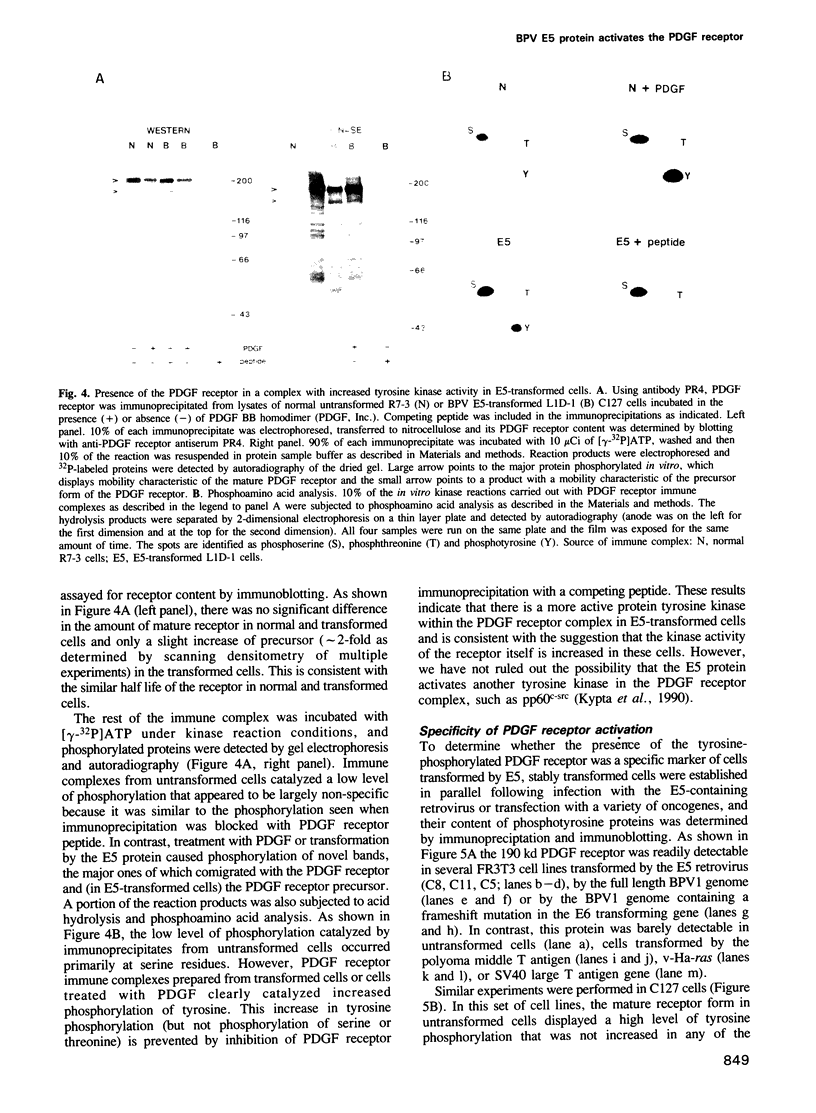
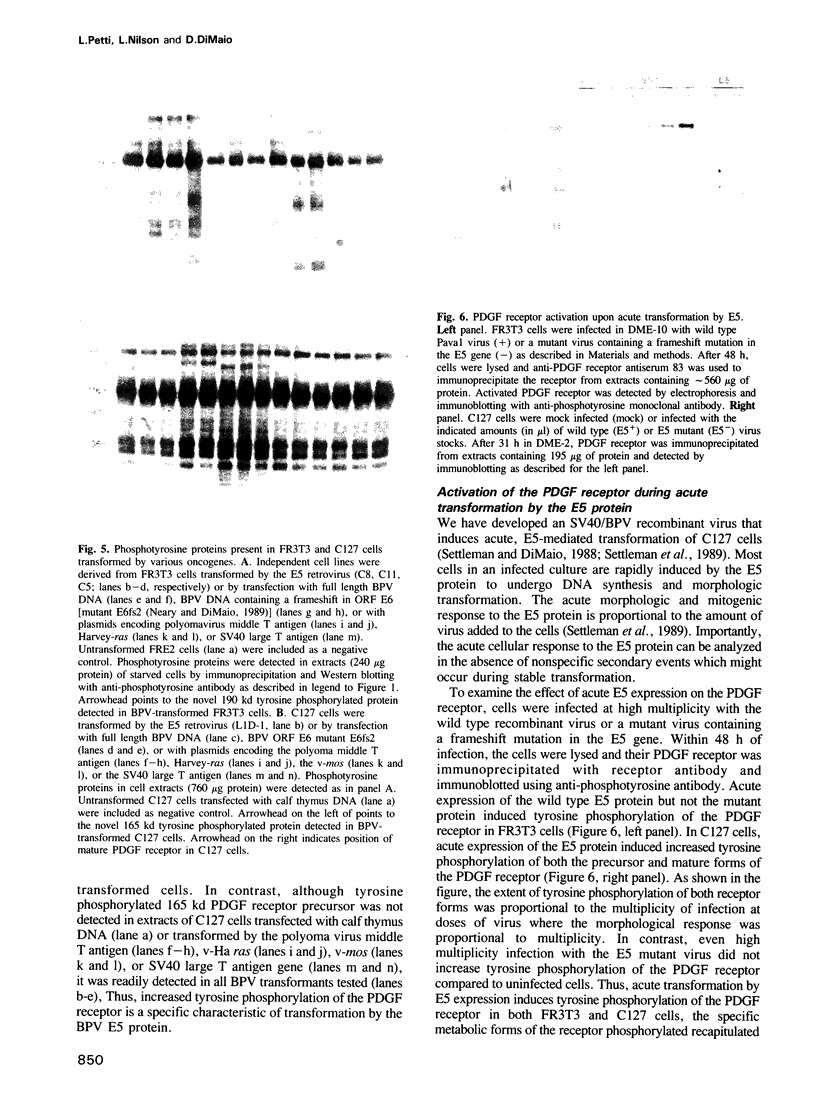
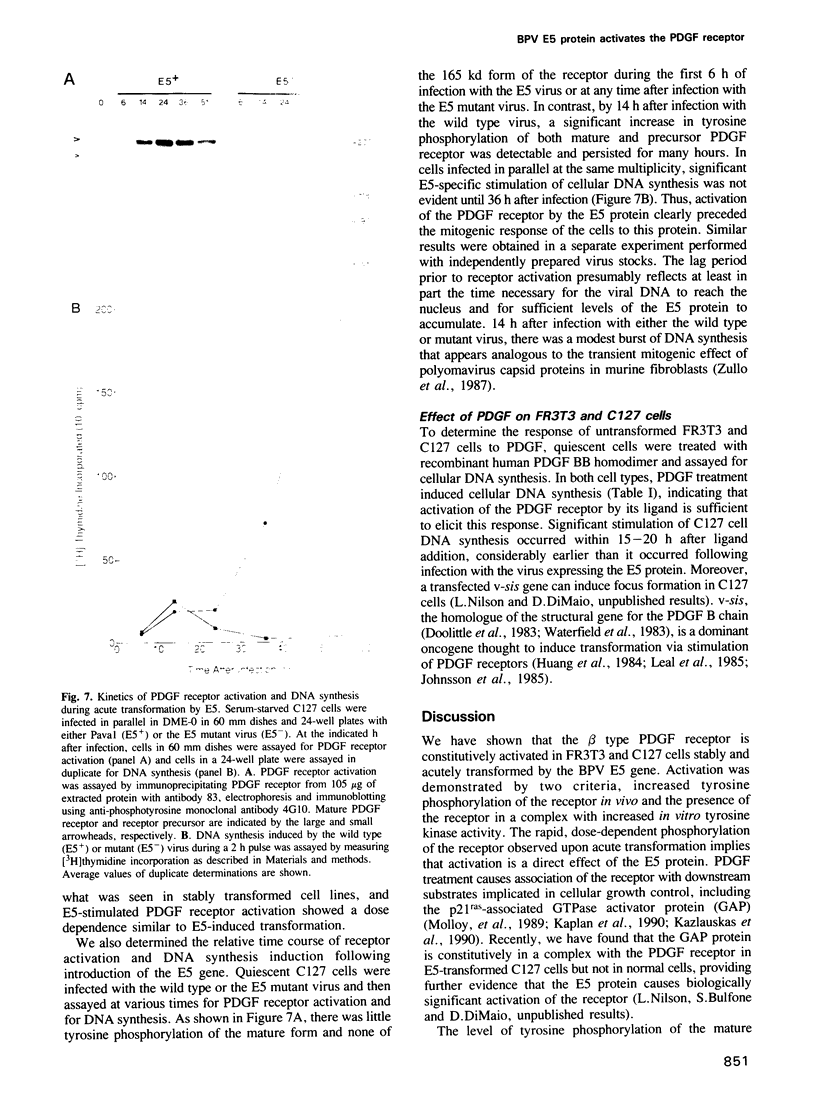
Abstract
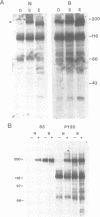
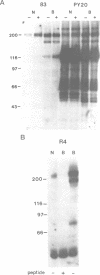
The bovine papillomavirus E5 gene encodes a 44 amino acid membrane-associated protein that can induce tumorigenic transformation of rodent fibroblast cell lines. Genetic studies suggest that the E5 protein may transform cells by influencing the activity of cellular proteins involved in growth regulation. We report here that the endogenous cellular beta type receptor for the platelet-derived growth factor (PDGF) is constitutively activated in C127 and FR3T3 cells stably transformed by the E5 protein, but not in these cell types transformed by a variety of other oncogenes. In C127 cells, a metabolic precursor as well as the mature form of the receptor is activated by E5 transformation. Activation of the receptor also occurs upon acute E5-mediated transformation of these cells and precedes mitogenic stimulation in this system. Moreover, activation of the receptor by addition of PDGF or the v-sis gene to untransformed cells is sufficient to induce DNA synthesis and stable growth transformation. We propose that the PDGF receptor is an important cellular intermediate in the transforming activity of the bovine papillomavirus E5 protein. There is a short region of sequence similarity between the fibropapillomavirus E5 proteins and PDGF, suggesting that the E5 proteins may activate the PDGF receptor by binding directly to it.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejcek B. E., Li D. Y., Deuel T. F. Transformation by v-sis occurs by an internal autoactivation mechanism. Science. 1989 Sep 29;245(4925):1496–1499. doi: 10.1126/science.2551043. [DOI] [PubMed] [Google Scholar]
