Abstract
Our purpose was to report efficacy of hypofractionated cavity stereotactic radiotherapy (HCSRT) in patients with and without prior whole brain radiotherapy (WBRT). 32 surgical cavities in 30 patients (20 patients/21 cavities had no prior WBRT and 10 patients/11 cavities had prior WBRT) were treated with image-guided linac stereotactic radiotherapy. 7 of the 10 prior WBRT patients had “resistant” local disease given prior surgery, post-operative WBRT and a re-operation, followed by salvage HCSRT. The clinical target volume was the post-surgical cavity, and a 2-mm margin applied as planning target volume. The median total dose was 30 Gy (range: 25-37.5 Gy) in 5 fractions. In the no prior and prior WBRT cohorts, the median follow-up was 9.7 months (range: 3.0-23.6) and 15.3 months (range: 2.9-39.7), the median survival was 23.6 months and 39.7 months, and the 1-year cavity local recurrence progression-free survival (LRFS) was 79 and 100%, respectively. At 18 months the LRFS dropped to 29% in the prior WBRT cohort. Grade 3 radiation necrosis occurred in 3 prior WBRT patients. We report favorable outcomes with HCSRT, and well selected patients with prior WBRT and “resistant” disease may have an extended survival favoring aggressive salvage HCSRT at a moderate risk of radiation necrosis.
Keywords: Stereotactic radiotherapy, Cavity radiosurgery, Hyofractionation, Radiation necrosis, Radiosurgery
Introduction
The standard of care for years following surgical resection of brain metastases has been post-operative whole brain radiotherapy (WBRT) (1–5). Randomized controlled trials (RCTs) have proven benefit with respect to local control and distant brain control as compared to surgery alone, however, no impact on overall survival (OS) (4, 5). Recently, we have observed based on formal neurocognitive testing that WBRT results in an independent adverse effect on memory function (6, 7). As a result, the shift in the treatment paradigm for the post-operative patient is to treat focally to the surgical cavity, and reserve WBRT as a salvage therapy (8, 9). In particular, in those patients who have already been treated with WBRT then post-operative options are limited to either no further radiation or re-treatment WBRT, neither of which are ideal. In principle, cavity radiation is analogous to the well-established practice of stereotactic radiosurgery (SRS) alone for patients presenting with limited brain metastases, and the use of SRS as a salvage treatment for WBRT failures (2, 10).
Although some have adopted single fraction SRS to the post-operative cavity (9), this approach is limited with respect to the deliverable total dose given the large target volumes associated with surgical cavities and the irregularity of the surgical cavity shape (11). With recent technologic advances allowing for frameless hypofractionated cavity stereotactic radiotherapy (HCSRT) (12), treatment with few fractions rather than a single fraction is gaining acceptance as routine clinical practice (8, 9, 13). The major advantage of HCSRT is to deliver high total doses in only a few fractions despite large treatment volumes, while minimizing the risk of radiation necrosis (RN). The latter is largely due to the protective effects of fractionation on the normal brain tissue (14). The purpose of this study is to report our outcomes for post-surgical HCSRT in patients with and without prior WBRT delivered in 5 daily fractions. In particular, we describe outcomes for a unique cohort of patients with resistant local disease who had undergone multiple surgeries and prior radiation to the same tumor and ultimately salvaged with HCSRT.
Methods and Materials
Patient Data
Institutional Research Board (IRB) approval was obtained from the Sunnybrook Health Sciences Centre for this retrospective review of a prospective database of patients treated with HCSRT. Thirty-two cavities in 30 consecutive patients treated with HCSRT were identified. All patients were treated by a single radiation oncologist (AS) between July 2009 and December 2011. The electronic medical records and treatment plans were retrospectively reviewed, toxicity was graded according to the National Cancer Institute Common Terminology Crieria for Adverse Events v. 4.0.
