Abstract
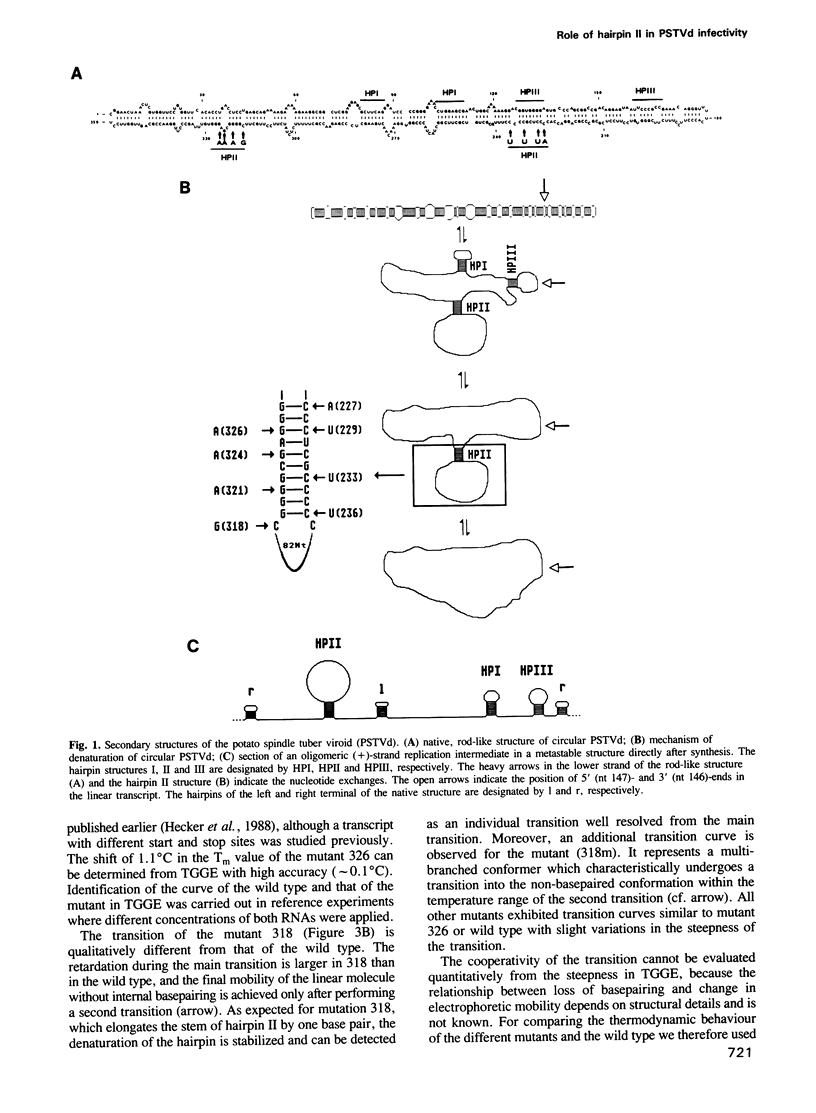
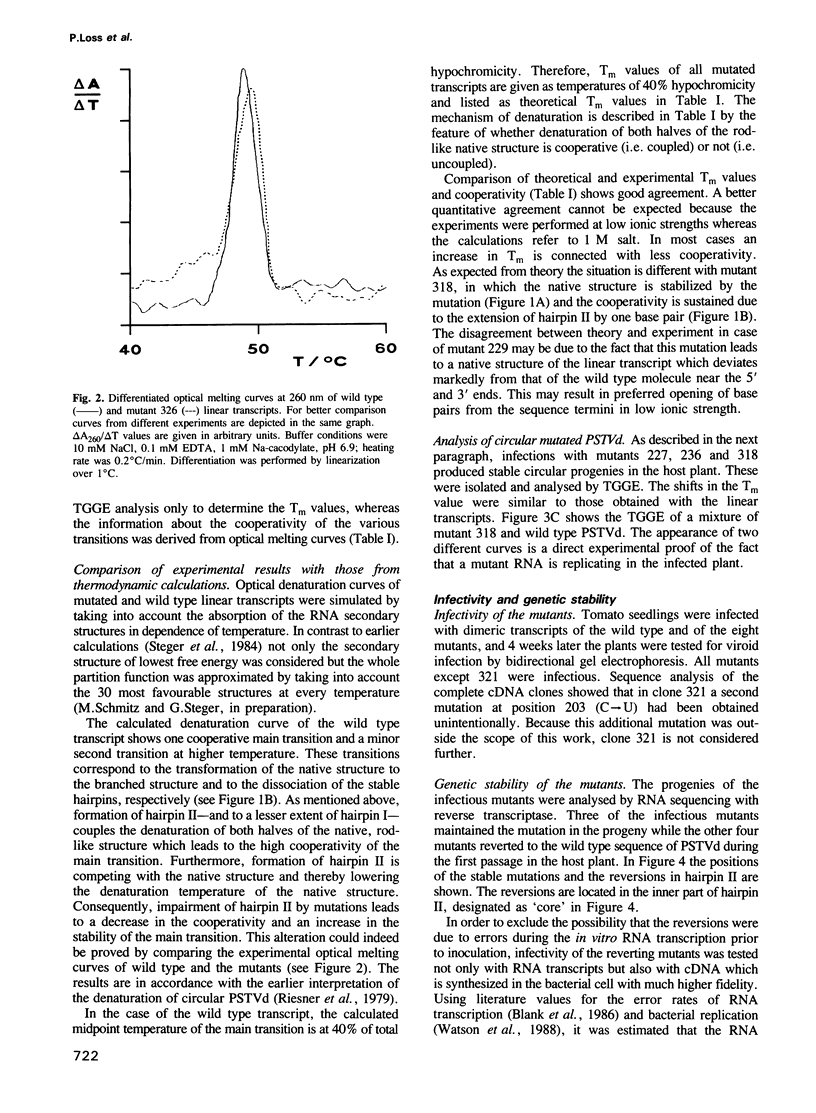
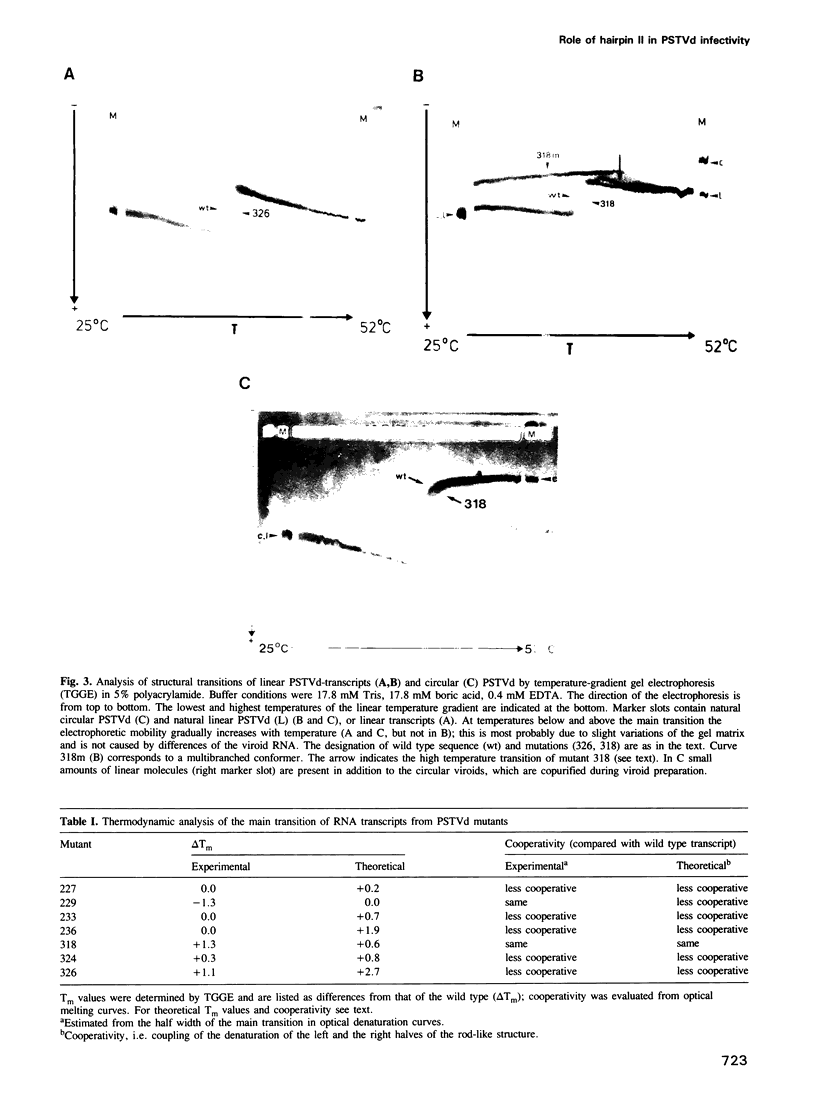
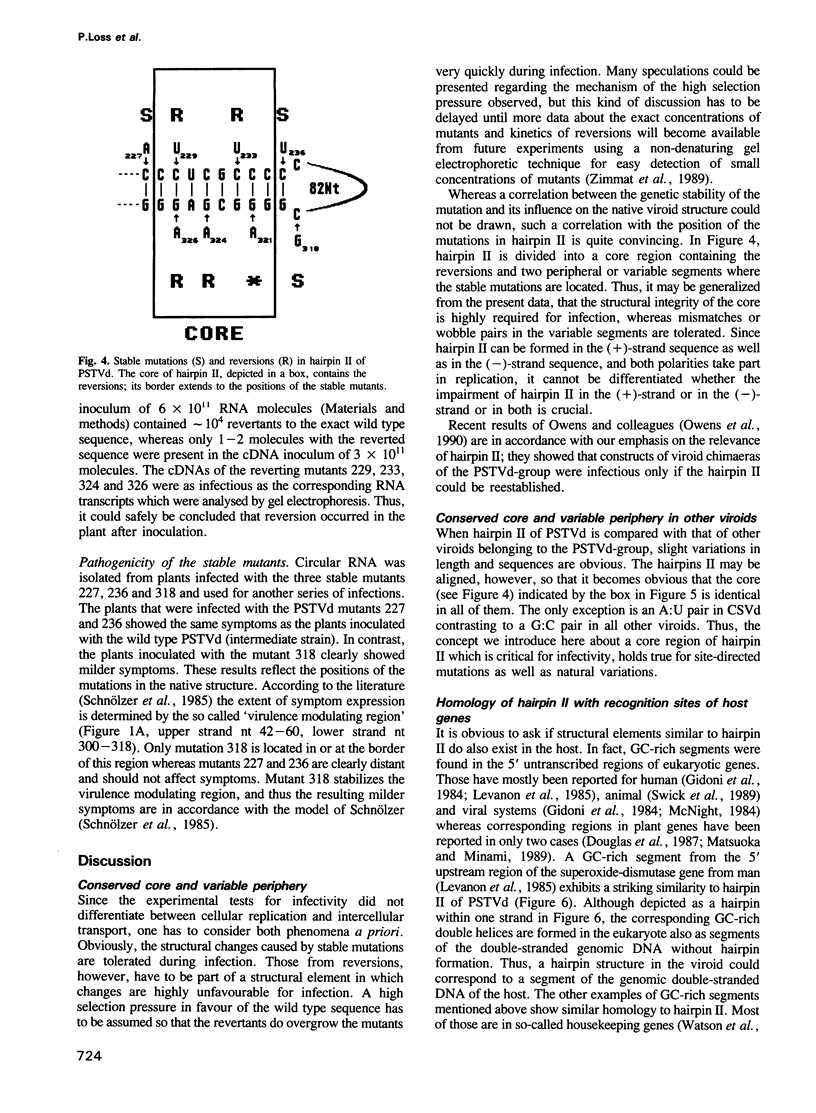
The functional relevance of a hairpin II-containing structure of viroid RNA was studied by site-directed mutagenesis, thermodynamic calculations, experimental denaturation curves and infectivity tests. Hairpin II is formed during thermal denaturation of circular viroids or as part of a metastable structure during synthesis of viroid replication intermediates. In potato spindle tuber viroid (PSTVd), eight single-site mutations were generated in the segments which form hairpin II. From the mutated viroid cDNA clones, linear RNA transcripts of PSTVd unit length were synthesized. The relevance of hairpin II for the mechanism of denaturation was confirmed quantitatively by optical denaturation curves and temperature-gradient gel electrophoresis. Infectivity tests showed that the mutations in the core region of hairpin II reverted to the wild type sequence whereas the mutations in the peripheral regions of hairpin II remained genetically stable. These data are in accordance with the natural variance of hairpin II in other viroids of the PSTVd class. Thus, the integrity of the core of hairpin II is critical for infectivity. Hairpin II exhibits a strong similarity in sequence as well as in three-dimensional structure to certain DNA GC-clusters found in the 5'-upstream regions of some genes in man, animals, viruses and plants. A hypothesis about a function of hairpin II as a binding site for host cell transcription factors is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank A., Gallant J. A., Burgess R. R., Loeb L. A. An RNA polymerase mutant with reduced accuracy of chain elongation. Biochemistry. 