- Bergman P., Ustav M., Sedman J., Moreno-Lopéz J., Vennström B., Pettersson U. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene. 1988 May;2(5):453–459. [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Burkhardt A., Willingham M., Gay C., Jeang K. T., Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989 May;170(1):334–339. doi: 10.1016/0042-6822(89)90391-7. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Decker S. J. Effects of epidermal growth factor and 12-O-tetradecanoylphorbol-13-acetate on metabolism of the epidermal growth factor receptor in normal human fibroblasts. Mol Cell Biol. 1984 Sep;4(9):1718–1724. doi: 10.1128/mcb.4.9.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D. Transforming activity of bovine and human papillomaviruses in cultured cells. Adv Cancer Res. 1991;56:133–159. doi: 10.1016/s0065-230x(08)60480-7. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Molloy C. J., Robbins K. C., Aaronson S. A. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese N. A., Robbins K. C., Aaronson S. A. The role of individual cysteine residues in the structure and function of the v-sis gene product. Science. 1987 Jun 5;236(4806):1315–1318. doi: 10.1126/science.3035718. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Schlegel R. The E5 oncoprotein of bovine papillomavirus binds to a 16 kd cellular protein. EMBO J. 1990 Jan;9(1):137–145. doi: 10.1002/j.1460-2075.1990.tb08089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M. Demonstration that a chemically synthesized BPV1 oncoprotein and its C-terminal domain function to induce cellular DNA synthesis. Cell. 1987 Dec 4;51(5):795–802. doi: 10.1016/0092-8674(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Molecular cloning and nucleotide sequence of deer papillomavirus. J Virol. 1985 Oct;56(1):85–91. doi: 10.1128/jvi.56.