Stereotactic Radiotherapy Technique
All patients were treated with a 4 mm multi-leaf collimator image-guided linac system equipped with a robotic couch (Elekta Axesse, Elekta AB, Stockholm, Sweden). Patients were immobilized supine with an aquaplast mask, and simulated with a 1 mm slice thickness computed tomogram (CT). A volumetric 1.5 mm slice thickness magnetic resonance imaging (MRI) scan was acquired with gadolinium contrast typically a few days prior to simulation for image-fusion to the planning CT scan. The time from surgery to treatment planning MRI was generally 2-3 weeks.
The surgical cavity was delineated based on the treatment planning MRI as the clinical target volume (CTV). In cases of a subtotal resection, the residual enhancing disease was delineated as the gross tumor volume (GTV). A 2 mm volumetric margin expansion beyond the CTV (and GTV) was used to define the planning target volume (PTV). The 2 mm PTV was based on our in-house published evaluation of precision in treatment delivery specific to this unit and immobilization system (15). All patients were treated with daily cone-beam CT (CBCT) image-guided radiotherapy with online corrections for any translation greater than 1 mm or rotations greater than 1 degree. A radiation oncologist verified the first day treatment setup and CBCT. Figure 1 illustrates a typical patient treated with HCSRT.
Figure 1:
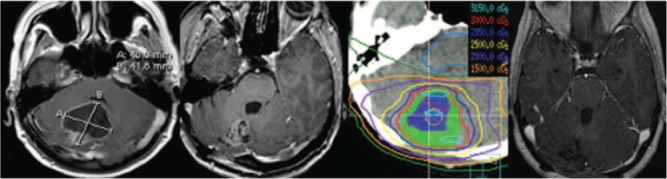
HCSRT in a patient with cerebellar metastases from a primary lung cancer. The left-most image is an axial T1 post-gadolinium MR illustrating the pre-operative tumor, and the subsequent images indicate the postop axial MRI (3 weeks post-op), the treatment plan with representative isodose lines (CTV in blue color wash and PTV in green color wash), and the right-most image is the patient’s axial MRI 1-year post-HCSRT illustrating no evidence of disease.
Statistical Analysis
Local recurrence was defined as the presence of a new enhancing mass within, or directly adjacent to, the resection cavity. In cases where it was difficult to differentiate local recurrence versus RN, surgery was undertaken for pathologic confirmation. Distant recurrence was defined as the presence of new enhancing lesions consistent with brain metastases beyond the resection cavity, along the dura or within the leptomeninges. Time to progression was calculated from the date of HCSRT to the first MRI that showed evidence of progression. Statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC). The Fisher’s exact test and Pearson’s chi-square test were used to compare proportions. The Wilcoxon rank-sum test was used to compare continuous variables. OS, progression-free survival (PFS), local recurrence progression-free survival (LRFS) and distant recurrence progression-free survival (DRFS) were calculated using the Kaplan-Meier method. Differences between survival curves were analyzed by the log-rank test. Results were considered significant if the p-value was less <0.05. No patient was lost to follow-up and all were meticulously followed with MRI every 2-3 months post-HCSRT.
Results
Patient Characteristics
32 surgical cavities in 30 consecutive patients were identified. 20 patients (21 cavities) had no prior WBRT while 10 patients (11 cavities) had a history of prior WBRT. 7 of these 10 patients had locally resistant disease having had prior surgery and post-operative WBRT who subsequently locally relapsed and were re-operated followed by salvage HCSRT. Patient characteristics are listed in Table I. The median age at diagnosis was 70 years (range: 41-90) in the no prior WBRT cohort which was significantly older than 57 years (range: 24-71) in the prior WBRT cohort (p = 0.006). In both groups, the most common primary site was nonsmall cell lung cancer (NSCLC) and most patients had a recursive partitioning analysis (RPA) class of 2 (66%). A gross total resection was achieved in 17 cavities (81%) in the no prior WBRT cohort, and 6 cavities (55%) in the prior WBRT group.
Table I.
