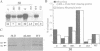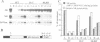Abstract
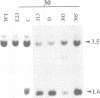
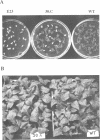
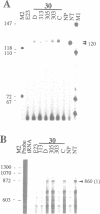
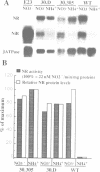
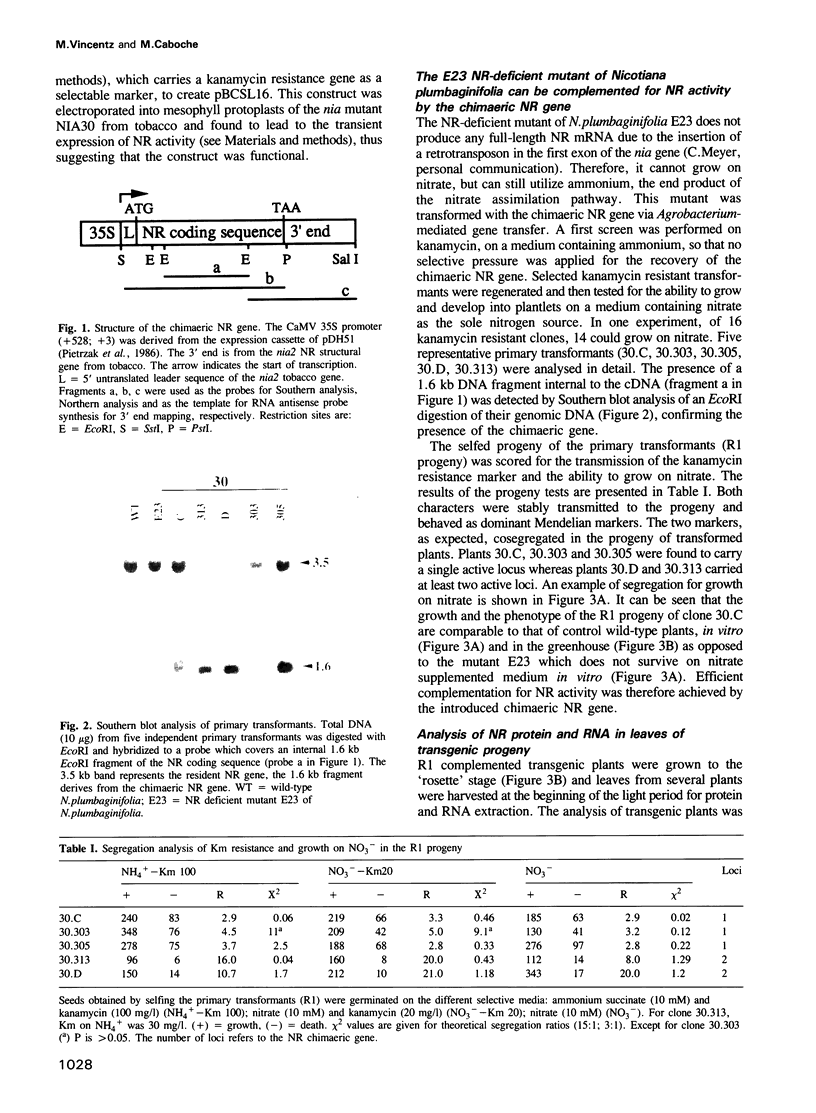
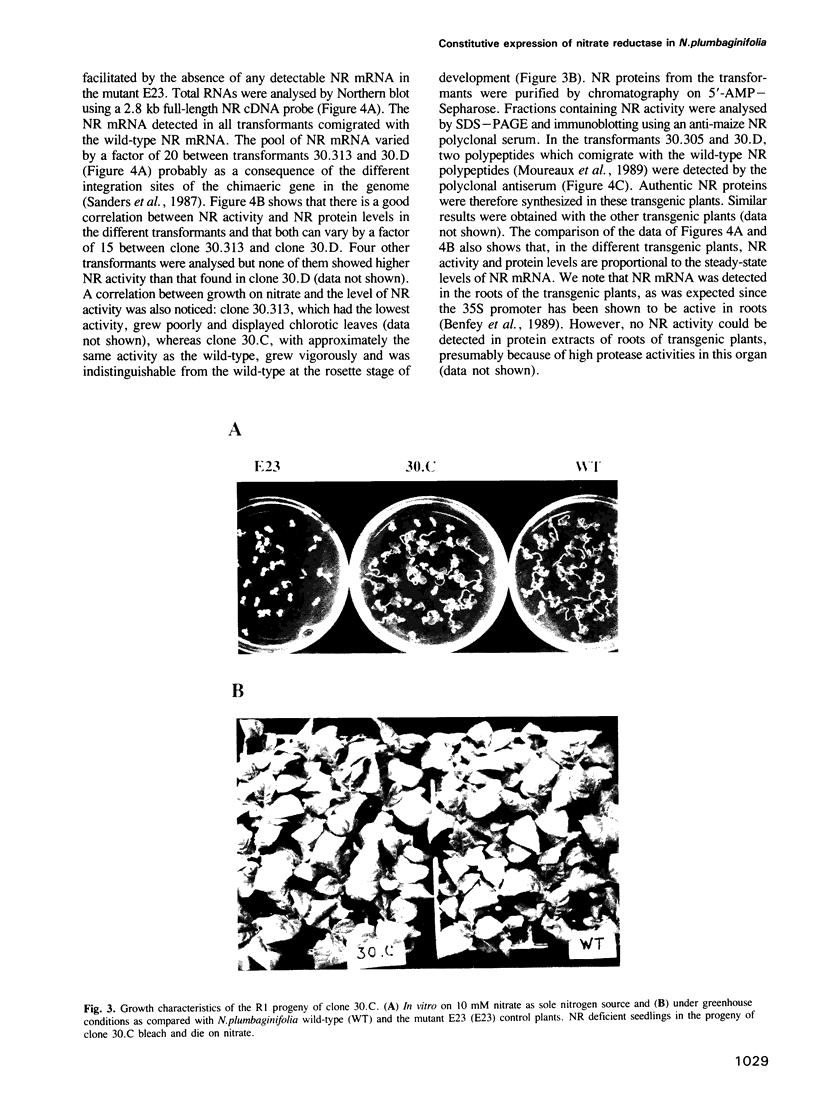
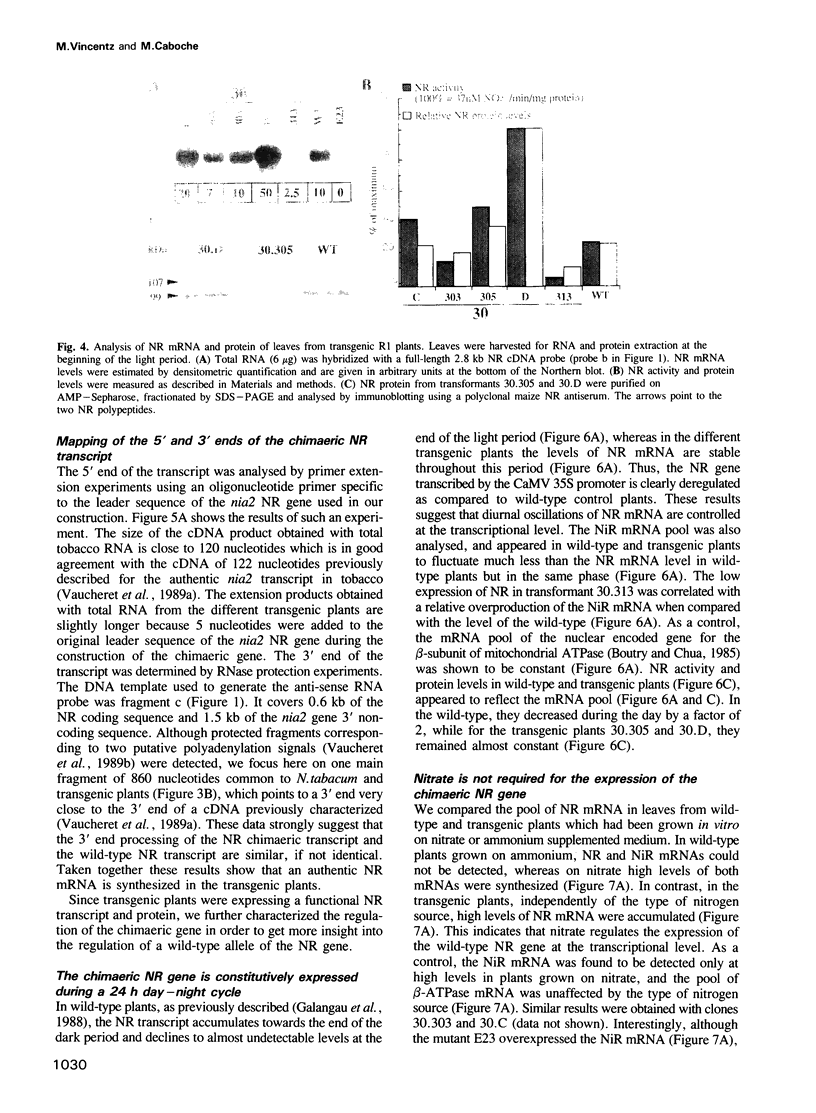
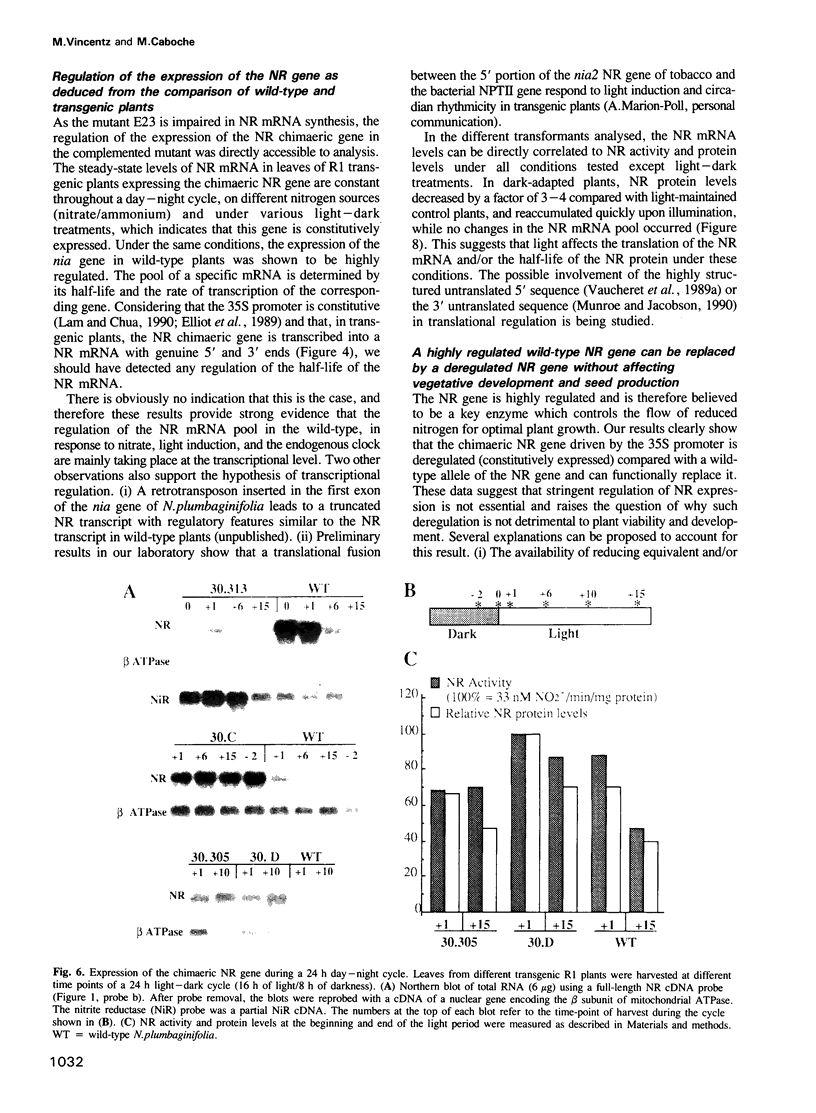
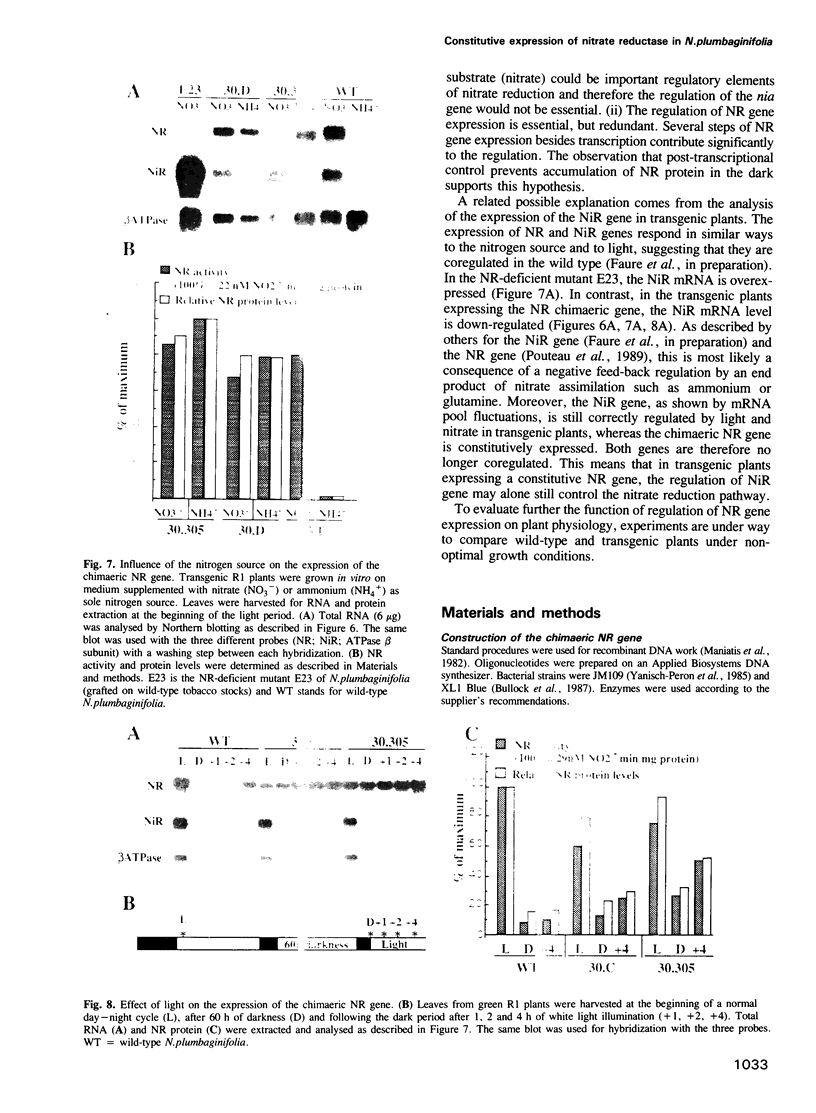
A nitrate reductase (NR) deficient mutant of Nicotiana plumbaginifolia totally impaired in the production of NR transcript and protein was restored for NR activity by transformation with a chimaeric NR gene. This gene was composed of a full-length tobacco NR cDNA fused to the CaMV 35S promoter and to termination signals from the tobacco NR gene. The transgenic plants we obtained were viable and fertile and expressed from one-fifth to three times the wild-type NR activity in their leaves. The analysis of chimeric NR gene expression in these plants showed, by comparison with wild-type plants, that the regulation of NR gene expression by light, nitrate and circadian rhythm takes place at the transcriptional level. However, unlike nitrate, light was required for the accumulation of NR protein in transgenic plants, suggesting that NR expression is also controlled at the