Abstract
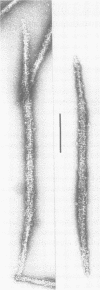
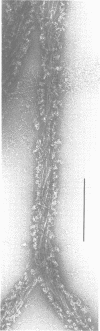
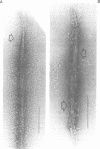
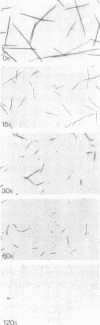
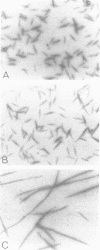
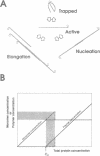
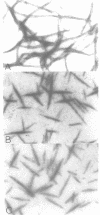
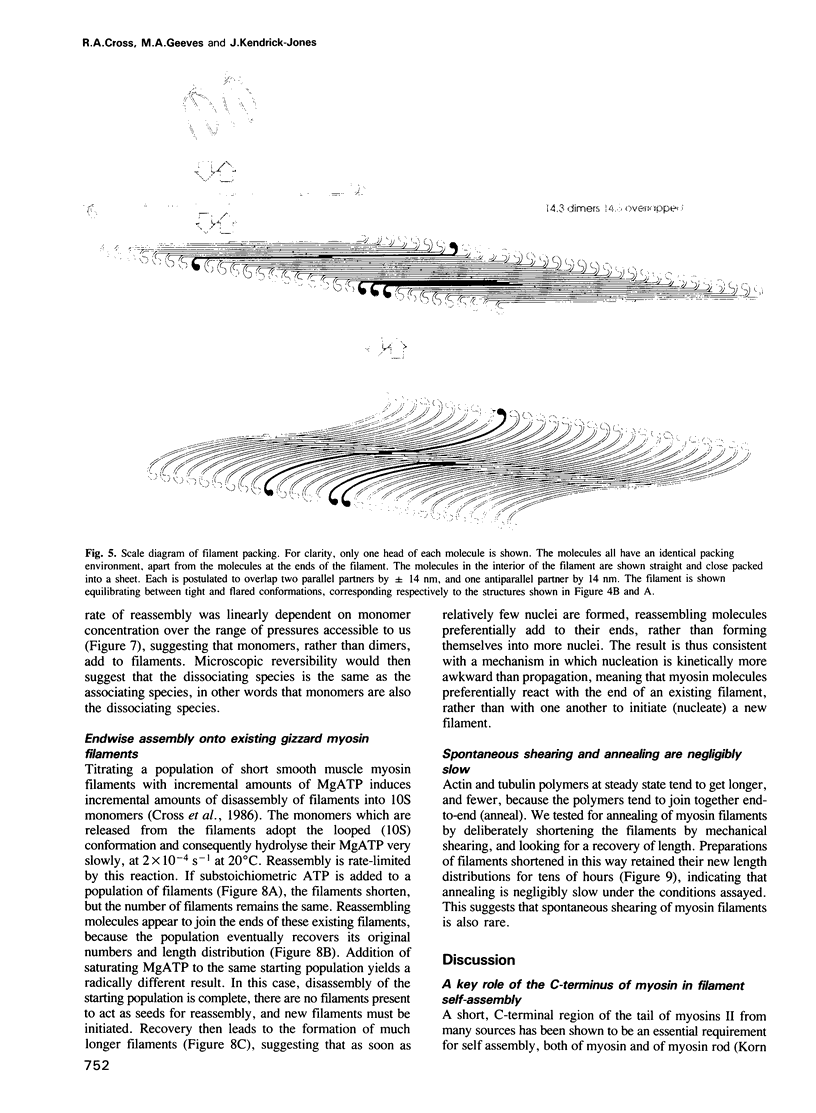
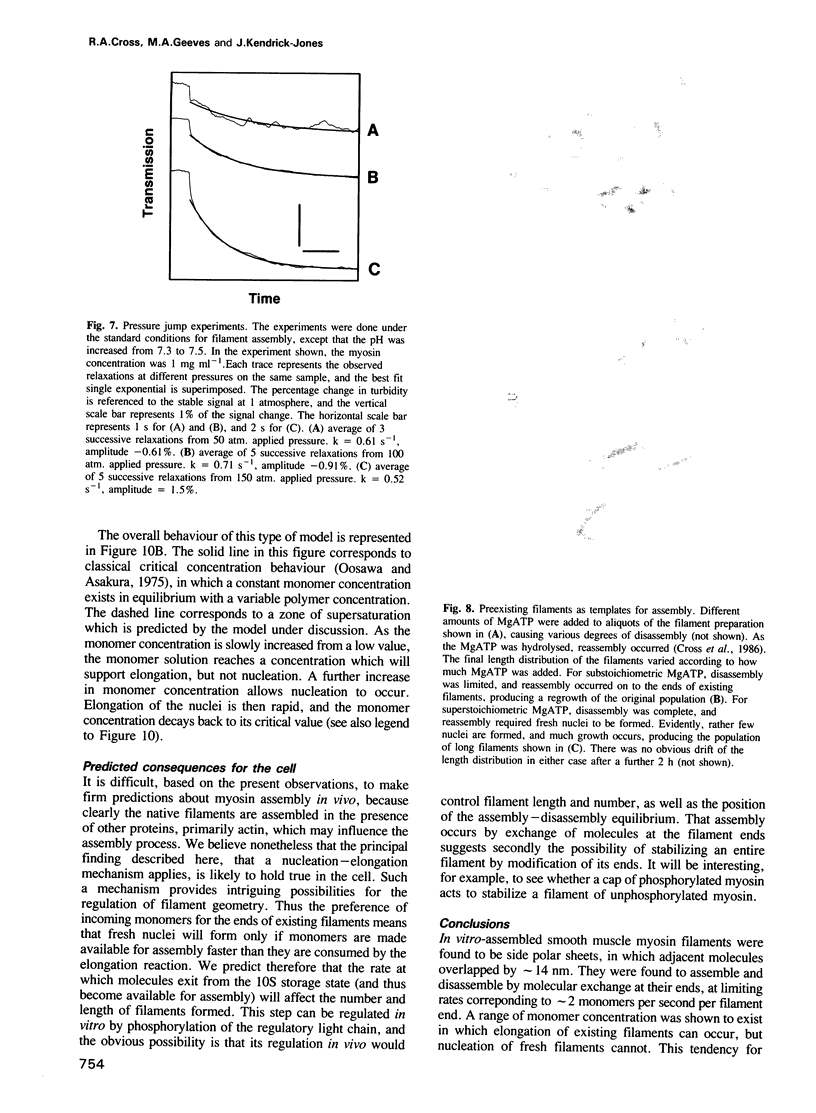
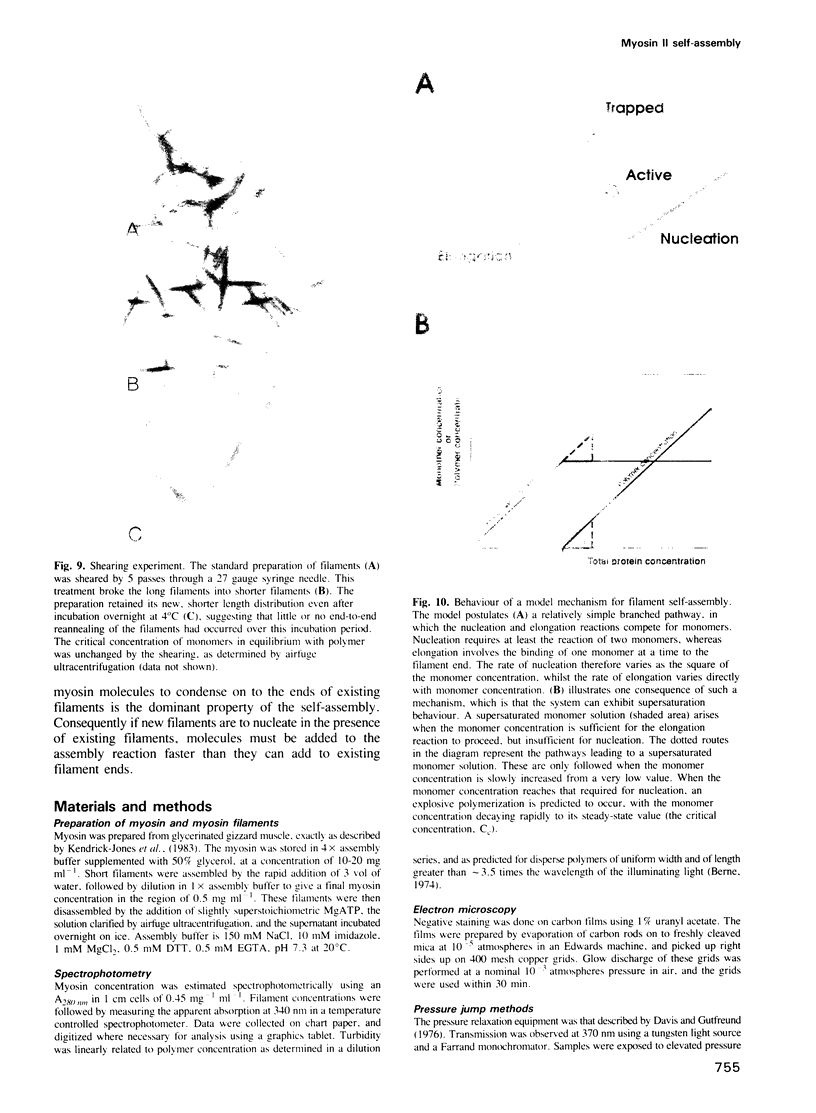
Self-assembled filaments of smooth muscle myosin were observed by low dose electron microscopy to be flat side-polar sheets, in which the component molecules appeared straight and close-packed. Fraying experiments released small oligomers, in which molecules were staggered in parallel by about +/- 14 nm relative to two immediate neighbours, and were bound also to an antiparallel partner via a approximately 14 nm overlap at the very tip of the tail. We suggest a filament model which preserves these packing relationships. Adding stoichiometric amounts of MgATP to the filaments caused them to disassemble completely by progressive loss of material from their ends, at a limiting rate equivalent to about 2 monomers per second per end in physiological saline. The rate of the competing association reaction varied linearly with the monomer concentration, as determined in pressure-jump experiments. This suggests that myosin monomers, rather than dimers or higher oligomers, are the building blocks of these filaments. Shearing and annealing of assembled filaments appeared negligible on a time scale of a few hours. In consequence, filament number and filament length were dependent on the rate at which monomers were supplied to the assembly reaction, and on the number of filaments already present at the start of the assembly reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Castellani L., Elliott B. W., Jr, Cohen C. Phosphorylatable serine residues are located in a non-helical tailpiece of a catch muscle myosin. J Muscle Res Cell Motil. 1988 Dec;9(6):533–540. doi: 10.1007/BF01738758. [DOI] [PubMed] [Google Scholar]