A comparison of baseline patient and tumor characteristics according to the treatment cohort.
| Characteristic | No prior WBRT cohort 20 patients/21 cavities | Prior WBRT cohort 10 patients/11 cavities | p-value |
|---|---|---|---|
| Median age (range) | 70 (41-90) | 57 (24-71) | 0.006 |
| Male | 7 | 3 | 0.45 |
| Female | 13 | 7 | |
| NSCLC | 10 | 4 | 0.27 |
| Breast | 3 | 3 | |
| Other | 8 | 4 | |
| Frontal | 6 | 1 | 0.27 |
| Temporal | 2 | 3 | |
| Parietal | 4 | 4 | |
| Occipital | 1 | 1 | |
| Cerebellum | 7 | 1 | |
| Pre-op median tumor | 3.4 cm | 3.2 cm | 0.49 |
| Diameter (range) | (2.0-4.7) | (2.5-6.2) | |
| GTR | 17 | 6 | 0.70 |
| STR | 4 | 5 | |
| ECOG 0 | 3 | 2 | 0.73 |
| ECOG 1 | 17 | 8 | |
| RPA Class I | 7 | 3 | 0.78 |
| RPA Class II | 13 | 7 | |
| GPA 0-1 | 0 | 3 | 0.55 |
| GPA 1.5-2.5 | 17 | 6 | |
| GPA 3-4 | 3 | 1 |
WBRT refers to whole brain radiotherapy, RPA refers to the brain metastases recursive partioning analysis, GPA refers to the brain metastases graded prognostic analysis, NSCLC refers to non-small cell lung cancer.
Treatment Characteristics
Treatment characteristics are listed in Table II. The total dose ranged from 25 to 37.5 Gy (median 30 Gy) in 5 fractions in the prior WBRT group and 25-35 Gy (median 30 Gy) in 5 fractions in the no prior WBRT group. The median PTV was 34.5 cc (range: 5.0-179.8) in the prior WBRT group and 23.6 cc (range: 3.1-42.1) in the no prior WBRT.
Table II.
Tumor cavity dosimetric characteristics.
| No prior WBRT cohort | Prior WBRT cohort | p-value | |
|---|---|---|---|
| Median PTV volume (range) | 23.6 cc (3.1-42.1) | 34.5 cc (5.0-179.8) | 0.10 |
| Median PTV V95% (range) | 99.9% (95.8-99.9) | 99.3% (39.5-100) | 0.09 |
| Median PTV V90% (range) | 100% (97.3-100) | 99.9% (44.9-100) | 0.22 |
| Median PTV D95 (range) | 30.3 Gy (24.22-35.6) | 31.0 Gy (6.7-36.1) | 0.09 |
| Median PTV D90 (range) | 30.5 Gy (25.2-35.8) | 31.2 Gy (8.2-36.6) | 0.08 |
| Mean PTV dose | 30.9 Gy (25.5-37.1) | 31.6 Gy (19.9-37.8) | 0.20 |
| Homogeneity index | 0.05 (0.01-0.10) | 0.06 (0.03 -1.2) | 0.36 |
| Conformity index | 1.6 (1.3-3.2) | 1.4 (0.4 -1.9) | 0.02 |
PTV refers to planning target volume, V95% and V90% refers to the percent volume encompassed by 95% and 90% of the prescribed dose, respectively, D95 and D90 refer to the absolute dose within 95% and 90% of the PTV volume, respectively.
Clinical Outcomes
In the no prior and prior WBRT cohorts, the median follow-up was 9.7 months (range: 3-23.6) and 15.3 months (range: 2.9-39.7), and the median survival was 23.6 months and 39.7 months and the 1-year OS rate was 71 and 80%, respectively. Figure 2 describe the OS, LRFS and the DRFS according to cohort. In total, seven patients had local progression with 3 in the no prior and 4 in the prior WBRT groups. 3/4 failures in the prior WBRT cohort occurred in those 7 patients with “resistant” local disease. In the no prior WBRT and prior WBRT cohorts, the 1-year cavity LRFS was 79 and 100%, and at 18 months had not been reached and 29%, respectively. The clinical details and salvage treatments for each patient who failed within the treated cavity are summarized in Table III. In the no prior WBRT and prior WBRT cohorts, the DRFS at 12 months was 63 and 52%, and the median time to distant failure had not been reached and 17 months, respectively. No statistically significant differences in outcomes were observed between the two cohorts.
