Abstract
Posttraumatic stress disorder (PTSD) manifests after exposure to a traumatic event and is characterized by avoidance/numbing, intrusive symptoms and flashbacks, mood and cognitive disruptions, and hyperarousal/reactivity symptoms. These symptoms reflect dysregulation of the fear system likely caused by poor fear inhibition/extinction, increased generalization, and/or enhanced consolidation or acquisition of fear. These phenotypes can be modeled in animal subjects using Pavlovian fear conditioning, allowing investigation of the underlying neurobiology of normative and pathological fear. Pre-clinical studies reveal a number of neurotransmitter systems and circuits critical for aversive learning and memory, which have informed the development of therapies used in human clinical trials. In this review, we discuss the evidence for a number of established and emerging pharmacotherapies and device-based treatments for PTSD that have been developed via a bench to bedside translational model.
Keywords: Exposure, Extinction, Fear, Antidepressant, Morphine, Opioid, D-Cycloserine, Hydrocortisone, Cannabinoid, Glucocorticoid, Propranolol
Introduction
Posttraumatic stress disorder (PTSD) manifests after exposure to a traumatic event and is characterized by four core clusters of symptoms: avoidance/numbing, intrusive symptoms and flashbacks, mood and cognitive disruptions and hyperarousal/reactivity symptoms (1). An event is considered traumatic if it involves exposure to death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence. The estimated lifetime prevalence of PTSD in the United States is between 7–9% (2).
Data suggests that 94% of individuals who experience trauma develop acute PTSD-like symptoms (2, 3). For most, these symptoms will abate over time. Some hypothesize that increased generalization and deficits in extinction underlie symptomatology of PTSD. Enhanced acquisition and consolidation of trauma-related fear may also precipitate the development of PTSD, additionally. Animal studies using Pavlovian fear conditioning and extinction paradigms offer insight on the neurobiology of these fear-related dimensions, allowing identification of functional circuitry and molecular signaling pathways critical for normative and pathological fear, (see Supplementary for Fig S1 and detailed review of Neurobiological Approaches to Fear).
Through these pre-clinical studies, researchers have identified the amygdala, interacting critically with the hippocampus and medial prefrontal cortex (mPFC), as the primary anatomical loci of fear learning and extinction (4–6). Furthermore, manipulations of various transmitter systems during different phases of aversive learning point to a number of potential pharmacotherapies and specific treatment windows. Based on pre-clinical indications, pilot and large-scale clinical studies have now been conducted on a number of treatments with a variety of administration protocols, e.g. chronically, administered in the immediate aftermath of trauma, in conjunction with exposure therapy, or during reconsolidation. Additionally, researchers are exploring the efficacy of device-based treatments for PTSD and PTSD-like symptoms in humans and rodents, given the success of deep-brain stimulation (DBS) for the treatment of depression (7).
In this review, we explore established and emerging treatment strategies for PTSD that are supported by pre-clinical and clinical data. Although the number of approved treatments is small, with SSRIs as the only class of drug approved for treatment of PTSD, exciting new evidence points to a number of promising pharmacotherapies and device-based treatments with a variety of treatment protocols.
Pharmacotherapy approaches to Fear- and Anxiety-related disorders
The following sections review pre-clinical and clinical evidence for a variety of established and emerging pharmacotherapies, especially focusing on underlying transmitter and receptor systems, as well targeted brain regions. In discussing the pre-clinical data, we focus on outlining evidence from studies of cued and contextual fear conditioning, but include discussion of evidence from alternative fear and anxiety paradigms where relevant.
Serotonin (5-HT)/selective serotonin reuptake inhibitors (SSRIs)
Use of SSRIs in PTSD stems from the observed efficacy of SSRIs for depression and the high incidence of depression co-morbid with anxiety and PTSD (8). SSRI efficacy in the treatment of depression contributed to the previously accepted biogenic amine hypothesis, which postulates that disturbances in serotonin, dopamine, and norepinephrine underlie the pathology of depression (9–13).
Similarly, evidence from rodent and human studies implicates brain serotonin systems in the neurobiology of PTSD (14–16). The amygdala, hippocampus, and frontal cortex areas with a demonstrated role in PTSD - receive serotonergic input via projections from the dorsal and median raphe nucleus (17–19). A recent meta-analysis supports an association between the lesser expressing, short allele of 5-HTTLPR (serotonin transporter gene) and PTSD in high-trauma exposed individuals (20). Conclusions from PET analysis are consistent with this model, where individuals with PTSD exhibit reduced amygdala 5-HTT (serotonin transporter protein) binding (14). Additional PET studies observe an association between early trauma exposure and serotonin type 1B receptor binding, as well as higher serotonin 1A binding in PTSD, however these findings have not been uniformly replicated (21–23).
Several studies find increased cued fear acquisition and expression in rodents and humans with acute SSRI administration (24–26). An effect on cued fear acquisition and expression may be mediated by the serotonin 2A receptor, as administration of a serotonin 2A receptor agonist after fear conditioning increases cued fear expression (27), and administration prior to extinction enhances within-session extinction (27). Similarly, serotonin 2A receptor antagonist administration blocks cued fear acquisition (28).
Conversely, chronic SSRI administration impairs fear learning, in particular cued fear acquisition and extinction (29). However, chronic fluoxetine may also prevent return of extinguished fear and facilitate extinction in female rats (30–32). Ultimately, an effect of chronic SSRI administration on extinction, as well as SSRI efficacy for treatment of depression and PTSD, may be driven by a change in glutamatergic transmission, as supported by recent DCS and ketamine findings.
Somewhat consistent with rodent pre-clinical data, several studies indicate that outcomes for individuals treated with SSRIs and CBT outcomes may be worse compared to CBT alone (33–35). Other studies report comparable or modest benefits with combinatorial treatment (36–42). While a 2008 report from the Institute of Medicine (IOM) concludes that SSRIs, among other all other classes of drugs, do not demonstrate efficacy in the treatment of PTSD, a recent meta-analysis supports the efficacy of long-term treatment of PTSD with SSRIs (43). While some suggest that SSRIs are as effective as psychotherapy as a first-line treatment, others recommend SSRIs as a second-line treatment after CBT (41), clearly further investigation of SSRIs is needed (44).
N-methyl-D-aspartic acid (NMDA)/D-cycloserine (DCS)
In combination with cognitive behavioral therapy, D-cycloserine (DCS) - a compound that acts as a partial agonist at the strychnine-insensitive glycine-recognition site of the NMDA receptor has helped the field consider targeted pharmacological augmentation of psychotherapy. In rodents, systemic or intra-amygdala administration of DCS has repeatedly been shown to facilitate extinction of fear-potentiated startle and cued freezing in rats (FPS) (45–48). Furthermore, DCS blocks increases in freezing caused by reinstatement, but has no effect on renewal processes (49, 50). DCS is thought to act on consolidation of emotional learning, as post-training administration of DCS similarly facilitates extinction (45). Importantly, DCS reverses deficits in fear extinction caused by the single prolonged stress model that is hypothesized to more thoroughly instantiate PTSD-like symptoms and the accompanying underlying pathology (51, 52). Similarly, DCS enhances extinction in 129S1/SvImJ (S1), an alternative genetic mouse model of PTSD that exhibits persistent impairment of fear extinction (53).