- Citi S., Cross R. A., Bagshaw C. R., Kendrick-Jones J. Parallel modulation of brush border myosin conformation and enzyme activity induced by monoclonal antibodies. J Cell Biol. 1989 Aug;109(2):549–556. doi: 10.1083/jcb.109.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S., Kendrick-Jones J. Regulation in vitro of brush border myosin by light chain phosphorylation. J Mol Biol. 1986 Apr 5;188(3):369–382. doi: 10.1016/0022-2836(86)90161-0. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S., Craig R. Myosin filaments isolated from skinned amphibian smooth muscle cells are side-polar. J Muscle Res Cell Motil. 1989 Jun;10(3):206–220. doi: 10.1007/BF01739811. [DOI] [PubMed] [Google Scholar]
- Craig R., Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977 Dec;75(3):990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. A., Citi S., Kendrick-Jones J. How phosphorylation controls the self-assembly of vertebrate smooth and non-muscle myosins. Biochem Soc Trans. 1988 Aug;16(4):501–503. doi: 10.1042/bst0160501. [DOI] [PubMed] [Google Scholar]
- Cross R. A., Cross K. E., Sobieszek A. ATP-linked monomer-polymer equilibrium of smooth muscle myosin: the free folded monomer traps ADP.Pi. EMBO J. 1986 Oct;5(10):2637–2641. doi: 10.1002/j.1460-2075.1986.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. A., Vandekerckhove J. Solubility-determining domain of smooth muscle myosin rod. FEBS Lett. 1986 May 12;200(2):355–360. doi: 10.1016/0014-5793(86)81168-1. [DOI] [PubMed] [Google Scholar]
- Cross R. A. What is 10S myosin for? J Muscle Res Cell Motil. 1988 Feb;9(1):108–110. doi: 10.1007/BF01682153. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Assembly processes in vertebrate skeletal thick filament formation. Annu Rev Biophys Biophys Chem. 1988;17:217–239. doi: 10.1146/annurev.bb.17.060188.001245. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Buck J., Greene E. P. The myosin dimer: an intermediate in the self-assembly of the thick filament of vertebrate skeletal muscle. FEBS Lett. 1982 Apr 19;140(2):293–297. doi: 10.1016/0014-5793(82)80917-4. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Gutfreund H. The scope of moderate pressure changes for kinetic and equilibrium studies of biochemical systems. FEBS Lett. 1976 Dec 31;72(2):199–207. doi: 10.1016/0014-5793(76)80971-4. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Kinetics and thermodynamics of the assembly of the parallel- and antiparallel-packed sections of synthetic thick filaments of skeletal myosin: a pressure-jump study. Biochemistry. 1985 Sep 10;24(19):5263–5269. doi: 10.1021/bi00340a046. [DOI] [PubMed] [Google Scholar]
