Abstract
Objective:
We evaluated whether first-trimester high-sensitivity C-reactive protein (hsCRP), a suggested marker of pregnancy-associated hyperglycemia, predicts third-trimester impaired glucose tolerance (IGT) in a secondary analysis of a prospective cohort of nondiabetic singletons enrolled at <26 weeks gestation.
Study Design:
We measured the association between hsCRP collected at <14 weeks among women classified as IGT (gestational diabetes screening results, 135 to <200 mg/dL) and those among normoglycemic women. Multivariable modeling estimated the association between log hsCRP and IGT, adjusted for maternal body mass index (BMI).
Results:
Among 300 women, 13% (39 of 300) had IGT. The hsCRP was positively associated with glucose (P = .005). Compared with normoglycemic women, women with IGT had higher log hsCRP (0.87 ± 0.66 vs 0.67 ± 0.60, P = .04), but the association was not significant in adjusted models (adjusted odds ratio 1.20, 95% confidence interval 0.65-2.21). The hsCRP did not predict third-trimester IGT in this analysis when BMI is considered.
Conclusion:
Early identification of women at risk of IGT remains a priority, but the contribution of maternal BMI appears greater than hsCRP.
Keywords: high-sensitivity C-reactive protein, early pregnancy predictors, impaired glucose tolerance, maternal serum markers
Introduction
Impaired glucose tolerance (IGT) in pregnancy and gestational diabetes (GDM) are associated with adverse perinatal outcomes and is a significant, growing public health challenge.1 Maintaining glycemic control in pregnancy has short- and long-term health benefits for maternal and child health.2,3 However, current third-trimester glucose intolerance screening leaves limited time for intervention if IGT or GDM is diagnosed. Earlier identification of glucose intolerance risk may increase time for intervention and offer opportunities for prevention.4
Maternal serum markers, measureable early in pregnancy and in routine clinical practice, have been suggested as promising predictors of later pregnancy glucose intolerance.4,5 High-sensitivity C-reactive protein (hsCRP) is an acute-phase reactant that, at subclinical elevations, is a marker for endothelial damage, cardiovascular disease, and obesity in nonpregnant patients.6,7 In pregnancy, hsCRP is associated with maternal serum glucose when measured at the time of standard third-trimester GDM screening.5,8,9
Current data on hsCRP are limited to at-risk populations with few that assess early pregnancy measurements. Whether first-trimester hsCRP is associated with third-trimester glucose, and thus a potential early pregnancy serum marker, is unknown. We evaluated whether first-trimester hsCRP is predictive of third-trimester GDM screening result among women diagnosed with IGT.
Materials and Methods
We performed a secondary analysis of nondiabetic pregnant women enrolled in a cross-sectional prospective study of oral health in pregnancy. The study design and methods of the primary study have been described previously.10,11 As part of the primary study protocol, women with greater than singleton pregnancy, type 2 diabetes mellitus, chronic hypertension, or medical comorbidities likely to impact early pregnancy hsCRP values (ie, liver or renal diagnoses and HIV) were not eligible for study inclusion. Maternal demographic and medical history data, including measured height and weight for body mass index (BMI) calculation, were collected through interviews and written questionnaire at enrollment. Maternal race was recorded as white, black, or other by patient self-report.
During the 42-month study period of the primary study, starting in December 1997, 63% (1224 of 1945) of eligible women were consented at <26 weeks with ultrasound-confirmed gestational age. After exclusion of women without serum CRP, or fetal loss or spontaneous abortion >21 weeks, study withdrawal, or transfer for delivery at another hospital, 1020 remained enrolled. Women were followed through delivery and delivery outcomes were collected as part of the original study protocol. For the current secondary analysis, we included women with hsCRP collected at <14 weeks who also had GDM screening (50 g; 1-hour oral glucose load) at 24 to 28 weeks gestation. Women with GDM screening results of 135 to <200 mg/dL but without GDM were classified as IGT. Ten women diagnosed with GDM were excluded from the analysis.
Maternal serum was collected at enrollment to determine hsCRP, using a previously published technique.12 This was a commercially available, highly sensitive enzyme-linked immunosorbent assay (VIRGO C-reactive Protein Kit; Hemagen Diagnostics, Waltham, Massachusetts). This assay's range is 0.5 to 50 μg/mL, with inter- and intra-assay variability of 3% and 15%, respectively. Duke University Medical Center Institutional Review Board approval was obtained for the original study and for the current secondary analysis.