1986 Oct 7;25(20):5920–5928. doi: 10.1021/bi00368a013. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Benenfeld B. J., Robertson H. D. Evidence for a single rolling circle in the replication of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9128–9132. doi: 10.1073/pnas.85.23.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Colpan M., Schumacher J., Brüggemann W., Sänger H. L., Riesner D. Large-scale purification of viroid RNA using Cs2SO4 gradient centrifugation and high-performance liquid chromatography. Anal Biochem. 1983 May;131(1):257–265. doi: 10.1016/0003-2697(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Cress D. E., Kiefer M. C., Owens R. A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983 Oct 11;11(19):6821–6835. doi: 10.1093/nar/11.19.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroid processing: a model involving the central conserved region and hairpin I. Proc Natl Acad Sci U S A. 1986 Jan;83(1):58–62. doi: 10.1073/pnas.83.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C., Hoffmann H., Schulz W., Hahlbrock K. Structure and elicitor or u.v.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J. 1987 May;6(5):1189–1195. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco J. R., Adams A., Ascione R., Henley D., Lindahl T. Tertiary structure in transfer ribonucleic acids. Cold Spring Harb Symp Quant Biol. 1966;31:527–537. doi: 10.1101/sqb.1966.031.01.068. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Goodman T. C., Nagel L., Rappold W., Klotz G., Riesner D. Viroid replication: equilibrium association constant and comparative activity measurements for the viroid-polymerase interaction. Nucleic Acids Res. 1984 Aug 10;12(15):6231–6246. doi: 10.1093/nar/12.15.