- Davis J. S. The influence of pressure on the self-assembly of the thick filament from the myosin of vertebrate skeletal muscle. Biochem J. 1981 Aug 1;197(2):301–308. doi: 10.1042/bj1970301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinssen H., D'Haese J., Small J. V., Sobieszek A. Mode of filament assembly of myosins from muscle and nonmuscle cells. J Ultrastruct Res. 1978 Sep;64(3):282–302. doi: 10.1016/s0022-5320(78)90037-0. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. On the stability of myosin filaments. Biochemistry. 1968 Aug;7(8):2834–2847. doi: 10.1021/bi00848a020. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Cande W. Z., Tooth P. J., Smith R. C., Scholey J. M. Studies on the effect of phosphorylation of the 20,000 Mr light chain of vertebrate smooth muscle myosin. J Mol Biol. 1983 Mar 25;165(1):139–162. doi: 10.1016/s0022-2836(83)80247-2. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Smith R. C., Craig R., Citi S. Polymerization of vertebrate non-muscle and smooth muscle myosins. J Mol Biol. 1987 Nov 20;198(2):241–252. doi: 10.1016/0022-2836(87)90310-x. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szent-Gyorgyi A. S., Cohen C. Segments from vertebrate smooth muscle myosin rods. J Mol Biol. 1971 Aug 14;59(3):527–529. doi: 10.1016/0022-2836(71)90316-0. [DOI] [PubMed] [Google Scholar]
- Knight P., Trinick J. Structure of the myosin projections on native thick filaments from vertebrate skeletal muscle. J Mol Biol. 1984 Aug 15;177(3):461–482. doi: 10.1016/0022-2836(84)90295-x. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- Megerman J., Lowey S. Polymerization of myosin from smooth muscle of the calf aorta. Biochemistry. 1981 Apr 14;20(8):2099–2110. doi: 10.1021/bi00511a006. [DOI] [PubMed] [Google Scholar]
- Niederman R., Peters L. K. Native bare zone assemblage nucleates myosin filament assembly. J Mol Biol. 1982 Nov 15;161(4):505–517. doi: 10.1016/0022-2836(82)90404-1. [DOI] [PubMed] [Google Scholar]