In humans, DCS shows promise for the treatment of social anxiety, obsessive compulsive disorder (OCD), panic disorder, acrophobia, and nicotine dependence (54–59). Data on efficacy of DCS in the modulation of associative fear learning and treatment of PTSD, however, is mixed. In healthy human volunteers, DCS facilitates consolidation of fear acquisition of previously neutral cues and cued fear extinction (60, 61). Other studies, have also not observe a reduction in conditioned fear with administration of DCS (62–64). For individuals with PTSD, DCS seems particularly effective when administered with virtual reality exposure (VRE) (65, 66). Some studies have not reported increased remission with DCS compared to placebo (when administered in combination with cognitive behavioral therapy) (67, 68). Despite inconsistencies in the literature, meta-analyses suggest that DCS enhances fear extinction/exposure therapy in both animal subjects and humans (69, 70).
The current consensus is that its effects are modulated by a number of factors. DCS yields greater reductions in PTSD-symptoms in subjects with more severe pre-treatment PTSD (71). Furthermore, participants with high conscientiousness and low extraversion exhibit better outcomes with DCS and exposure therapy, compared to placebo (72). DCS also appears to selectively enhance exposure therapy when administered with successful sessions (73). This effect is reflected in rodent models, where subjects who exhibit successful within-session extinction show better long-term extinction with DCS (47, 74). These data suggest that DCS may be an efficacious adjunctive therapy, but only for a subset of the clinical population and with specifically tailored CBT sessions, among other factors (75, 76). Despite its limitations, DCS has been an important molecule in moving the field forward to directly addressing mechanisms of emotional learning from a translational perspective based on a behavioral neuroscience understanding of rodent emotion processing.
Glucocorticoids/Hydrocortisone
Under normative conditions, stress-induced activation of the (HPA)-axis causes an increase in the release of the adrenal hormone cortisol (corticosterone in rodents). Increased cortisol mobilizes biological resources needed to engage the flight or fight response to promote survival. These stress-related increases in cortisol eventually inhibit HPA-axis activity to terminate the stress response. Chronic or extreme stress, however, can contribute to HPA-axis dysregulation and a host of other adverse effects (77, 78).
HPA-axis dysregulation is observed in individuals with PTSD, where low baseline levels of cortisol (although higher levels or no differences have been observed as well (79–82)) and enhanced negative feedback in response to dexamethasone are reported (83–88). Prospective studies suggest that low cortisol in the face of trauma is a predisposing factor for the development of PTSD (87, 89–92). One hypothesis is that reduced cortisol signaling alters normal adaptive responses of the autonomic nervous system, including negative feedback to the pituitary and hypothalamus to terminate the stress response (93). Specifically, low cortisol could impede cortisol homeostasis, contributing to an inability to contain the stress response and resulting in runaway fear characteristic of PTSD.
A number of investigators have developed animal models that more thoroughly instantiate PTSD-like symptoms and replicate HPA-axis alteration. These approaches suggest that glucocorticoid system manipulations in healthy models, where the corticosterone response is adaptive, might be different from PTSD-like models, where corticosterone responses may be maladaptive. Importantly, these studies report that animals exposed to extreme stress exhibit poor fear extinction, greater acoustic startle, more anxiety-like behavior, and heightened fear expression, as in humans with PTSD (94–102).
For example, Lewis rats, which are more susceptible to PTSD-like behavior, do not exhibit an increase in circulating corticosterone several days after stressor exposure compared to other strains that are less vulnerable (95). Mice and rats exposed to extreme stress exhibit enhanced negative feedback in response to acute stress or cortisol (103–106). In other studies, however, subjects that exhibit PTSD-like behavior in response to extreme stress have increased circulating levels of corticosterone several days after stressor exposure compared to well adapted and non-exposed rats (96, 107, 108). Furthermore, other studies find that baseline corticosterone prior to stressor exposure does not predict subsequent behavioral responses to extreme stress (95, 109). Conflicting HPA-axis activity findings in PTSD-like models may reflect a problem of whether these models recapitulate PTSD and/or depression, similar to the problem seen in clinical studies where PTSD is often comorbid with depression.
PTSD-like models have also allowed researchers to test the hypothesis that glucocorticoid administration in the face of extreme stress might contain the stress response and prevent the development of PTSD-like effects. Indeed, acute pharmacological intervention with corticosterone after stress is able to rescue PTSD-like behavioral effects (95, 102, 110, 111). This is now being tested in humans. As in rodent models, administration of hydrocortisone during a critical window after trauma has been shown to reduce the risk of PTSD development (112–114). In humans, administration of hydrocortisone in combination with traumatic memory reactivation reduces PTSD symptoms (115); and greater patient retention was observed in the treatment group with hydrocortisone administered in combination with prolonged exposure therapy (116). Glucocorticoid modulation may also enhance extinction for other fear-related disorders besides PTSD, as seen in successful reduction of fear in combination with exposure therapy for social phobia, spider phobia, and phobia of heights (117, 118). These studies suggest that glucocorticoid modulation enhances extinction memory, in line with pre-clinical evidence implicating the glucocorticoids in memory consolidation (119, 120). It may also acutely reduce fear (118), as seen with daily hydrocortisone reducing re-experiencing and avoidance symptoms of PTSD (121).
Though promising, there remain some inconsistences in the HPA modulation field, consistent with the human literature, where the development of an extreme behavioral response to “trauma” is associated with an increase or decrease in circulating levels of glucocorticoids. Prospective studies evaluating glucocorticoid levels at baseline and in response to a stressor before and after trauma across species would be the most informative.
Opioids/Morphine
Along with marijuana and alcohol, opiates are one of the most commonly abused substances among individuals with PTSD, indicating that aberrant endogenous opioid signaling may underlie PTSD (122). In rodents, administration of opioid antagonists increase conditioned fear by enhancing fear acquisition or blocking fear extinction (123–125). Conversely, morphine (opioid receptor agonist) administration blocks conditioned fear acquisition in normal and prior-stress models (126, 127). Opioid signaling in ventrolateral periaqueductal gray matter (vlPAG) regulates conditioned fear extinction, potentially via activation of mPFC and the BLA (128, 129).
Research addressing specificity of opioid regulation of conditioned fear implicates the mu (μ) and kappa (κ) opioid and nociceptin (NOP)/orphanin FQ receptors. Antagonism of the μ opioid receptor facilitates contextual fear conditioning (130) and blocks extinction of cued fear (131). Similarly, antagonism of the κ opioid receptor blocks conditioned fear on a fear-potentiated startle paradigm (132, 133). In PTSD-like rodent models, differential levels of CSF nociceptin (NOP)/orphanin FQ and nociceptin (NOP)/orphanin FQ receptor mRNA are observed (134, 135). Furthermore, nociceptin (NOP)/orphanin FQ receptor agonist administration blocks contextual and cued fear consolidation in normal and PTSD-like rodent models (135–137).