translational and/or post-translational level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back E., Burkhart W., Moyer M., Privalle L., Rothstein S. Isolation of cDNA clones coding for spinach nitrite reductase: complete sequence and nitrate induction. Mol Gen Genet. 1988 Apr;212(1):20–26. doi: 10.1007/BF00322440. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Ren L., Chua N. H. The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J. 1989 Aug;8(8):2195–2202. doi: 10.1002/j.1460-2075.1989.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboche M., Rouzé P. Nitrate reductase: a target for molecular and cellular studies in higher plants. Trends Genet. 1990 Jun;6(6):187–192. doi: 10.1016/0168-9525(90)90175-6. [DOI] [PubMed] [Google Scholar]
- Calza R, Huttner E, Vincentz M, Rouzé P, Galangau F, Vaucheret H, Chérel I, Meyer C, Kronenberger J, Caboche M. Cloning of DNA fragments complementary to tobacco nitrate reductase mRNA and encoding epitopes common to the nitrate reductases from higher plants. Mol Gen Genet. 1987 Oct;209(3):552–562. doi: 10.1007/BF00331162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel I., Marion-Poll A., Meyer C., Rouze P. Immunological comparisons of nitrate reductase of different plant species using monoclonal antibodies. Plant Physiol. 1986 Jun;81(2):376–378. doi: 10.1104/pp.81.