- Onishi H., Wakabayashi T. Electron microscopic studies on myosin molecules from chicken gizzard muscle III. Myosin dimers. J Biochem. 1984 Mar;95(3):903–905. doi: 10.1093/oxfordjournals.jbchem.a134686. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Flicker P. F., Ravid S., Spudich J. A. Intermolecular versus intramolecular interactions of Dictyostelium myosin: possible regulation by heavy chain phosphorylation. J Cell Biol. 1989 Jul;109(1):203–210. doi: 10.1083/jcb.109.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Structure and polymerization of Acanthamoeba myosin-II filaments. J Cell Biol. 1982 Dec;95(3):816–825. doi: 10.1083/jcb.95.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisler E., Smith C., Seegan G. Myosin minifilaments. J Mol Biol. 1980 Oct 15;143(1):129–145. doi: 10.1016/0022-2836(80)90127-8. [DOI] [PubMed] [Google Scholar]
- Sinard J. H., Stafford W. F., Pollard T. D. The mechanism of assembly of Acanthamoeba myosin-II minifilaments: minifilaments assemble by three successive dimerization steps. J Cell Biol. 1989 Oct;109(4 Pt 1):1537–1547. doi: 10.1083/jcb.109.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Myosin filaments on the move. Nature. 1988 Feb 18;331(6157):568–569. doi: 10.1038/331568a0. [DOI] [PubMed] [Google Scholar]
- Sobieszek A. Cross-bridges on self-assembled smooth muscle myosin filaments. J Mol Biol. 1972 Oct 14;70(3):741–744. doi: 10.1016/0022-2836(72)90573-6. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. In pursuit of myosin function. Cell Regul. 1989 Nov;1(1):1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M., Lowey S. Conformational states of smooth muscle myosin. Effects of light chain phosphorylation and ionic strength. J Biol Chem. 1984 Jul 10;259(13):8564–8571. [PubMed] [Google Scholar]
- Trybus K. M., Lowey S. Subunit exchange between smooth muscle myosin filaments. J Cell Biol. 1987 Dec;105(6 Pt 2):3021–3030. doi: 10.1083/jcb.105.6.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura S., Fukui Y. Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature. 1985 Mar 14;314(6007):194–196. doi: 10.1038/314194a0. [DOI] [PubMed] [Google Scholar]