In humans, genetic analysis revealed a significant interaction between the OPRL1 (opioid receptor-like 1) gene and childhood trauma that is associated with PTSD and neural correlates of PTSD (135). Similarly, other studies suggest that a polymorphism in the OPRM1 gene (opioid receptor μ–1) is associated with less severe PTSD symptoms (138). Regarding specific PTSD-related symptoms, κ opioid receptor availability in the amygdala-anterior cingulate cortex-ventral striatal circuit mediates the expression of dysphoria where lower κ opioid receptor is associated with greater severity of trauma-related loss symptoms (139).
Interestingly, some evidence suggests that morphine may be effective for secondary prevention of PTSD. Children administered morphine after acute burns exhibit decreased PTSD symptoms months to years after treatment in a dose-dependent fashion (140–142). Studies of traumatized adults administered morphine mirror results found in pediatric data sets (143). Prospective studies find that patients who meet criteria for PTSD at 3 months post-trauma received significantly less morphine acutely after injury (144). Data from healthy volunteers, where opioid agonists inhibit and antagonists promote fear acquisition, support the hypothesis that morphine administration in the immediate aftermath of trauma may prevent the development of PTSD by inhibiting the acquisition of fear in response to trauma (145, 146), consistent with above data in rodents (126, 127).
A critical alternative explanation may be that a reduction in pain caused by morphine administration is able to mitigate the development of PTSD. This hypothesis is supported by several reports that pain after trauma is significantly associated with later development of PTSD (144, 147, 148). Nonetheless, previous and ongoing studies suggest that the effects on PTSD buffering may be independent of pain. Further prospective studies are needed to more safely establish morphine’s efficacy as a secondary preventative therapy. Concomitant pain monitoring, or comparison with non-opioid analgesics, will help determine the mechanism by which morphine may prevent the development of PTSD.
Cannabinoids/Nabilone/Delta-9-tetrahydrocannabinoil
Overwhelming evidence from rodent models suggest that the endocannabinoids are critically involved in stress, fear, and anxiety (149, 150). Knockout or antagonism of Cnr1 increases anxiety-like behavior on a number of different paradigms across a variety of species (149, 151, 152). Increased synthesis of the endocannabinoids and subsequent activation of Cnr1 in the amygdala is thought to mediate fear extinction in mice and rats, potentially via inhibition of the anxiogenic neuropeptide cholecystokinin (CCK) and/or modulation of the GABAergic system (151, 153, 154). Additionally, Cnr1 is critical for acquisition, retrieval, and extinction of both cue and context fear, as well as reconsolidation of cued fear memory (153, 155–157). Cnr1 involvement in cued fear is thought to be mediated primarily by the amygdala and mPFC (154, 156, 157). Furthermore, the endocannabinoid system is implicated in stress and stress-sensitization of fear behavior, where Cnr1 is thought to modulate glutamatergic and GABAergic signaling primarily in the bed nucleus of the stria terminalis, the basolateral amygdala, and the central amygdala (158–164). Administration of a Cnr1 agonist acutely after shock prevents PTSD-like symptoms in rats, suggesting that cannabinoid drugs might be administered acutely after trauma to prevent development of PTSD (163).
Evidence implicating Cnr1 involvement in stress, fear, and anxiety in rodent models has stimulated investigation of Cnr1 involvement in PTSD and fear processes in humans. Studies suggest delta-9-tetrahydrocannabinoil (Δ9-THC) facilitates extinction of conditioned fear in healthy human volunteers (64, 165). As mentioned, PTSD diagnosis is significantly associated with greater marijuana use, indicating that Δ9-THC is used as a form of self-medication to compensate for cannabinoid system dysregulation (166). In fact, several genetic association studies reveal specific Cnr1 and FAAH (fatty acid amide hydrolase, an anandamide degradative enzyme) allelic risk factors for threat processing, anxiety, extinction, stress-coping, and PTSD (167–170). Furthermore, PET studies suggest that individuals with PTSD have increased brain Cnr1 availability, possibly due to changes in peripheral levels of the endocannabinoids (171–173). Although the data is preliminary, several studies show that cannabinoid receptor agonists, including nabilone and Δ9-THC, improve insomnia, subjective chronic pain, nightmares, and other symptoms related to PTSD (174–177). While these studies suggest that chronic administration of Cnr1 agonists can improve general mood and symptoms related to PTSD, more studies are needed to address the role of the cannabinoid system in memory processes, as they relate to PTSD and traumatic memory consolidation. As the cannabinoids have been implicated in primary consolidation, extinction, and reconsolidation across rodents and humans, it is of great interest to determine an effect, if any, of drugs targeting the cannabinoid system on PTSD development and treatment when administered during trauma consolidation, in combination with exposure therapy, and/or traumatic memory reactivation.
Norepinephrine/Propranolol/Yohimbine
Researchers and clinicians hypothesize that hyper-consolidation of trauma and/or poor extinction might contribute to development of PTSD. Given the vast amount of data implicating the noradrenergic/norepinephrine (NE) system in memory consolidation, some suggest that noradrenergic dysfunction might underlie pathology of PTSD, in particular, deficits in fear acquisition and extinction, as well as symptoms of hyperarousal (119, 178–180). Indeed, multiple studies find evidence of abnormal noradrenergic function in PTSD (181–185).
In rodents, stress-induced release of norepinephrine (NE) into the amygdala, specifically the BLA, is critical for emotional memory consolidation (186). Although numerous studies implicate NE, via β-adrenergic receptors, in consolidation of aversive memory (inhibitory avoidance learning, in particular), the role of NE in associative fear learning is less clear (179, 187). Studies find evidence of noradrenergic activity in consolidation of associative fear learning and extinction (188–194). Others, however, report that treatment with NE or propranolol, a β-adrenergic receptor antagonist, has no effect on consolidation of auditory fear learning. Furthermore, propranolol administration significantly impairs auditory fear acquisition (whereas, treatment with an α1-adrenergic receptor antagonist facilitates fear acquisition) (195–197).
Interestingly, noradrenergic signaling is critical for reconsolidation of fear learning across multiple paradigms (197–199). Reconsolidation involves transiently rendering memories labile through memory reactivation (200). Through this reactivation (and as in the primary consolidation phase), memories undergo a stabilization process that is sensitive to protein-synthesis inhibitors. Propranolol administered systemically or intra-amygdala blocks reconsolidation of cue and context fear conditioning (197, 198). Intra-LA infusion of isoproterenol, a β-adrenergic receptor agonist, enhances reconsolidation, blocking extinction of cued fear (201). Similarly, yohimbine, an α2-adrenoceptor antagonist that increases release of norepinephrine from the locus coeruleus, enhances reconsolidation, whereas clonidine, an α2-adrenoceptor agonist, blocks reconsolidation of conditioned fear (194, 202, 203).
Studies in healthy human subjects support a role for norepinephrine in memory consolidation, additionally. Propranolol attenuates responses to aversively conditioned stimuli and memory for emotionally arousing stories when administered during the consolidation window (204–206). Memory retrieval, however, is not impaired by propranolol (207, 208).