Figure 2:
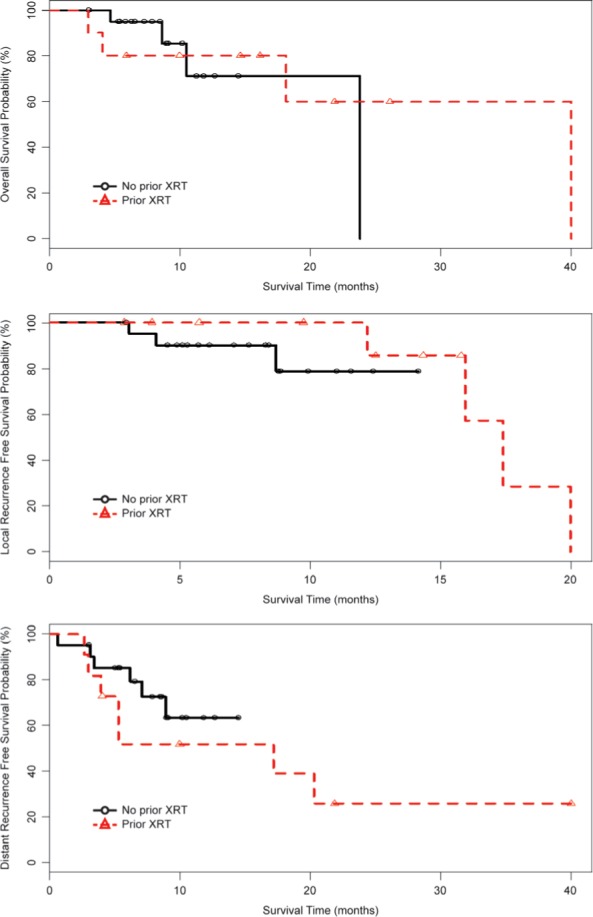
Overall survival (OS), local recurrence progression-free survival (LRFS) and distant recurrence progression-free survival (DRFS) according to the no prior WBRT (n = 20 patients and 21 cavities) and prior WBRT (n = 10 patients and 11 cavities) cohorts.
Table III.
Patient and treatment characteristics of local failures and outcomes following salvage therapy.
| Patients | Cohort | Tumor size (cm) | Pattern of failure | Time to failure (months) | Salvage treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | No prior WBRT | 3.9 X 3.0 | Local and distant | 8.2 | WBRT: 30 Gy/10 with simultaneous boost to GTV of 40 Gy/10 | Died 2 months post-salvage from systemic disease, brain locally controlled |
| 2 | No prior WBRT | 3.4 X 3.6 | Local | 2.4 | WBRT: 20 Gy/5 with simultaneous boost to GTV of 25 Gy/5 | Died 6 months post-salvage from CNS disease progression |
| 3 | No prior WBRT | 2.5 X 2.5 | Local | 6.0 | SRT: 30 Gy/5 | Locally controlled 1 month post-SRT |
| 4* | Prior WBRT | 3.7 X 3.8 | Local and distant | 2.2 | Surgery alone | Died 3 months post-salvage from CNS disease progression |
| 5* | Prior WBRT | 3.5 X 2.9 | Local | 13.9 | Surgery alone | Locally controlled at 4 months |
| 6 | Prior WBRT | 2.5 X 2.4 | Local | 6.0 | None | Died from CNS disease progression |
| 7* | Prior WBRT | 4.3 X 2.9 | Local | 8.0 | Surgery plus WBRT 25 Gy/10 | Locally controlled at 5 months |
*Patients with resistant disease having had prior surgery followed by WBRT +/− further SRS and subsequently locally relapsed and treated with re-excision and HCSRT, WBRT refers to whole brain radiotherapy, CNS refers to central nervous system, SRT refers to stereotactic radiotherapy.
Four patients (19%) died in the no prior WBRT cohort and the median time to death was 8.5 months (range: 4.0-10.1). Two out of the four patients died of neurologic progression and other two died of uncontrolled systemic disease. Four patients (36%) in the prior WBRT group died and the median time to death was 9.2 months (range: 3.3-24.5 months). Two out of the four patients died of neurologic progression and the other two died of uncontrolled systemic disease.