We described the final cohort, comparing women with and without IGT, using Student's t-test and chi-square analysis. The hsCRP values were not normally distributed and were log-transformed to approximate a normal distribution. We assessed the unadjusted and adjusted linear relationship of log hsCRP and glucose. We evaluated test-for-trend of mean hsCRP result for each one standard deviation (SD) increase in glucose. Mean log hsCRP was compared between normal women and women with IGT. Multivariable modeling estimated the association of log hsCRP and IGT. Of potential confounders assessed in bivariate analysis, only maternal BMI remained significant and was included in adjusted linear and multivariable models. Logistic regression was used to generate a receiver–operating curve (ROC) to identify whether a specific cut point existed for hsCRP, maternal BMI, or the combination of both to optimally predict IGT. The c-statistic or area under the curve (AUC) is reported.
Results
Among 300 women meeting inclusion criteria for this secondary analysis, mean glucose result was 107 ± 26 mg/dL, and 13% (39 of 300) had IGT. Women with IGT, compared with normoglycemic women, had greater enrollment BMI (29.8 ± 7.4 kg/m2 vs 26.1 ± 5.7 kg/m2, P = .005), and the mean gestational age of enrollment among all women was 10.6 weeks. Women with IGT were approximately 2 years older than normoglycemic women (30.7 ± 6.3 vs 28.5 ± 6.1 years, P = .04). Other demographic variables were similar among women with and without IGT and are shown in Table 1.
Table 1.
Maternal Characteristics for Total Cohort and for Normoglycemic Women Versus Women With Impaired Glucose Tolerance.
| Characteristics | Total cohort, n = 300 | Normoglycemic, n= 261 | IGT, n = 39 | Normoglycemic vs IGT |
|---|---|---|---|---|
| Mean ± SD or n (%) | P | |||
| Maternal age at enrollment, years | 28.8 ± 6.1 | 28.5 ± 6.1 | 30.7 ± 6.3 | .04 |
| Ethnicity | ||||
| White | 165 (55) | 147 (56) | 18 (46) | |
| Black | 116 (39) | 100 (38) | 16 (41) | |
| Other | 19 (6) | 14 (5) | 5 (13) | .16 |
| Parous | 178 (59) | 153 (59) | 25 (64) | .52 |
| Gestational age at enrollment/serum collection, weeks | 10.6 ± 2.1 | 10.6 ± 2.1 | 10.5 ± 2.0 | .83 |
| Enrollment BMI, kg/m2 | 26.6 ± 6.0 | 26.1 ± 5.7 | 29.8 ± 7.4 | .005 |
| Gestational age at GDM screening, weeks | 26.8 ± 6.1 | 26.3 ± 2.2 | 24.7 ± 5.1 | .07 |
Abbreviations: IGT, impaired glucose tolerance; BMI, body mass index; GDM, gestational diabetes; SD, standard deviation.
In unadjusted linear regression, hsCRP was positively associated with glucose result (r2 = .03, P = .005), but the association was attenuated when enrollment BMI was considered (P = .25). Five categories of glucose result were created according to the SD of 26 mg/dL among the cohort. The test-for-trend was significant in unadjusted analysis (P = .01), demonstrating that first-trimester serum hsCRP correlated with third-trimester maternal glucose levels. The trend did not remain significant after adjusting for enrollment BMI (P = 0.31; Table 2)
Table 2.
Test-for-Trend of Mean Glucose and Mean High-Sensitivity C-Reactive Protein (hsCRP).a
| Glucose, mg/dL | N | Mean ± SD log hsCRP | Mean ± SD hsCRP, mg/Lb |
|---|---|---|---|
| <81 | 38 | 0.54 ± 0.56 | 8.10 ± 3.6 |
| 81-<107 | 128 | 0.66 ± 0.59 | 11.6 ± 2.0 |
| 107-<133 | 93 | 0.72 ± 0.59 | 12.9 ± 2.3 |
| 133-<159 | 28 | 0.85 ± 0.63 | 17.3 ± 4.2 |
| >150 | 13 | 0.92 ± 0.73 | 21.6 ± 6.2 |
Abbreviations: BMI, body mass index; SD, standard deviation.
aUnadjusted P = .01; adjusted for enrollment BMI P = .31.
bAbsolute hsCRP shown as clinical reference.
Compared with normal women, those with IGT had higher log hsCRP (0.87 ± 0.66 vs 0.67 ± 0.60, P = .04). In unadjusted multivariable logistic regression, log hsCRP was associated with IGT (odds ratio [OR] 1.70 95% confidence interval [CI] 1.01-2.99). Once adjusted for enrollment BMI, the association was not significant (adjusted OR 1.20 95% CI 0.65-2.21). As shown in Figure 1, hsCRP had an AUC of 0.59 as an individual marker of IGT. Maternal BMI had an AUC of 0.66. Combining hsCRP with maternal BMI marginally increased overall AUC to 0.67.
