Abstract
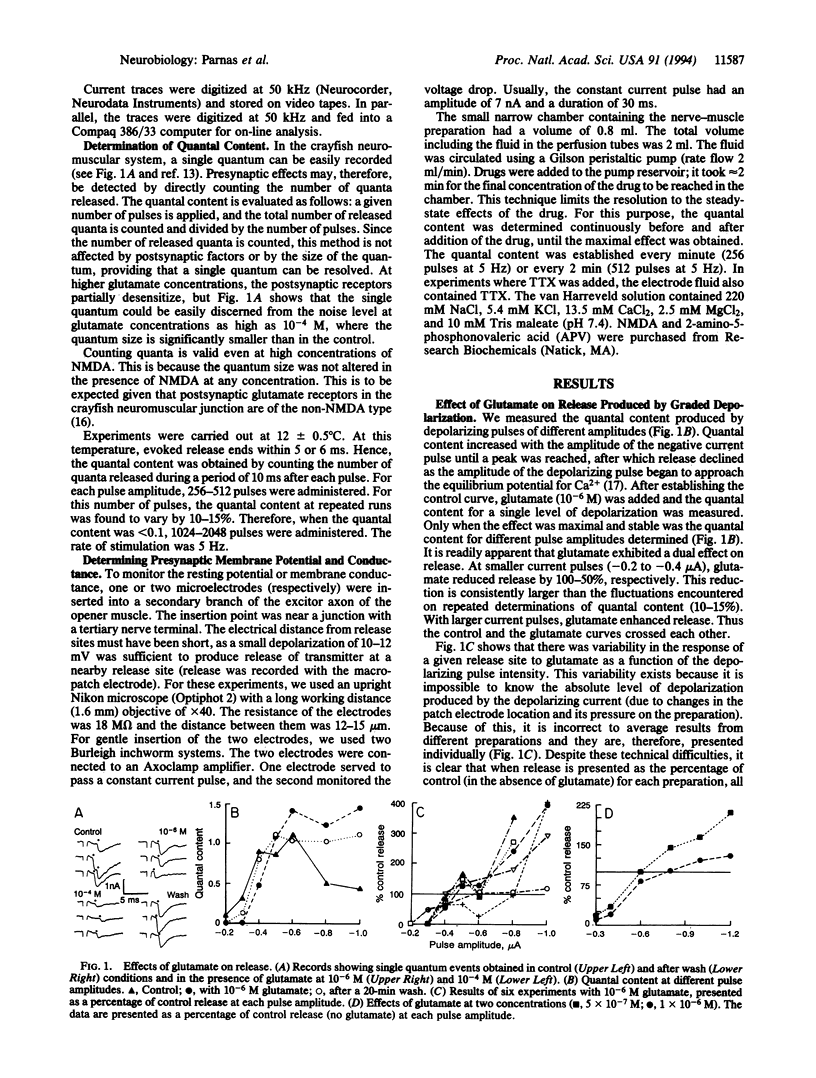
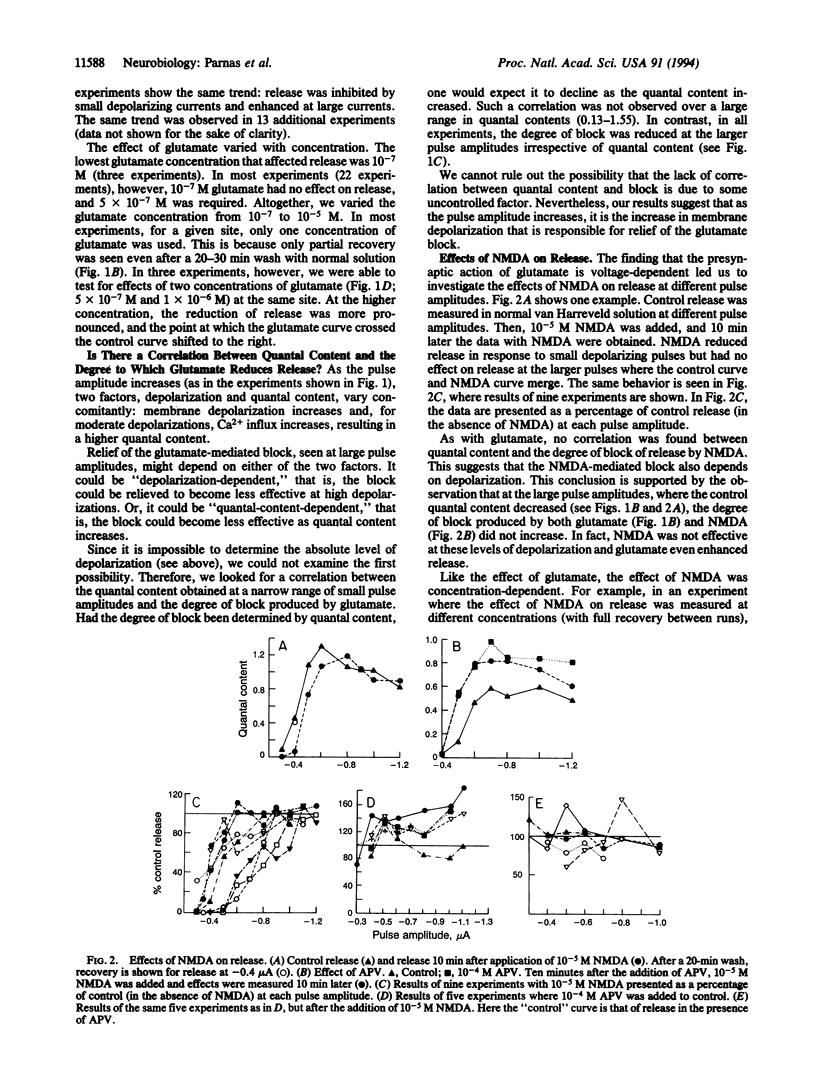
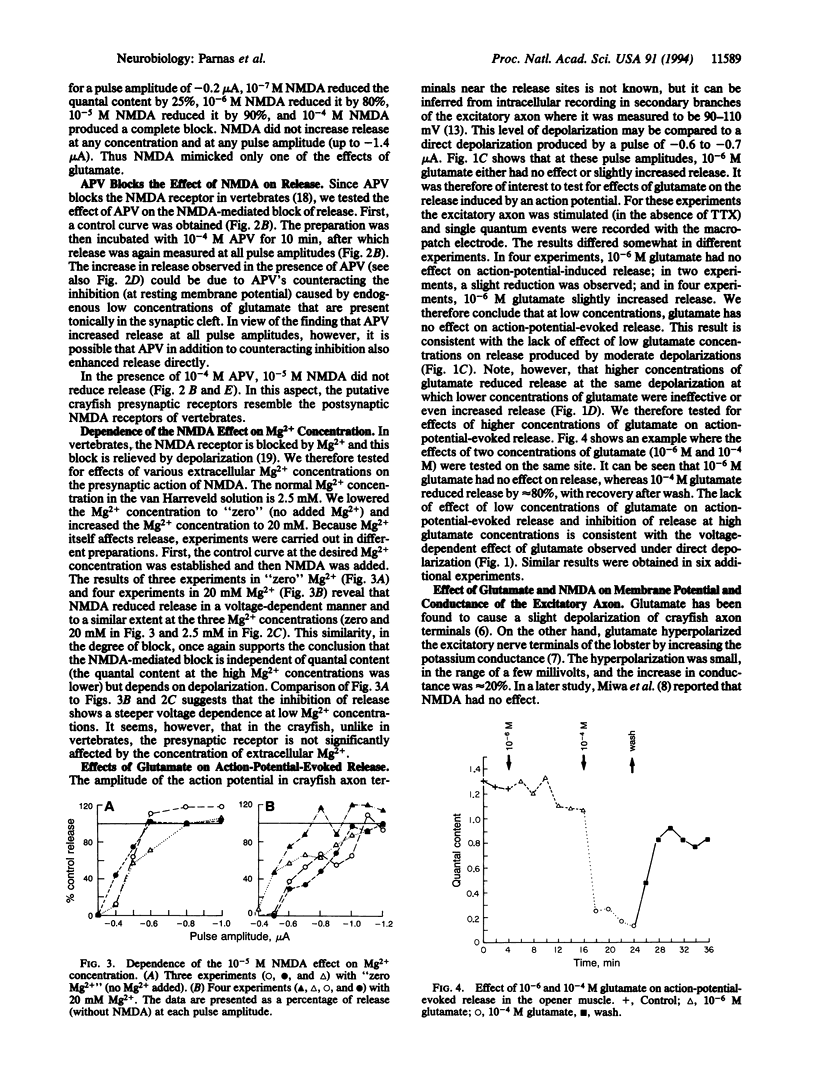
In the crayfish neuromuscular junction, the excitatory transmitter is glutamate. The present study shows that at concentrations as low as 5 x 10(-7) M, glutamate affects the depolarization-evoked release of neurotransmitter. Furthermore, the effect of glutamate on release is voltage-dependent and depends on the level of the depolarizing pulse. Nerve terminals were exposed to 5 x 10(-7) M tetrodotoxin and then depolarized to different levels by a macropatch electrode. Depending on the amplitude of the depolarizing pulse, glutamate (5 x 10(-7) to 1 x 10(-5) M) had a dual effect on release. At small depolarizing pulses, glutamate reduced release, whereas at large depolarizing pulses, it enhanced it. Glutamate at 10(-6) M had no significant effect on action-potential-induced release. At 10(-4) M glutamate, the action-potential-induced release was always inhibited. N-Methyl-D-aspartate was found to mimic one of the effects of glutamate: N-methyl-D-aspartate (10(-7) to 10(-5) M) reduced release at small depolarizing pulses but had no effect with larger depolarizations. 2-Amino-5-phosphonovaleric acid blocked the effect of N-methyl-D-aspartate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arechiga H., Cannone A., Parnas H., Parnas I. Blockage of synaptic release by brief hyperpolarizing pulses in the neuromuscular junction of the crayfish. J Physiol. 