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harders J., Lukács N., Robert-Nicoud M., Jovin T. M., Riesner D. Imaging of viroids in nuclei from tomato leaf tissue by in situ hybridization and confocal laser scanning microscopy. EMBO J. 1989 Dec 20;8(13):3941–3949. doi: 10.1002/j.1460-2075.1989.tb08577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker R., Wang Z. M., Steger G., Riesner D. Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene. 1988 Dec 10;72(1-2):59–74. doi: 10.1016/0378-1119(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Lauble H., Frank R., Blöcker H. Crystal structure analysis of an A-DNA fragment at 1.8 A resolution: d(GCCCGGGC). Nucleic Acids Res. 1987 Nov 25;15(22):9531–9550. doi: 10.1093/nar/15.22.9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Sänger H. L., Riesner D. Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Res. 1979 Jul 11;6(9):3041–3059. doi: 10.1093/nar/6.9.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985 Jan;4(1):77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Minami E. Complete structure of the gene for phosphoenolpyruvate carboxylase from maize. Eur J Biochem. 1989 May 15;181(3):593–598. doi: 10.1111/j.1432-1033.1989.tb14765.x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. C., Spence A., Smith M. The distal transcription signals of the herpesvirus tk gene share a common hexanucleotide control sequence. Cell. 1984 May;37(1):253–262. doi: 10.1016/0092-8674(84)90321-0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mühlbach H. P., Sänger H. L. Viroid replication is inhibited by alpha-amanitin. Nature. 1979 Mar 8;278(5700):185–188. doi: 10.1038/278185a0. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Candresse T., Diener T. O. Construction of novel viroid chimeras containing portions of tomato apical stunt and citrus exocortis viroids. Virology. 1990 Mar;175(1):238–246. doi: 10.1016/0042-6822(90)90204-5. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Riesner D., Colpan M., Goodman T. C., Nagel L., Schumacher J., Steger G., Hofmann H. Dynamics and interactions of viroids. J Biomol Struct Dyn. 1983 Dec;1(3):669–688. doi: 10.1080/07391102.1983.10507474. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum V., Riesner D. Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys Chem. 1987 May 9;26(2-3):235–246. doi: 10.1016/0301-4622(87)80026-1. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Harper K. L. Optimal conditions for cell-free synthesis of citrus exocortis viroid and the question of specificity of RNA polymerase activity. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4429–4433. doi: 10.1073/pnas.81.14.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Swick A. G., Blake M. C., Kahn J. W., Azizkhan J. C. Functional analysis of GC element binding and transcription in the hamster dihydrofolate reductase gene promoter. Nucleic Acids Res. 1989 Nov 25;17(22):9291–9304. doi: 10.1093/nar/17.22.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Forster A. C., Symons R. H. Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucleic Acids Res. 1985 Aug 26;13(16):5843–5856. doi: 10.1093/nar/13.16.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader Jane E., Symons Robert H. Replication of in vitro constructed viroid mutants: location of the pathogenicity-modulating domain of citrus exocortis viroid. EMBO J. 1986 Sep;5(9):2051–2055. doi: 10.1002/j.1460-2075.1986.tb04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]