Toxicity
HCSRT was well tolerated with no treatment interruptions or unexpected acute side effects. There were no grade 3 or 4 late toxicities in the no prior WBRT; however, three patients (30%) developed grade 3 RN in the prior WBRT group, and each patient underwent surgery to confirm the diagnosis. Furthermore, a central pathology review was performed for each case to confirm this diagnosis.
The first patient with RN had metastatic endometrial cancer and been initially treated with surgery followed by WBRT (20 Gy in 5 fractions), and following a local recurrence treated with salvage SRS (15 Gy in 1 fraction). Ten months later there was radiographic evidence of local disease progression and the patient was re-resected followed by HCSRT to a 12.4 cc PTV. Five months later the patient developed symptomatic RN within the resection cavity requiring surgical resection. The second patient with RN had metastatic melanoma and been initially treated with surgery followed by WBRT (30 Gy in 10 fractions with a simultaneous integrated boost to the cavity to 40 Gy in 10 fractions). Five months later, the patient underwent reresection for local recurrence followed by HCSRT (30 Gy in 5 fractions) to a 36.3 cc PTV. Three months later the patient developed symptomatic RN and required surgical resection. The third patient with RN had metastatic NSCLC and been initially treated with WBRT (20 Gy in 5 fractions) and progressed locally two months later. The patient then underwent re-resection followed by HSCRT (30 Gy in 5 fractions) to a PTV of 17.8 cc (3 months following the initial WBRT). Symptomatic RN developed 13 months later requiring surgical resection (Figure 3).
Figure 3:
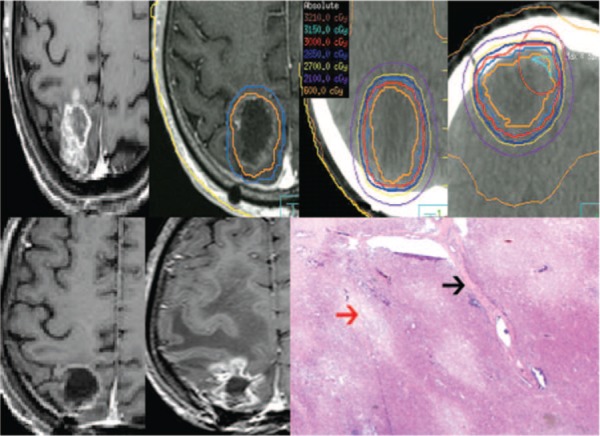
Radiation necrosis post-HCSRT in a patient previously treated with WBRT. The top left is the pre-operative metastases secondary to lung cancer followed by the post-operative MRI with the CTV (orange) and PTV (blue) delineated. The subsequent top panel images illustrate the treatment planning CT with representative isodose lines on the axial and coronal images. The bottom panel left-most image shows the 12 month post-HCSRT axial MRI and the 13 month MRI taken as a result of symptoms of increased intra-cranial pressure that was surgically resected and proven to be RN. The bottom right image is a low power H&E stained histology photomicrograph showing well circumscribed areas of necrosis of the brain parenchyma (red arrow) and vascular hyalinization (black arrow) with no evidence of residual or recurrent tumor.
Discussion
The aim of cavity radiation is to reduce the risk of local relapse and reserve WBRT as a salvage therapy (8, 9). Furthermore, cavity radiation has been developed with the intent to improve local control by treating with doses consistent with SRS (16) and HCSRT practice (13), as opposed to those lower doses common to WBRT (1). Although we do not have any RCT to prove equivalence or superiority for cavity radiation as compared to WBRT, the literature is suggestive of efficacy and randomized studies are ongoing (8).