As the noradrenergic system is implicated in PTSD and, more generally, in memory consolidation processes, drugs that target the noradrenergic system are being tested for their efficacy in blocking primary consolidation or reconsolidation of traumatic memory, or alternatively, strengthening extinction of traumatic memory, in individuals with PTSD and other anxiety disorders. Studies show that propranolol administration in the immediate aftermath of trauma might be effective at secondary prevention of PTSD, as rates and symptoms of PTSD are lower over a period of weeks to months post-trauma in individuals who receive propranolol (209–211). However, a recent double-blind pilot study in children finds weak evidence for a decrease in PTSD symptoms in boys acutely administered propranolol, but an increase in symptoms in similarly-treated girls (212).
Increasingly, research is examining the effect of propranolol on reconsolidation to weaken the strength of emotional salience of traumatic memory. Propranolol administration with trauma reactivation decreases physiological responses, such as heart rate and skin conductance, during subsequent mental imagery of the event (213). In separate studies, propranolol administered in combination with six brief trauma reactivation sessions significantly improves PTSD symptoms compared to placebo (214). Several other studies report improvement of PTSD symptoms with propranolol treatment, however, dosage and administration is either unknown or not reported (215, 216). Notably, there are a number of negative trials across rodents and humans examining an effect of propranolol on reconsolidation (198, 208, 217). While a meta-analysis suggests that propranolol blocks primary consolidation and reconsolidation of long-term emotional memory in healthy humans, inconsistencies in the propranolol literature will benefit from a similar analysis examining individuals with PTSD (218).
New evidence indicates that modulation of the noradrenergic system may be able to facilitate exposure therapy, additionally. Individuals with Social Anxiety Disorder and claustrophobia exhibit better outcomes when administered yohimbine in conjunction with exposure sessions, compared to outcomes in individuals administered placebo plus exposure (219, 220). An additional study, however, finds no effect of yohimbine on extinction of fear of flying using virtual reality (221). Further study, specifically examining an effect on extinction in individuals with PTSD, is needed to more confidently assess therapeutic efficacy of yohimbine.
Device-based treatments
Increasingly, researchers are investigating device-based treatments to alter pathological brain activity and connectivity in psychiatric disease. A number of different stimulation tools - including deep-brain stimulation (DBS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation (TMS) - are under investigation and each are at various stages of development and testing at the pre-clinical and clinical level (222, 223). Similar to traditional pharmaceutical drugs, device-based treatments are being tested in combination with Pavlovian fear conditioning to determine efficacy in the treatment of PTSD.
Relative to other device-based treatments, DBS is the most extensively studied therapy with a comparatively large amount of evidence accumulated supporting efficacy in the treatment of psychiatric disorders. DBS is demonstrably efficacious for the treatment of Parkinson’s Disease, its original indication, and is now being investigated for the treatment of and depression, obsessive compulsive disorder (OCD), and PTSD (7, 222, 224–226). At the pre-clinical level, several studies find enhanced cued fear extinction with DBS of the ventral striatum that may be mediated by enhanced BDNF expression (53, 227, 228). Others find decreased PTSD-like symptoms and cued fear expression in rats with DBS of the amygdala (229–231). Based on these studies, participants are now being recruited to evaluate the efficacy of DBS targeting the amygdala for the treatment of PTSD (224). Because the mechanism of action is still relatively unclear (i.e. whether DBS activates or inhibits targeted brain regions), future pre-clinical studies are important for the interpretation, and thus refinement, of DBS and DBS treatment protocols.
Additionally, transcranial magnetic stimulation shows promise for the treatment of psychiatric disorders, where non-invasive electrical current is delivered via magnetic coil placed on the scalp (232). In combination with a brief trauma re-exposure with script driven imagery, and in combination with exposure therapy, TMS of mPFC ameliorates PTSD symptoms when administered repetitively over two weeks (233–235). These studies, along with evidence that transcranial direct current stimulation (tDCS) of dorsolateral prefrontal cortex modulates consolidation of cued fear, underline the importance of mPFC in fear learning, which has been extensively studied in rodents (6, 236, 237).
While the efficacy of device-based treatments for PTSD is still being evaluated in pre-clinical and clinical studies, largely in combination with classical Pavlovian fear extinction/exposure therapy, DBS, VNS, tDCS, and TMS may be viable treatment options, particularly for individuals with treatment-resistant PTSD.
Discussion
We have examined the evidence regarding efficacy of some specific treatment strategies for PTSD informed by rodent pre-clinical studies. We have focused on Pavlovian fear conditioning and extinction experiments in animals, which allow researchers to model aversive learning processes that may underlie development of PTSD in response to trauma, as well as extinction of pathological fear via exposure therapy. Even in prior stress models, which are thought to more thoroughly model PTSD, fear acquisition and extinction using fear conditioning is often assessed to determine the extent to which prior stress instantiates a PTSD-like phenotype. In this way, fear conditioning and extinction have initiated the discovery of promising therapies for the treatment of PTSD.
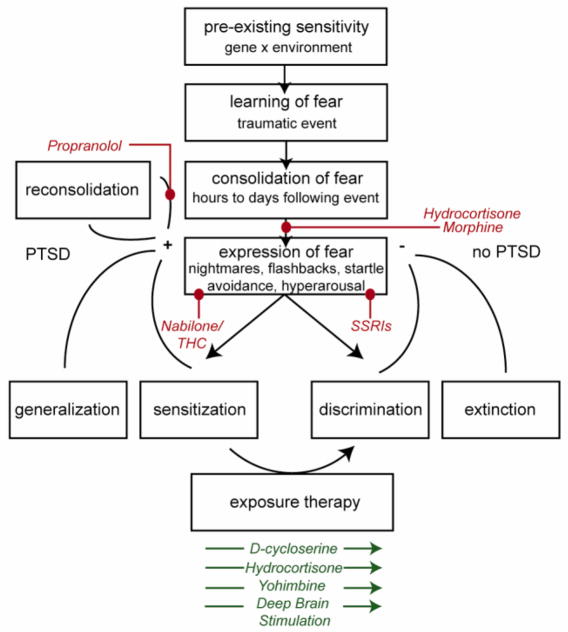
PTSD can be conceptualized as involving a number of transitional steps, from pre-existing vulnerability prior to trauma to expression of pathological fear after traumatic memory consolidation. Each of these steps can be targeted by various drugs or device-based therapies (Fig. 1). Individuals who experience trauma may be rendered more vulnerable to the development of PTSD by pre-existing sensitivities, including genetic makeup and prior environmental context (e.g. abuse during childhood). Fear memory is consolidated in the hours and days following traumatic experience, and those with PTSD are thought to over-consolidate traumatic fear memory. Morphine has been shown to block fear acquisition in animals and is now being evaluated for secondary prevention of PTSD (126, 127, 143, 144). Hydrocortisone has also been shown to ameliorate PTSD symptoms when administered after extreme stress; however a mechanism is still being determined (113). According to one hypothesis, hydrocortisone normalizes low cortisol, thought to be a risk factor for PTSD, in order to contain the stress response and maintain homeostasis (93). In line with evidence from pre-clinical models, hydrocortisone, D-cycloserine, yohimbine, and deep brain stimulation, in combination with exposure therapy, appear to enhance extinction (53, 69, 70, 115–118, 219, 220, 227, 228). Alternatively, traumatic fear memory may be rendered labile through brief reactivation via reconsolidation. Propranolol shows some promise in improving PTSD symptoms by blocking reconsolidation processes (218), although these studies require replication.