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Smith M., Bellissimo D., Davis R. W. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5006–5010. doi: 10.1073/pnas.85.14.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. C., Dickey L. F., White M. J., Thompson W. F. cis-Acting Elements for Light Regulation of Pea Ferredoxin I Gene Expression Are Located within Transcribed Sequences. Plant Cell. 1989 Jul;1(7):691–698. doi: 10.1105/tpc.1.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gabard J, Marion-Poll A, Chérel I, Meyer C, Müller A, Caboche M. Isolation and characterization of Nicotiana plumbaginifolia nitrate reductase-deficient mutants: genetic and biochemical analysis of the NIA complementation group. Mol Gen Genet. 1987 Oct;209(3):596–606. doi: 10.1007/BF00331169. [DOI] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Wiebauer K., Filipowicz W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 1990;181:148–161. doi: 10.1016/0076-6879(90)81117-d. [DOI] [PubMed] [Google Scholar]
- Guerche P., Bellini C., Le Moullec J. M., Caboche M. Use of a transient expression assay for the optimization of direct gene transfer into tobacco mesophyll protoplasts by electroporation. Biochimie. 1987 Jun-Jul;69(6-7):621–628. doi: 10.1016/0300-9084(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Kudla B., Caddick M. X., Langdon T., Martinez-Rossi N. M., Bennett C. F., Sibley S., Davies R. W., Arst H. N., Jr The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. Mutations affecting specificity of gene activation alter a loop residue of a putative zinc finger. EMBO J. 1990 May;9(5):1355–1364. doi: 10.1002/j.1460-2075.1990.tb08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. GT-1 binding site confers light responsive expression in transgenic tobacco. Science. 1990 Apr 27;248(4954):471–474. doi: 10.1126/science.2330508. [DOI] [PubMed] [Google Scholar]
- Moureaux T., Leydecker M. T., Meyer C. Purification of nitrate reductase from Nicotiana plumbaginifolia by affinity chromatography using 5'AMP-sepharose and monoclonal antibodies. Eur J Biochem. 1989 Feb 15;179(3):617–620. doi: 10.1111/j.1432-1033.1989.tb14591.x. [DOI] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. Tales of poly(A): a review. Gene. 1990 Jul 16;91(2):151–158. doi: 10.1016/0378-1119(90)90082-3. [DOI] [PubMed] [Google Scholar]
- Pietrzak M., Shillito R. D., Hohn T., Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986 Jul 25;14(14):5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S., Cherel I., Vaucheret H., Caboche M. Nitrate Reductase mRNA Regulation in Nicotiana plumbaginifolia Nitrate Reductase-Deficient Mutants. Plant Cell. 1989 Nov;1(11):1111–1120. doi: 10.1105/tpc.1.11.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar V. K., Gowri G., Campbell W. H. Phytochrome-mediated light regulation of nitrate reductase expression in squash cotyledons. Plant Physiol. 1988 Oct;88(2):242–244. doi: 10.1104/pp.88.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartoris S., Cohen E. B., Lee J. S. A rapid and improved method for generating cDNA libraries in plasmid and phage lambda vectors. Gene. 1987;56(2-3):301–307. doi: 10.1016/0378-1119(87)90148-x. [DOI] [PubMed] [Google Scholar]
- Shih M. C., Goodman H. M. Differential light regulated expression of nuclear genes encoding chloroplast and cytosolic glyceraldehyde-3-phosphate dehydrogenase in Nicotiana tabacum. EMBO J. 1988 Apr;7(4):893–898. doi: 10.1002/j.1460-2075.1988.tb02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R., Matzeit V., Gronenborn B., Schell J., Steinbiss H. H. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987 Jul 24;15(14):5890–5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H., Vincentz M., Kronenberger J., Caboche M., Rouzé P. Molecular cloning and characterisation of the two homologous genes coding for nitrate reductase in tobacco. Mol Gen Genet. 1989 Mar;216(1):10–15. doi: 10.1007/BF00332224. [DOI] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]