There have been multiple studies reporting outcomes for single fraction surgical cavity SRS (8, 9); however, no dedicated series for five fraction HCSRT. The issue with single fraction SRS is the practice of applying the same principles of SRS dose selection to the cavity as would otherwise for intact lesions and; therefore, as the target volume increases the dose prescribed decreases (16). This dose-volume relationship is based on respecting normal tissue tolerance to maintain an acceptable risk of RN (16). In our series the median pre-operative tumor diameter and PTV in the no prior and prior WBRT cohorts were 3.4 cm and 3.2 cm, and 23.6 cc and 34.5 cc, respectively, which translates to a comparatively sub-optimal SRS dose of 15 Gy (10, 16). Our approach was developed to treat cavities with 5 fraction HCSRT and deliver radical doses while maintaining a low risk of RN due to the protective effects of fractionation. The dose of 30 Gy in 5 fractions was most often chosen as the peripheral dose fall-off is relatively steep such that the 20 Gy isodose line would typically lie ~6-10 mm beyond the PTV, and we are very comfortable with large volumes of normal brain tissue receiving 20 Gy in 5 fractions (common WBRT dose schedule). Therefore, we postulated a priori that this regimen would minimize the risk of RN, despite the large volume of normal tissue irradiation.
With respect to cavity single fraction SRS outcomes in patients with no prior WBRT (9), a recent review of 10 series comprising a total of 380 patients estimated crude local control at 79% and a 1-year actuarial LRFS rate of 70%. The estimated risk of RN was ~5% (9). We report comparable crude local control at 76% (relapse in 3 of 21 cavities) and a 1-year actuarial LRFS rate of 79%. It is important to note that our median pre-operative tumor diameter was 3.4 cm (range: 2.0-4.7 cm) and PTV was 23.6 cc (3.1-42.1), and we have yet to have a single case of RN in this cohort. With further follow-up we will see if our practice does in fact prove superior to single fraction SRS as the rates of local control have been recently reported to drop significantly beyond a year with SRS (11). Hartford et al. reported on only 50% and 40% of cavities free of relapse at 2-years for those pre-operative tumors measuring >2 cm and >3 cm, respectively (11). Lastly, the evidence is gathering to support hypofractionated SRT in both patients with intact metastases (13) and cavities.
For example, Kim et al. reported better outcomes for SRT using 36 Gy in 6 fractions (daily over 1.5 weeks) as compared to single fraction SRS (median dose 20 Gy) despite those tumors treated with SRT being of greater volume (5.0 cc vs. 2.21 cc, respectively) (13). Katz et al. reported in abstract form better outcomes for 24 Gy in 3 fractions as HCSRT as compared to single fraction SRS (median 18 Gy), and again volumes were greater for the HCSRT cohort (8.9 cc vs 2.2 cc, respectively) (17). Ultimately, a RCTs will be required to determine optimal dosing.
When comparing the baseline characteristics of the no prior WBRT cohort to the prior WBRT cohort, we observe a statistically significant difference in age. Patients were older in the prior WBRT cohort, and this reflected our initial practice of offering older patients HCSRT to spare them from the adverse memory effects inherent to WBRT. As our experience matured we now offer HSCRT to all patients regardless of age. Our prior WBRT cohort was also unique and well selected for aggressive therapy, such that these patients had controlled extra-cranial disease and an expected prolonged survival (median survival of 39.7 months was observed). We determined “resistant disease” in 7 of the 10 prior WBRT patients given that they had multiple prior treatments to the same brain metastases that included combinations of prior surgery, postoperative WBRT, some had salvage SRS, and ultimately salvage surgery followed by post-operative HCSRT. There is also some experimental literature to support the idea of treatmentinduced radiation resistance (18) and a lack of literature detailing clinical outcomes in such a cohort. Although the small number of patients in this cohort is a limitation to making firm conclusions, the lack of outcomes in such a cohort of heavily pre-treated patients is also a strength of our report.
In our prior WBRT cohort, the pre-operative diameter and PTV were also large with a median of 3.2 cm (range: 2.5-6.2) and 34.5 cc (5.0-179.8), respectively. We report at one year a 100% LRFS rate. However, by 18 months this rate dropped to 29% (Figure 2). The drop in local control was driven by four of the seven local failures observed within the prior WBRT cohort. Three of the four prior WBRT failures occurred in those with resistant disease (n = 7). These results suggest that we have the ability with aggressive therapy to gain control of what could be considered as the uncontrollable brain metastases, and remarkably for up to a year in all patients.