Figure 1.
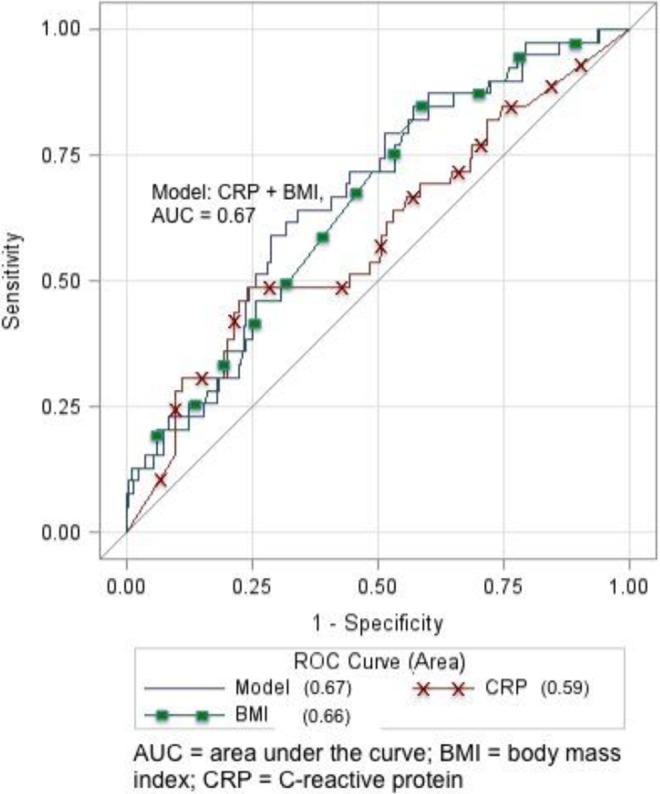
Receiver–operating curve (ROC) for unadjusted and adjusted logistic regression models.
Discussion
Among women undergoing routine third-trimester GDM screening, IGT was a prevalent outcome. The hsCRP, measured in the first trimester, has some association with GDM screening result among women with IGT. However, when early pregnancy maternal BMI is considered, the contribution of hsCRP is attenuated and not stronger than BMI.
Strengths of our analysis include its analysis of rigorously collected prospective data, avoiding potential errors in clinical chart abstraction or participant recall bias after an IGT diagnosis. The original prospective cohort included data on potential mediators and confounders that could impact CRP or IGT diagnosis, and we were able to consider these in our analysis. The original cohort was not specified as high risk for glucose intolerance or GDM so mirrors clinical practice where providers do not clearly know who is at risk as early as first trimester. Nonetheless, we were able to measure a prevalent clinical outcome, IGT, that typically goes untreated but has known negative consequences for maternal, fetal, and child health.
Study limitations must also be considered. Although we have early pregnancy measured height and weight to calculate BMI, we do not know gestational weight gain through time of GDM screening. If women diagnosed with IGT, compared with normoglycemic women, had gained more weight as of GDM screening, this may further attenuate the adjusted association between hsCRP and GDM screening result. We do know, however, that total gestational weight gain was similar between normoglycemic and IGT women. Thus, it is plausible that weight gain among this cohort was similar at time of GDM screening. In this sample size, we were unable to measure the association between hsCRP and a diagnosis of GDM. However, finding an association with IGT suggests an even greater association if GDM versus normoglycemic women were compared in a larger sample. In addition, data were not collected on family history of diabetes mellitus or personal history of GDM. However, as the primary analysis was the association between first-trimester hsCRP and IGT, this history would not likely have impacted overall findings.
Others have shown an association between hsCRP and degrees of glucose intolerance. Smirnakis et al reported a first-trimester nonfasting hsCRP was greater among women who later developed GDM than with normoglycemic women.8 Retnakaran, et al., found hsCRP was more closely associated with BMI than components of an oral glucose tolerance test (OGTT).9 However, this association was specific to hsCRP and an OGTT, each measured in the third trimester, so may not parallel findings when hsCRP is measured earlier in pregnancy, as hsCRP is known to increase with advancing gestational age.13
Each of these studies included smaller sample sizes, compared with ours, and dichotomized outcomes of glucose intolerance versus normoglycemia, instead of measuring the adjusted linear relationship between exposure and outcome as we did. The large Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study did assess linear relationships in a secondary analysis of inflammatory mediators. The authors reported a linear relationship between second-trimester hsCRP and components of the 2-hour OGTT test, even when adjusted for BMI.14 That large analysis of third-trimester measurements helps establish the plausibility of our analysis, seeking whether early pregnancy hsCRP predicts later IGT.