1990 Nov;430:119–133. doi: 10.1113/jphysiol.1990.sp018285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Wojtowicz J. M. Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int Rev Neurobiol. 1986;28:275–362. doi: 10.1016/s0074-7742(08)60111-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M., Sokolovsky M. Inhibition of pertussis toxin catalyzed ADP-ribosylation of G-proteins by membrane depolarization in rat brain synaptoneurosomes. Neurosci Lett. 1991 May 13;126(1):87–90. doi: 10.1016/0304-3940(91)90378-7. [DOI] [PubMed] [Google Scholar]
- Dale N., Kandel E. R. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Parnas I., Parnas H. Neurotransmitter release and its facilitation in crayfish. III. Amplitude of facilitation and inhibition of entry of calcium into the terminal by magnesium. Pflugers Arch. 1982 May;393(3):237–242. doi: 10.1007/BF00584076. [DOI] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- Hochner B., Parnas H., Parnas I. Effects of intra-axonal injection of Ca2+ buffers on evoked release and on facilitation in the crayfish neuromuscular junction. Neurosci Lett. 1991 Apr 29;125(2):215–218. doi: 10.1016/0304-3940(91)90032-o. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Bean B. P. G-protein modulation of ion permeation through N-type calcium channels. Nature. 1993 Sep 16;365(6443):258–262. doi: 10.1038/365258a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M., Tyler E., Fidler S., Merritt A. Properties of a presynaptic metabotropic glutamate receptor in rat neostriatal slices. J Neurophysiol. 