Figure 1. Overview of translationally informed treatments for PTSD and mechanism of action.
The development of PTSD can be organized into a framework of pre- and post-trauma risk factors and pathological learning, each of which can be uniquely targeted by therapeutics. Hydrocortisone and morphine have been shown to interrupt primary consolidation of conditioned fear and trauma across species. While evidence is inconsistent, propranolol has been suggested to block reconsolidation, a process which renders previously consolidated memories labile, and thus vulnerable to interference. Exposure therapy, the recommended first line treatment for PTSD, is facilitated by D-cycloserine, yohimbine, hydrocortisone, and deep brain stimulation. Furthermore, nabilone and THC (Cnr1 agonists) and SSRIs have been shown to reduce expression of fear with chronic administration. The color red indicates that a specific drug blocks the indicated process, while green indicates a facilitating effect.
In surveying the literature, we make several tentative conclusions about the current status and future of treatment strategies for PTSD. First, the time surrounding exposure therapy may be the best target window for administering treatment, for several reasons. However, not all people who experience trauma will develop PTSD, there is the question of whether resilient individuals should receive treatment. Clinical trials investigating the efficacy of secondary preventatives, such as opiates or hydrocortisone in the aftermath of trauma, may benefit from a better understanding of pre-trauma risk factors that predispose individuals to PTSD. At this stage, the use of secondary preventatives might be best for administration after trauma in known high risk populations.
Additionally, the window surrounding exposure therapy may be the best time for administering adjunctive therapies, as exposure therapy is the gold standard treatment for PTSD. Drug or device-based treatment in combination with exposure therapy may allow real-time assessment of its success. This is critical, as short exposure therapy sessions or sessions where individuals insufficiently inhibit fear can limit the effectiveness of adjunctive treatment, or, worse, strengthen traumatic fear memory (73). Thus a better approach may focus on therapies administered after individual exposure therapy sessions, allowing clinicians to assess the potential success of the therapy session before administering the adjunctive treatment.
Although it is clear that rodent models inform clinical studies, the traditional bench to bedside translational paradigm has shifted. Pharmacotherapies currently being tested in humans, stemming from rodent studies using Pavlovian fear conditioning paradigms, are being further developed in their original model systems to safely refine 1) dosages, 2) targeted molecular epitopes, and 3) treatment windows. In the case of SSRIs or DBS, for instance, rodent models are now being developed based on indications from human studies. In another shift away from the traditional pre-clinical to clinical translation model, more studies are now focusing on the effects of specific treatments on intermediate phenotypes - such as amygdala activation using fMRI or fear inhibition - rather than overall symptoms of PTSD. These types of studies will increase with the establishment of Research Domain Critieria (RDoc) by NIMH, a move that emphasizes the classification of psychiatric disorders based on behavioral dimensions and neurobiological measures.
While the number of approved treatments for PTSD is minimal, and research appears to be moving away from the traditional translational model, fear conditioning and extinction may still offer hope for the development of new therapies, as this model is among the best-validated in psychiatric research. Through these models, researchers and clinicians have established efficacious treatment strategies and are beginning to develop a number of promising pharmacotherapies and device-based treatments for PTSD.
Supplementary Material
Acknowledgments
Support was provided by NIH (T32-GM08605, 1F31MH097397, and R01MH096764), the Burroughs Wellcome Fund, and by an NIH/NCRR base grant (P51RR000165) to Yerkes National Primate Research Center.
Footnotes
Financial disclosures:
Dr. Ressler is a founding member of Extinction Pharmaceuticals/Therapade Technologies to develop D-cycloserine, a generically available compound, for use to augment the effectiveness of psychotherapy. He has received no equity or income from this relationship within the last 5 years. Dr. Bowers reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R. Risk and resilience in posttraumatic stress disorder. The Journal of clinical psychiatry. 2004;65(Suppl 1):29–36. [PubMed] [Google Scholar]
- 4.Davis M. The role of the amygdala in fear and anxiety. Annual review of neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux J. The amygdala. Current biology : CB. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DG, Felker BL, Liu CF, Yano EM, Kirchner JE, Chan D, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. Journal of general internal medicine. 2007;22:711–718. doi: 10.1007/s11606-006-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey BH. The neurobiology and pharmacology of depression. A comparative overview of serotonin selective antidepressants. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1997;87:540–550. 552. [PubMed] [Google Scholar]
- 10.El Mansari M, Sanchez C, Chouvet G, Renaud B, Haddjeri N. Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:1269–1277. doi: 10.1038/sj.npp.1300686. [DOI] [PubMed] [Google Scholar]
- 11.Blier P, Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. Journal of clinical psychopharmacology. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundamental & clinical pharmacology. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 13.Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang YY, Mann JJ, et al. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biological psychiatry. 2013;74:26–31. doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murrough JW, Huang Y, Hu J, Henry S, Williams W, Gallezot JD, et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biological psychiatry. 2011;70:1033–1038. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of general psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. The Journal of comparative neurology. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 18.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. The Journal of comparative neurology. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 19.McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. Journal of neurochemistry. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- 20.Gressier F, Calati R, Balestri M, Marsano A, Alberti S, Antypa N, et al. The 5-HTTLPR polymorphism and posttraumatic stress disorder: a meta-analysis. Journal of traumatic stress. 2013;26:645–653. doi: 10.1002/jts.21855. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan GM, Ogden RT, Huang YY, Oquendo MA, Mann JJ, Parsey RV. Higher in vivo serotonin-1a binding in posttraumatic stress disorder: a PET study with [11C]WAY-100635. Depression and anxiety. 2013;30:197–206. doi: 10.1002/da.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Archives of general psychiatry. 2011;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, et al. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. The American journal of psychiatry. 2005;162:383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- 24.Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- 25.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biological psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biological psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, Asgeirsdottir HN, Cohen SJ, Munchow AH, Barrera MP, Stackman RW., Jr Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology. 2013;64:403–413. doi: 10.1016/j.neuropharm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh SE, Romano AG, Harvey JA. Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacology. 1998;137:157–163. doi: 10.1007/s002130050605. [DOI] [PubMed] [Google Scholar]
- 29.Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biological psychiatry. 2013;73:1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology. 2011;215:231–237. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 31.Lebron-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behavioural brain research. 2013;253:217–222. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience. 2013;247:253–272. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 33.Haug TT, Blomhoff S, Hellstrom K, Holme I, Humble M, Madsbu HP, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. The British journal of psychiatry : the journal of mental science. 2003;182:312–318. doi: 10.1192/bjp.182.4.312. [DOI] [PubMed] [Google Scholar]
- 34.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 35.Marks IM, Swinson RP, Basoglu M, Kuch K, Noshirvani H, O’Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. The British journal of psychiatry : the journal of mental science. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 36.van Apeldoorn FJ, van Hout WJ, Mersch PP, Huisman M, Slaap BR, Hale WW, 3rd, et al. Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta psychiatrica Scandinavica. 2008;117:260–270. doi: 10.1111/j.1600-0447.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- 37.van Balkom AJ, de Haan E, van Oppen P, Spinhoven P, Hoogduin KA, van Dyck R. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive compulsive disorder. The Journal of nervous and mental disease. 1998;186:492–499. doi: 10.1097/00005053-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Foa EB, Franklin ME, Moser J. Context in the clinic: how well do cognitive-behavioral therapies and medications work in combination? Biological psychiatry. 2002;52:987–997. doi: 10.1016/s0006-3223(02)01552-4. [DOI] [PubMed] [Google Scholar]