Although our data examined a much smaller sample size, our exposure, hsCRP, was measured in the first trimester. We explored whether this serum marker could be an early pregnancy predictor of later IGT. Measurement of IGT, a prevalent outcome that is primarily left untreated but also linked to the same adverse outcomes as GDM, highlights a different group who may be amenable to treatment or prevention efforts. In a nonpregnant population at risk of type 2 diabetes mellitus, some data suggest modifying diet and exercise can prevent diagnosis. Thus far, similar efforts have not been consistent among pregnant women, and development of IGT has not been prioritized as an outcome. We speculate that, before interventions can be critically appraised, we need to better identify at-risk women. Inflammatory mediators may extend the predictive ability of traditional risk factors such as BMI.
High-sensitivity C-reactive protein has been implicated as a predictor of type 2 diabetes mellitus, given that subclinical inflammation is part of diabetes mellitus pathophysiology. Gestational diabetes or IGT, as potential precursors to type 2 diabetes mellitus, may also have subclinical CRP elevation. Perhaps glucose intolerance in pregnancy is a transient unmasking of a latent metabolic syndrome.
Our data, in conjunction with these associations outside of pregnancy, have implications for future research. Current clinical practice leaves little time for intervention that may decrease the poor perinatal outcomes associated with hyperglycemia. Perhaps the debate over third-trimester GDM screening nuances should shift to identifying earlier pregnancy variables that can predict IGT or GDM before these diagnoses occur. The hsCRP may be one inflammatory and insulin resistance marker present as early as the first trimester. Our findings support maternal BMI as an integral component of the association between first-trimester hsCRP and IGT, although neither individually predicted IGT in our sample. The greatest AUC shown in our ROC curve was 0.67 and predominantly driven by maternal BMI, not hsCRP. We speculate that a combination of maternal characteristics in conjunction with early pregnancy inflammatory markers may optimize IGT prediction. Impaired glucose tolerance and GDM remain a public health priority, and earlier pregnancy identification of at-risk women has the potential to increase time for intervention, or prevention, efforts.
Footnotes
Authors’ Note: This work was completed in full at 2 institutions, the University of North Carolina at Chapel Hill and Duke University. Preliminary data were presented in abstract format at the Society for Gynecologic Investigation 60th Annual Scientific Meeting, Orlando, Florida, March 29, 2013.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 (suppl 1):S61–S78. [DOI] [PubMed] [Google Scholar]
- 2. Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50 (4):972–929. [DOI] [PubMed] [Google Scholar]
- 3. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192 (4):989–997. [DOI] [PubMed] [Google Scholar]
- 4. Sattar N. Biomarkers for diabetes prediction, pathogenesis or pharmacotherapy guidance? Past, present and future possibilities. Diabet Med. 2011;29 (1):5–13. [DOI] [PubMed] [Google Scholar]
- 5. Lowe LP, Metzger BE, Lowe WL, Jr, , Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95 (12):5427–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107 (3):363–369. [DOI] [PubMed] [Google Scholar]
- 7. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282 (22):2131–2135. [DOI] [PubMed] [Google Scholar]
- 8. Smirnakis KV, Plati A, Wolf M, Thadhani R, Ecker JL. Predicting gestational diabetes: choosing the optimal early serum marker. Am J Obstet Gynecol. 2007;196(4):410 e1–e6. [DOI] [PubMed] [Google Scholar]
- 9. Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab. 2003;88 (8):3507–3512. [DOI] [PubMed] [Google Scholar]
- 10. Lieff S, Boggess KA, Murtha AP, et al. The oral conditions and pregnancy study: periodontal status of a cohort of pregnant women. J Periodontol. 2004;75 (1):116–126. [DOI] [PubMed] [Google Scholar]
- 11. Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107 (1):29–36. [DOI] [PubMed] [Google Scholar]
- 12. Boggess KA, Lieff S, Murtha AP, et al. Maternal serum C-reactive protein concentration early in pregnancy and subsequent pregnancy loss. Am J Perinatol. 2005;22 (6):299–304. [DOI] [PubMed] [Google Scholar]
- 13. Picklesimer AH, Jared HL, Moss K, Offenbacher S, Beck JD, Boggess KA. Racial differences in C-reactive protein levels during normal pregnancy. Am J Obstet Gynecol. 2008;199(5):523.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358 (19):1991–2002. [DOI] [PubMed] [Google Scholar]