Importantly, three cases of RN were observed, and all in the prior WBRT cohort. Two of these three patients were those with highly resistant disease having had multiple courses of radiation and surgery and, therefore, at greater baseline risk of RN. The third patient was treated with HCSRT at only 3 months following prior WBRT and to a large volume (17.8 cc). We suspect that both of these factors increased this patients’ risk. No patient in the de novo group has developed RN; however, with long-term follow-up we may see this toxicity emerge.
The only other series with dedicated outcomes for patients with prior WBRT and salvage cavity radiation was reported by the Wake Forest group in 2006 (19). They treated 79 patients with single fraction cavity SRS and reported crude local recurrence in 5.1% of patients and RN in 3.8%. These data indicate efficacy, however, the cohort is not largely comparable to our prior WBRT cohort given the “resistant disease” in the majority of the 10 patients (prior WBRT and multiple surgeries for the same tumor eventually treated with HCSRT).
Conclusion
We report favorable outcomes with HCSRT in patients with and without prior WBRT. In patients with recurrent brain metastases despite prior surgery and WBRT, further surgery and salvage HCSRT yields prolonged local control at a moderate risk of RN. Further follow-up and a greater sample size is necessary to confirm our observations.
Abbreviations
- HCSRT:
Hypofractionated Cavity Stereotactic Radiotherapy
- WBRT:
Whole Brain Radiotherapy
- RCTs:
Randomized Controlled Trails
- CTV:
Clinical Target Volume
- PTV:
Planning Target Volume
- GTV:
Gross Tumor Volume
- LRFS:
Local Recurrence Progression-free Survival
- SRS:
Stereotactic Radiosurgery
- CT:
Computed Tomogram
- CBCT:
Cone-beam CT
- OS:
Overall Survival
- PFS:
Progression-free Survival
- DRFS:
Distant Recurrence Progression-free Survival
- MRI:
Magnetic Resonance Imaging
- NSCLC:
Non-small Cell Lung Cancer
- RPA:
Recursive Partioning Analysis
- RN:
Radiation Necrosis
- GPA:
Graded Prognostic Index.
Footnotes
Conflict of Interest: None of the authors have any conflict of interest to report.
References
- 1. Sahgal A., Soliman H., Larson D. A. Whole-brain radiation therapy of brain metastasis. Prog Neurol Surg 25, 82–95 (2012). DOI: 10.1159/000331179. [DOI] [PubMed] [Google Scholar]
- 2. Tsao M., Xu W., Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 118, 2486–2493 (2012). DOI: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 3. Tsao M. N., Lloyd N., Wong R. K., Chow E., Rakovitch E., Laperriere N., Xu W., Sahgal A. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 4, (2012). DOI: 10.1002/14651858CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patchell R. A., Tibbs P. A., Regine W. F., Dempsey R. J., Mohiuddin M., Kryscio R. J., Markesbery W. R., Foon K. A., Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. Jama 280, 1485–1489 (1998). DOI: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 5. Kocher M., Soffietti R., Abacioglu U., Villa S., Fauchon F., Baumert B. G., Fariselli L., Tzuk-Shina T., Kortmann R. D., Carrie C., Ben Hassel M., Kouri M., Valeinis E., van den Berge D., Collette S., Collette L., Mueller R. P. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29, 134–141 (2010). DOI: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang E. L., Wefel J. S., Hess K. R., Allen P. K., Lang F. F., Kornguth D. G., Arbuckle R. B., Swint J. M., Shiu A. S., Maor M. H., Meyers C. A. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The Lancet Oncology 10, 1037–1044 (2009). DOI: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 7. Sun A., Bae K., Gore E. M., Movsas B., Wong S. J., Meyers C. A., Bonner J. A., Schild S. E., Gaspar L. E., Bogart J. A., Werner-Wasik M., Choy H. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol 29, 279–286 (2011). DOI: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberge D., Parney I., Brown P. D. Radiosurgery to the post-operative surgical cavity: who needs evidence? International Journal of Radiation Oncology, Biology, Physics 83, 486–493 (2012). DOI: 10.1016/j.ijrobp.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 9. Roberge D., Souhami L. Tumor bed radiosurgery following resection of brain metastases: a review. Technology in Cancer Research & Treatment 9, 597–602 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Follwell M. J., Khu K. J., Cheng L., Xu W., Mikulis D. J., Millar B. A., Tsao M. N., Laperriere N. J., Bernstein M., Sahgal A. Volume specific response criteria for brain metastases following salvage stereotactic radiosurgery and associated predictors of response. Acta oncologica (Stockholm, Sweden: ) (2012). DOI: 10.3109/0284186X.2012.681066. [DOI] [PubMed] [Google Scholar]
- 11. Hartford A. C., Paravati A. J., Spire W. J., Li Z., Jarvis L. A., Fadul C. E., Rhodes C. H., Erkmen K., Friedman J., Gladstone D. J., Hug E. B., Roberts D. W., Simmons N. E. Postoperative stereotactic radiosurgery without whole-brain radiation therapy for brain metastases: potential role of preoperative tumor size. International Journal of Radiation Oncology, Biology, Physics (2012). DOI: 10.1016/j.ijrobp.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 12. Sahgal A., Ma L., Chang E., Shiu A., Larson D. A., Laperriere N., Yin F. F., Tsao M., Menard C., Basran P. S., Letourneau D., Heydarian M., Beachey D., Shukla V., Cusimano M., Hodaie M., Zadeh G., Bernstein M., Schwartz M. Advances in technology for intracranial stereotactic radiosurgery. Technology in Cancer Research & Treatment 8, 271–280 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Kim Y. J., Cho K. H., Kim J. Y., Lim Y. K., Min H. S., Lee S. H., Kim H. J., Gwak H. S., Yoo H. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. International Journal of Radiation Oncology, Biology, Physics 81, 483–489 (2011). DOI: 10.1016/j.ijrobp.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 14. Kim J. H., Khil M. S., Kolozsvary A., Gutierrez J. A., Brown S. L. Fractionated radiosurgery for 9L gliosarcoma in the rat brain. International Journal of Radiation Oncology, Biology, Physics 45, 1035–1040 (1999). DOI: 10.1016/S0360-3016(99)00273-4. [DOI] [PubMed] [Google Scholar]
- 15. Lightstone A. W., Tsao M., Baran P. S., Chan G., Pang G., Ma L., Lochray F., Sahgal A. Cone Beam CT (CBCT) evaluation of inter-and intra-fraction motion for patients undergoing brain radiotherapy immobilized using a commercial thermoplastic mask on a robotic couch. Technology in Cancer Research & Treatment 11, 203–209 (2012). DOI: 10.7785/tcrt.2012.500288. [DOI] [PubMed] [Google Scholar]
- 16. Shaw E., Scott C., Souhami L., Dinapoli R., Kline R., Loeffler J., Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. International Journal of Radiation Oncology, Biology, Physics 47, 291–298 (2000). DOI: http://dx.doi.org/10.1016/S0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 17. Katz J., Knisely J., Ghaly M., Shulder M. Adjuvant tumor bed radiosurgery following surgical resection of intracranial metastases. J Neurooncol Abstract (2012) in press. [Google Scholar]
- 18. Qutob S. S., Multani A. S., Pathak S., McNamee J. P., Bellier P. V., Liu Q. Y., Ng C. E. Fractionated X-radiation treatment can elicit an inducible-like radioprotective response that is not dependent on the intrinsic cellular X-radiation resistance/sensitivity. Radiat Res 166, 590–599 (2006). DOI: http://dx.doi.org/10.1667/RR0514.1. [DOI] [PubMed] [Google Scholar]
- 19. Kim P. K., Ellis T. L., Stieber V. W., McMullen K. P., Shaw E. G., McCoy T. P., D’Agostino R. B., Bourland J. D., DeGuzman A. F., Ekstrand K. E., Raber M. R., Tatter S. B. Gamma Knife surgery targeting the resection cavity of brain metastasis that has progressed after whole-brain radiotherapy. Journal of Neurosurgery 105 (Suppl), 75–78 (2006). DOI: 10.3171/sup.2006.105.7.75. [DOI] [PubMed] [Google Scholar]