- 39.Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. Journal of consulting and clinical psychology. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- 40.Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of general psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- 41.Difede J, Olden M, Cukor J. Evidence-based treatment of post-traumatic stress disorder. Annual review of medicine. 2014;65:319–332. doi: 10.1146/annurev-med-051812-145438. [DOI] [PubMed] [Google Scholar]
- 42.Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62:617–627. doi: 10.1016/j.neuropharm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2012;15:825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- 44.Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD) The Cochrane database of systematic reviews. 2010:CD007316. doi: 10.1002/14651858.CD007316.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biological psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiology of learning and memory. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral neuroscience. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 50.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behavioral neuroscience. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 51.Yamada D, Wada K, Sekiguchi M. Facilitating actions of an AMPA receptor potentiator upon extinction of contextually conditioned fear response in stressed mice. Neuroscience letters. 2011;488:242–246. doi: 10.1016/j.neulet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 53.Whittle N, Schmuckermair C, Gunduz Cinar O, Hauschild M, Ferraguti F, Holmes A, et al. Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model. Neuropharmacology. 2013;64:414–423. doi: 10.1016/j.neuropharm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biological psychiatry. 2010;68:1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Archives of general psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 56.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biological psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 58.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of general psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 59.Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, et al. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug and alcohol dependence. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuriyama K, Honma M, Soshi T, Fujii T, Kim Y. Effect of D-cycloserine and valproic acid on the extinction of reinstated fear-conditioned responses and habituation of fear conditioning in healthy humans: a randomized controlled trial. Psychopharmacology. 2011;218:589–597. doi: 10.1007/s00213-011-2353-x. [DOI] [PubMed] [Google Scholar]
- 61.Kalisch R, Holt B, Petrovic P, De Martino B, Kloppel S, Buchel C, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex. 2009;19:187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuriyama K, Honma M, Yoshiike T, Kim Y. Valproic acid but not D-cycloserine facilitates sleep-dependent offline learning of extinction and habituation of conditioned fear in humans. Neuropharmacology. 2013;64:424–431. doi: 10.1016/j.neuropharm.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 63.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behaviour research and therapy. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Delta9-THC and D-cycloserine on extinction of conditioned fear in humans. J Psychopharmacol. 2012;26:471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. The American journal of psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of psychiatric research. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Scheeringa MS, Weems CF. Randomized placebo-controlled D-cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. Journal of child and adolescent psychopharmacology. 2014;24:69–77. doi: 10.1089/cap.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigues H, Figueira I, Lopes A, Goncalves R, Mendlowicz MV, Coutinho ES, et al. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS one. 2014;9:e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biological psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 71.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 72.de Kleine RA, Hendriks GJ, Smits JA, Broekman TG, van Minnen A. Prescriptive variables for d-cycloserine augmentation of exposure therapy for posttraumatic stress disorder. Journal of psychiatric research. 2014;48:40–46. doi: 10.1016/j.jpsychires.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biological psychiatry. 2013;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bolkan SS, Lattal KM. Opposing effects of D-cycloserine on fear despite a common extinction duration: interactions between brain regions and behavior. Neurobiology of learning and memory. 2014;113:25–34. doi: 10.1016/j.nlm.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofmann SG. D-cycloserine for treating anxiety disorders: making good exposures better and bad exposures worse. Depression and anxiety. 2014;31:175–177. doi: 10.1002/da.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues in clinical neuroscience. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yehuda R. Post-traumatic stress disorder. The New England journal of medicine. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 79.Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, et al. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta psychiatrica Scandinavica. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- 80.Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biological psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 82.Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biological psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- 83.Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. The American journal of psychiatry. 2004;161:1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- 84.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 85.Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biological psychiatry. 2002;52:393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- 86.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. The American journal of psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 87.Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of nervous and mental disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 88.de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, Westenberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 89.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, et al. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. The American journal of psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- 90.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 91.Pineles SL, Rasmusson AM, Yehuda R, Lasko NB, Macklin ML, Pitman RK, et al. Predicting emotional responses to potentially traumatic events from pre-exposure waking cortisol levels: a longitudinal study of police and firefighters. Anxiety, stress, and coping. 2013;26:241–253. doi: 10.1080/10615806.2012.672976. [DOI] [PubMed] [Google Scholar]
- 92.Walsh K, Nugent NR, Kotte A, Amstadter AB, Wang S, Guille C, et al. Cortisol at the emergency room rape visit as a predictor of PTSD and depression symptoms over time. Psychoneuroendocrinology. 2013;38:2520–2528. doi: 10.1016/j.psyneuen.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, Pare D. Impact of predatory threat on fear extinction in Lewis rats. Learn Mem. 2010;17:494–501. doi: 10.1101/lm.1948910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biological psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- 97.Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem. 2012;19:43–49. doi: 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012;37:1531–1545. doi: 10.1016/j.psyneuen.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of psychiatric research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. The American journal of psychiatry. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- 102.Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biological psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 103.Lebow M, Neufeld-Cohen A, Kuperman Y, Tsoory M, Gil S, Chen A. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 105.Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, et al. Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology. 2012;63:1118–1126. doi: 10.1016/j.neuropharm.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daniels WM, de Klerk Uys J, van Vuuren P, Stein DJ. The development of behavioral and endocrine abnormalities in rats after repeated exposure to direct and indirect stress. Neuropsychiatric disease and treatment. 2008;4:451–464. doi: 10.2147/ndt.s2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. A distinct pattern of intracellular glucocorticoid-related responses is associated with extreme behavioral response to stress in an animal model of post-traumatic stress disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2009;19:759–771. doi: 10.1016/j.euroneuro.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Cohen H, Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Annals of the New York Academy of Sciences. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- 109.Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z. Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacol. 2008;11:331–349. doi: 10.1017/S1461145707007912. [DOI] [PubMed] [Google Scholar]