1993 Apr;69(4):1236–1244. doi: 10.1152/jn.1993.69.4.1236. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Miwa A., Kawai N. Presynaptic glutamate receptor--possible involvement of a K+ channel. Brain Res. 1986 Oct 15;385(1):161–164. doi: 10.1016/0006-8993(86)91559-3. [DOI] [PubMed] [Google Scholar]
- Miwa A., Robinson H. P., Kawai N. Presynaptic glutamate receptors depress inhibitory postsynaptic transmission in lobster neuromuscular synapse. J Neurophysiol. 1993 Sep;70(3):1159–1167. doi: 10.1152/jn.1993.70.3.1159. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Parnas I., Atwood H. L. Phasic and tonic neuromuscular systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol. 1966 Aug;18(4):701–723. doi: 10.1016/0010-406x(66)90206-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C., Glantz R. M. An arthropod NMDA receptor. Synapse. 1991 Sep;9(1):35–42. doi: 10.1002/syn.890090106. [DOI] [PubMed] [Google Scholar]
- Thieffry M., Bruner J. Direct evidence for a presynaptic action of glutamate at a crayfish neuromuscular junction. Brain Res. 1978 Nov 10;156(2):402–406. doi: 10.1016/0006-8993(78)90528-0. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Dun N. J. Excitatory amino acids depress synaptic currents in neonate rat sympathetic preganglionic neurons. J Neurophysiol. 1993 Jun;69(6):2030–2038. doi: 10.1152/jn.1993.69.6.2030. [DOI] [PubMed] [Google Scholar]


