluation of beat-to-beat fetal heart rate variability. Am J Obstet Gynecol. 1981;141:521–526. doi: 10.1016/s0002-9378(15)33272-5. [DOI] [PubMed] [Google Scholar]
- 41.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 42.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85:I77–I91. [PubMed] [Google Scholar]
- 44.Garrard CS, Kontoyannis DA, Piepoli M. Spectral analysis of heart rate variability in the sepsis syndrome. Clin Auton Res. 1993;3:5–13. doi: 10.1007/BF01819137. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, Prondzinsky R, Loppnow H, Buerke M, Hoyer D, Werdan K. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33:1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 46.Aydemir M, Yazisiz V, Basarici I, Avci AB, Erbasan F, Belgi A, Terzioglu E. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus. 2010;19:255–261. doi: 10.1177/0961203309351540. [DOI] [PubMed] [Google Scholar]
- 47.Stojanovich L, Milovanovich B, de Luka SR, Popovich-Kuzmanovich D, Bisenich V, Djukanovich B, Randjelovich T, Krotin M. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus. 2007;16:181–185. doi: 10.1177/0961203306076223. [DOI] [PubMed] [Google Scholar]
- 48.Sharma P, Makharia GK, Ahuja V, Dwivedi SN, Deepak KK. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig Dis Sci. 2009;54:853–861. doi: 10.1007/s10620-008-0424-6. [DOI] [PubMed] [Google Scholar]
- 49.Lindgren S, Stewenius J, Sjolund K, Lilja B, Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand J Gastroenterol. 1993;28:638–642. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 50.Bruchfeld A, Goldstein RS, Chavan S, Patel NB, Rosas-Ballina M, Kohn N, Qureshi AR, Tracey KJ. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. 2010;268:94–101. doi: 10.1111/j.1365-2796.2010.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, Huston BJ, Chavan S, Rosas-Ballina M, Gregersen PK, Czura CJ, Sloan RP, Sama AE, Tracey KJ. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF. Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock. 2010;33:363–368. doi: 10.1097/SHK.0b013e3181b66bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoeger S, Bergstraesser C, Selhorst J, Fontana J, Birck R, Waldherr R, Beck G, Sticht C, Seelen MA, van Son WJ, Leuvenink H, Ploeg R, Schnuelle P, Yard BA. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am J Transplant. 2010;10:477–489. doi: 10.1111/j.1600-6143.2009.02951.x. [DOI] [PubMed] [Google Scholar]
- 54.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Bajbouj M, Merkl A, Schlaepfer TE, Frick C, Zobel A, Maier W, O’Keane V, Corcoran C, Adolfsson R, Trimble M, Rau H, Hoff HJ, Padberg F, Muller-Siecheneder F, Audenaert K, van den AD, Matthews K, Christmas D, Eljamel S, Heuser I. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. 2010;30:273–281. doi: 10.1097/JCP.0b013e3181db8831. [DOI] [PubMed] [Google Scholar]
- 59.Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, Barry JJ. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, Nahas Z, Lisanby SH. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 61.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 62.Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:759–7. doi: 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carney RM, Freedland KE, Stein PK, Miller GE, Steinmeyer B, Rich MW, Duntley SP. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res. 2007;62:463–467. doi: 10.1016/j.jpsychores.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janszky I, Ericson M, Lekander M, Blom M, Buhlin K, Georgiades A, Ahnve S. Inflammatory markers and heart rate variability in women with coronary heart disease. J Intern Med. 2004;256:421–428. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 66.Frasure-Smith N, Lesperance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009;23:1140–1147. doi: 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Soares-Miranda L, Negrao CE, Antunes-Correa LM, Nobre TS, Silva P, Santos R, Vale S, Mota J. High levels of C-reactive protein are associated with reduced vagal modulation and low physical activity in young adults. Scand J Med Sci Sports. 2010 doi: 10.1111/j.1600-0838.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 68.Sloan RP, Shapiro PA, Demeersman RE, McKinley PS, Tracey KJ, Slavov I, Fang Y, Flood PD. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 69.Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]