- 110.Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1401660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2286–2302. doi: 10.1038/npp.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 113.Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DDEQ, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Annals of the New York Academy of Sciences. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 115.Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2010;22:274–279. [PMC free article] [PubMed] [Google Scholar]
- 116.Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–597. doi: 10.1016/j.psyneuen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 117.de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, et al. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 120.Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 121.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. The American journal of psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 122.Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of Mental Health and Well-Being. The American journal of psychiatry. 2006;163:652–658. doi: 10.1176/ajp.2006.163.4.652. [DOI] [PubMed] [Google Scholar]
- 123.McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behavioral neuroscience. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez LL, Powell DA. Effects of anloxone on Pavlovian conditioning of eyeblink and heart rate responses in rabbits. Life sciences. 1980;27:863–869. doi: 10.1016/0024-3205(80)90081-8. [DOI] [PubMed] [Google Scholar]
- 125.Fanselow MS, Calcagnetti DJ, Helmstetter FJ. Peripheral versus intracerebroventricular administration of quaternary naltrexone and the enhancement of Pavlovian conditioning. Brain research. 1988;444:147–152. doi: 10.1016/0006-8993(88)90921-3. [DOI] [PubMed] [Google Scholar]
- 126.Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behavioral neuroscience. 1995;109:631–641. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- 127.Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. Morphine prevents the development of stress-enhanced fear learning. Pharmacology, biochemistry, and behavior. 2013;103:672–677. doi: 10.1016/j.pbb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 128.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Frontiers in behavioral neuroscience. 2010:4. doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fanselow MS, Kim JJ, Young SL, Calcagnetti DJ, DeCola JP, Helmstetter FJ, et al. Differential effects of selective opioid peptide antagonists on the acquisition of pavlovian fear conditioning. Peptides. 1991;12:1033–1037. doi: 10.1016/0196-9781(91)90056-u. [DOI] [PubMed] [Google Scholar]
- 131.McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behavioral neuroscience. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- 132.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. The Journal of pharmacology and experimental therapeutics. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 133.Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biological psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, Gandhi PR, Standifer KM. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Molecular pain. 2012;8:76. doi: 10.1186/1744-8069-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Science translational medicine. 2013;5:188ra173. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiology of learning and memory. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fornari RV, Soares JC, Ferreira TL, Moreira KM, Oliveira MG. Effects of nociceptin/orphanin FQ in the acquisition of contextual and tone fear conditioning in rats. Behavioral neuroscience. 2008;122:98–106. doi: 10.1037/0735-7044.122.1.98. [DOI] [PubMed] [Google Scholar]
- 138.Nugent NR, Lally MA, Brown L, Knopik VS, McGeary JE. OPRM1 and diagnosis-related posttraumatic stress disorder in binge-drinking patients living with HIV. AIDS and behavior. 2012;16:2171–2180. doi: 10.1007/s10461-011-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pietrzak RH, Naganawa M, Huang Y, Corsi-Travali S, Zheng MQ, Stein MB, et al. Association of in vivo kappa-opioid receptor availability and the transdiagnostic dimensional expression of trauma-related psychopathology. JAMA Psychiatry. 2014;71:1262–1270. doi: 10.1001/jamapsychiatry.2014.1221. [DOI] [PubMed] [Google Scholar]
- 140.Saxe G, Stoddard F, Courtney D, Cunningham K, Chawla N, Sheridan R, et al. Relationship between acute morphine and the course of PTSD in children with burns. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:915–921. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 141.Sheridan RL, Stoddard FJ, Kazis LE, Lee A, Li NC, Kagan RJ, et al. Long-term posttraumatic stress symptoms vary inversely with early opiate dosing in children recovering from serious burns: effects durable at 4 years. The journal of trauma and acute care surgery. 2014;76:828–832. doi: 10.1097/TA.0b013e3182ab111c. [DOI] [PubMed] [Google Scholar]
- 142.Stoddard FJ, Jr, Sorrentino EA, Ceranoglu TA, Saxe G, Murphy JM, Drake JE, et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. Journal of burn care & research : official publication of the American Burn Association. 2009;30:836–843. doi: 10.1097/BCR.0b013e3181b48102. [DOI] [PubMed] [Google Scholar]
- 143.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. The New England journal of medicine. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 144.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biological psychiatry. 2009;65:438–440. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 145.Eippert F, Bingel U, Schoell E, Yacubian J, Buchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5465–5472. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, et al. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38:166–170. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 147.Norman SB, Stein MB, Dimsdale JE, Hoyt DB. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychological medicine. 2008;38:533–542. doi: 10.1017/S0033291707001389. [DOI] [PubMed] [Google Scholar]
- 148.Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. Journal of traumatic stress. 2007;20:401–412. doi: 10.1002/jts.20256. [DOI] [PubMed] [Google Scholar]
- 149.Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS spectrums. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruehle S, Rey AA, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol. 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 152.Moise AM, Eisenstein SA, Astarita G, Piomelli D, Hohmann AG. An endocannabinoid signaling system modulates anxiety-like behavior in male Syrian hamsters. Psychopharmacology. 2008;200:333–346. doi: 10.1007/s00213-008-1209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bowers ME, Ressler KJ. Interaction between the Cholecystokinin and Endogenous Cannabinoid Systems in Cued Fear Expression and Extinction Retention. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, et al. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- 155.Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–777. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ratano P, Everitt BJ, Milton AL. The CB1 Receptor Antagonist AM251 Impairs Reconsolidation of Pavlovian Fear Memory in the Rat Basolateral Amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2529–2537. doi: 10.1038/npp.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kuhnert S, Meyer C, Koch M. Involvement of cannabinoid receptors in the amygdala and prefrontal cortex of rats in fear learning, consolidation, retrieval and extinction. Behavioural brain research. 2013;250:274–284. doi: 10.1016/j.bbr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Laricchiuta D, Centonze D, Petrosini L. Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behavioural brain research. 2013;256:101–107. doi: 10.1016/j.bbr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 159.Ganon-Elazar E, Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:456–466. doi: 10.1038/npp.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campos AC, Ferreira FR, Guimaraes FS. Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. Journal of psychiatric research. 2012;46:1501–1510. doi: 10.1016/j.jpsychires.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 161.Reich CG, Iskander AN, Weiss MS. Cannabinoid modulation of chronic mild stress-induced selective enhancement of trace fear conditioning in adolescent rats. J Psychopharmacol. 2013;27:947–955. doi: 10.1177/0269881113499207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Puente N, Elezgarai I, Lafourcade M, Reguero L, Marsicano G, Georges F, et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PloS one. 2010;5:e8869. doi: 10.1371/journal.pone.0008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Korem N, Akirav I. Cannabinoids Prevent the Effects of a Footshock Followed by Situational Reminders on Emotional Processing. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–1125. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Calhoun PS, Sampson WS, Bosworth HB, Feldman ME, Kirby AC, Hertzberg MA, et al. Drug use and validity of substance use self-reports in veterans seeking help for posttraumatic stress disorder. Journal of consulting and clinical psychology. 2000;68:923–927. [PubMed] [Google Scholar]