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Autocrine stimulation by the v-sis gene product requires a ligand-receptor interaction at the cell surface. J Cell Biol. 1988 Jul;107(1):287–298. doi: 10.1083/jcb.107.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Weinstat D. L., DiMaio D. Transforming activity of a 16-amino-acid segment of the bovine papillomavirus E5 protein linked to random sequences of hydrophobic amino acids. J Virol. 1989 Nov;63(11):4515–4519. doi: 10.1128/jvi.63.11.4515-4519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Kaczmarek L., DiMaio D. Stimulation of cellular DNA synthesis by wild type and mutant bovine papillomavirus DNA. Biochem Biophys Res Commun. 1987 Oct 14;148(1):86–91. doi: 10.1016/0006-291x(87)91079-5. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Escobedo J. A., Williams L. T. Ligand activation causes a phosphorylation-dependent change in platelet-derived growth factor receptor conformation. J Biol Chem. 1988 Sep 15;263(26):12805–12808. [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Processing of the platelet-derived growth factor receptor. Biosynthetic and degradation studies using anti-receptor antibodies. J Biol Chem. 1987 Jun 5;262(16):7932–7937. [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Leal F., Williams L. T., Robbins K. C., Aaronson S. A. Evidence that the v-sis gene product transforms by interaction with the receptor for platelet-derived growth factor. Science. 1985 Oct 18;230(4723):327–330. doi: 10.1126/science.2996133. [DOI] [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Mercola M., Melton D. A., Stiles C. D. Platelet-derived growth factor A chain is maternally encoded in Xenopus embryos. Science. 1988 Sep 2;241(4870):1223–1225. doi: 10.1126/science.3413486. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Neary K., DiMaio D. Open reading frames E6 and E7 of bovine papillomavirus type 1 are both required for full transformation of mouse C127 cells. J Virol. 