- 167.Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature communications. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pardini M, Krueger F, Koenigs M, Raymont V, Hodgkinson C, Zoubak S, et al. Fatty-acid amide hydrolase polymorphisms and post-traumatic stress disorder after penetrating brain injury. Translational psychiatry. 2012;2:e75. doi: 10.1038/tp.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lu AT, Ogdie MN, Jarvelin MR, Moilanen IK, Loo SK, McCracken JT, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2008;147B:1488–1494. doi: 10.1002/ajmg.b.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bailey CR, Cordell E, Sobin SM, Neumeister A. Recent progress in understanding the pathophysiology of post-traumatic stress disorder: implications for targeted pharmacological treatment. CNS drugs. 2013;27:221–232. doi: 10.1007/s40263-013-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS neuroscience & therapeutics. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. Journal of clinical psychopharmacology. 2014;34:559–564. doi: 10.1097/JCP.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clinical drug investigation. 2014;34:587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 177.Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug testing and analysis. 2012;4:649–659. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- 178.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annual review of neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 179.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual review of neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 180.O’Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- 181.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Archives of general psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 182.Perry BD, Giller EL, Jr, Southwick SM. Altered platelet alpha 2-adrenergic binding sites in posttraumatic stress disorder. The American journal of psychiatry. 1987;144:1511–1512. doi: 10.1176/ajp.144.11.1511a. [DOI] [PubMed] [Google Scholar]
- 183.Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of general psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 184.Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. The Journal of nervous and mental disease. 1991;179:371–373. doi: 10.1097/00005053-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 185.Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. The Journal of nervous and mental disease. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 186.Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of learning and memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 187.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiology of learning and memory. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 189.Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiology of learning and memory. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 190.LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gazarini L, Stern CA, Carobrez AP, Bertoglio LJ. Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting alpha1- and beta-adrenergic receptors. Learn Mem. 2013;20:210–219. doi: 10.1101/lm.030007.112. [DOI] [PubMed] [Google Scholar]
- 195.Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Frontiers in behavioral neuroscience. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 2010;17:489–493. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 198.Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 201.Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats--a possible mechanism for the persistence of traumatic memories in PTSD. Depression and anxiety. 2011;28:186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gamache K, Pitman RK, Nader K. Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2789–2796. doi: 10.1038/npp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Progress in neurobiology. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 204.Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology. 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- 205.Orr SP, Milad MR, Metzger LJ, Lasko NB, Gilbertson MW, Pitman RK. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biological psychology. 2006;73:262–271. doi: 10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 206.Reist C, Duffy JG, Fujimoto K, Cahill L. beta-Adrenergic blockade and emotional memory in PTSD. Int J Neuropsychopharmacol. 2001;4:377–383. doi: 10.1017/S1461145701002607. [DOI] [PubMed] [Google Scholar]
- 207.Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Psychophysiological responding to emotional memories in healthy young men after cortisol and propranolol administration. Psychopharmacology. 2009;203:793–803. doi: 10.1007/s00213-008-1427-x. [DOI] [PubMed] [Google Scholar]
- 208.Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiology of learning and memory. 2009;91:23–31. doi: 10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 209.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 210.Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biological psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 211.Taylor F, Cahill L. Propranolol for reemergent posttraumatic stress disorder following an event of retraumatization: a case study. Journal of traumatic stress. 2002;15:433–437. doi: 10.1023/A:1020145610914. [DOI] [PubMed] [Google Scholar]
- 212.Nugent NR, Christopher NC, Crow JP, Browne L, Ostrowski S, Delahanty DL. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. Journal of traumatic stress. 2010;23:282–287. doi: 10.1002/jts.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of psychiatric research. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 214.Brunet A, Poundja J, Tremblay J, Bui E, Thomas E, Orr SP, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. Journal of clinical psychopharmacology. 2011;31:547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 215.McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH. The effect of propranolol on posttraumatic stress disorder in burned service members. Journal of burn care & research : official publication of the American Burn Association. 2009;30:92–97. doi: 10.1097/BCR.0b013e3181921f51. [DOI] [PubMed] [Google Scholar]
- 216.Famularo R, Kinscherff R, Fenton T. Propranolol treatment for childhood posttraumatic stress disorder, acute type. A pilot study. Am J Dis Child. 1988;142:1244–1247. doi: 10.1001/archpedi.1988.02150110122036. [DOI] [PubMed] [Google Scholar]
- 217.Spring JD, Wood NE, Mueller-Pfeiffer C, Milad MR, Pitman RK, Orr SP. Prereactivation propranolol fails to reduce skin conductance reactivity to prepared fear-conditioned stimuli. Psychophysiology. 2014 doi: 10.1111/psyp.12326. [DOI] [PubMed] [Google Scholar]
- 218.Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. Journal of psychiatry & neuroscience : JPN. 2013;38:222–231. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biological psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 220.Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. Journal of anxiety disorders. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 221.Meyerbroeker K, Powers MB, van Stegeren A, Emmelkamp PM. Does yohimbine hydrochloride facilitate fear extinction in virtual reality treatment of fear of flying? A randomized placebo-controlled trial. Psychotherapy and psychosomatics. 2012;81:29–37. doi: 10.1159/000329454. [DOI] [PubMed] [Google Scholar]
- 222.Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depression and anxiety. 2014;31:269–278. doi: 10.1002/da.22252. [DOI] [PubMed] [Google Scholar]
- 223.Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Frontiers in behavioral neuroscience. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koek RJ, Langevin JP, Krahl SE, Kosoyan HJ, Schwartz HN, Chen JW, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD) study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356. doi: 10.1186/1745-6215-15-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yu H, Neimat JS. The treatment of movement disorders by deep brain stimulation. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2008;5:26–36. doi: 10.1016/j.nurt.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mian MK, Campos M, Sheth SA, Eskandar EN. Deep brain stimulation for obsessive-compulsive disorder: past, present, and future. Neurosurgical focus. 2010;29:E10. doi: 10.3171/2010.4.FOCUS10107. [DOI] [PubMed] [Google Scholar]
- 227.Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Frontiers in behavioral neuroscience. 2013;7:102. doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sui L, Huang S, Peng B, Ren J, Tian F, Wang Y. Deep brain stimulation of the amygdala alleviates fear conditioning-induced alterations in synaptic plasticity in the cortical-amygdala pathway and fear memory. J Neural Transm. 2014;121:773–782. doi: 10.1007/s00702-014-1183-5. [DOI] [PubMed] [Google Scholar]
- 230.Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. Journal of psychiatric research. 2010;44:1241–1245. doi: 10.1016/j.jpsychires.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 231.Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain stimulation. 2013;6:837–844. doi: 10.1016/j.brs.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 232.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanha C, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. The Journal of clinical psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. Journal of anxiety disorders. 2009;23:54–59. doi: 10.1016/j.janxdis.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder--a pilot study. Brain stimulation. 2013;6:377–383. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 236.Asthana M, Nueckel K, Muhlberger A, Neueder D, Polak T, Domschke K, et al. Effects of transcranial direct current stimulation on consolidation of fear memory. Frontiers in psychiatry. 2013;4:107. doi: 10.3389/fpsyt.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.