1989 Jan;63(1):259–266. doi: 10.1128/jvi.63.1.259-266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. A., Loewenstein P. M., Green M. Mutational analysis of bovine papillomavirus type 1 E5 peptide domains involved in induction of cellular DNA synthesis. J Virol. 1989 Nov;63(11):4962–4964. doi: 10.1128/jvi.63.11.4962-4964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M. K., Donoghue D. J. Identification of nonessential disulfide bonds and altered conformations in the v-sis protein, a homolog of the B chain of platelet-derived growth factor. Mol Cell Biol. 1988 Mar;8(3):1011–1018. doi: 10.1128/mcb.8.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J., DiMaio D. Efficient transactivation and morphologic transformation by bovine papillomavirus genes expressed from a bovine papillomavirus/simian virus 40 recombinant virus. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9007–9011. doi: 10.1073/pnas.85.23.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J., Fazeli A., Malicki J., Horwitz B. H., DiMaio D. Genetic evidence that acute morphologic transformation, induction of cellular DNA synthesis, and focus formation are mediated by a single activity of the bovine papillomavirus E5 protein. Mol Cell Biol. 1989 Dec;9(12):5563–5572. doi: 10.1128/mcb.9.12.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Heffernan P. A., Weinberg R. A. p185, a product of the neu proto-oncogene, is a receptorlike protein associated with tyrosine kinase activity. Mol Cell Biol. 1986 May;6(5):1729–1740. doi: 10.1128/mcb.6.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P., Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988 Sep;8(9):3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Zullo J., Stiles C. D., Garcea R. L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]