Abstract
Substance use and abuse begins during adolescence. Male and female adolescent humans initiate use at comparable rates, but males increase use faster. In adulthood, more men than women use and abuse addictive drugs. However, some women progress more rapidly from initiation of use to entry into treatment. In animal models, adolescent males and females consume addictive drugs similarly. However, reproductively mature females acquire self-administration faster, and in some models, escalate use more. Sex/gender differences exist in neurobiologic factors mediating both reinforcement (dopamine, opioids) and aversiveness (CRF, dynorphin), as well as intrinsic factors (personality, psychiatric co-morbidities) and extrinsic factors (history of abuse, environment especially peers and family) which influence the progression from initial use to abuse., Many of these important differences emerge during adolescence, and are moderated by sexual differentiation of the brain. Estradiol effects which enhance both dopaminergic and CRF-mediated processes contribute to the female vulnerability to substance use and abuse. Testosterone enhances impulsivity and sensation seeking in both males and females. Several protective factors in females also influence initiation and progression of substance use including hormonal changes of pregnancy as well as greater capacity for self-regulation and lower peak levels of impulsivity/sensation seeking. Same sex peers represent a risk factor more for males than females during adolescence, while romantic partners increase risk for women during this developmental epoch. In summary, biologic factors, psychiatric co-morbidities as well as personality and environment present sex/gender-specific risks as adolescents begin to initiate substance use.
Keywords: Sex, Gender, Addiction, Adolescence, Substance Abuse, Dopamine, CRF, Impulsivity, Self-regulation, Peers
1. Introduction: Sex/Gender Differences in Substance Use and Abuse in Humans
The term sex/gender will be used throughout this review to indicate that both a person’s biologic sex as well as her or her gender role influence the development of substance use or abuse. It will begin with an overview of sex/gender differences in drug use patterns and a description of the emergence of these patterns during development. Then there is a brief review of factors which contribute to the initiation and progression of drug use followed by the main body which describes how sex/gender influence the major factors influencing initiation of substance use. The review focuses on adolescence, as this is the developmental epoch during which substance use begins in human populations. It will emphasize commonalities driven by developmental stage across cultures and geographic location, as a complete exploration of cross-cultural differences in initiation of sex/gender differences in substance abuse is beyond the scope of the present review.
“Adolescence” throughout is considered to be the developmental epoch between childhood and adulthood (Spear, 2000). It is the development period when animals attain their adult physical potential, become reproductively competent and undergo the final stages of cognitive development. Puberty occurs during adolescence and profoundly influences the physical and cognitive changes that occur during this time (Blakemore, Burnett, & Dahl, 2010; Romeo, 2003; Romeo, Richardson, & Sisk, 2002; Sisk & Foster, 2004; Veldhuis et al., 2005), However, it is only a component of the changes that occur. For human, this occurs between 11–14 in females, and slightly later, between 13–16 in males (Parent et al., 2003). Similarly in rodents, pubertal development in female rodents occurs abruptly at about a month of age when females have their first estrus cycle, while males gradually attain adult reproductive capacity gradually over the second month of life (Ojeda, Andrews, Advis, & White, 1980). Many behavioral and neurobiologic changes that influence the initiation and progression of substance use occur during adolescence, and a primary goal of this review is to describe which of these are influenced importantly by the endocrine changes associated with puberty.
2. Sex Differences in Substance Use and Abuse in Humans
According to the most recently published National Household Survey on Drug Use and Health (2013), men in the United States use more of every category of psychoactive drug than women, including alcohol, tobacco, marijuana, cocaine, methamphetamine, prescription stimulants, heroin and pain relievers ("National Household Survey on Drug Use and Health," 2013). Differences are greatest at high levels of consumption: two to three times as many males as females report alcohol, marijuana, stimulant or narcotic abuse or dependence. Disparities are smallest for use of pain relievers and prescription stimulants, for which men and women report close to the same amount of use. Men consume more alcohol than women and they are disproportionately represented in the highest drinking fraction of the population (Holdcraft & Iacono, 2002; "National Household Survey on Drug Use and Health," 2013; Wilsnack et al., 2000). Men are more likely to become alcohol dependent than women, although rates of alcohol dependence are equalizing (Grant et al., 2004; Health, 2005; Kerr, Greenfield, Bond, Ye, & Rehm, 2009; Keyes, Grant, & Hasin, 2008; Simons-Morton et al., 2009; York, Welte, Hirsch, Hoffman, & Barnes, 2004).
The gender disparity in drug and alcohol use is not so obviously male dominated as it appears. These numbers are equalizing in younger age cohorts world-wide (Degenhardt et al., 2008; Geels et al., 2013; Johnson & Gerstein, 2000; Kerr et al., 2009; Keyes et al., 2008; Pitel, Geckova, van Dijk, & Reijneveld, 2010; York et al., 2004) and gender differences are somewhat exaggerated in college students, an over-sampled population, compared to non-college attending late adolescents (Bingham, Shope, & Tang, 2005; White, Kraus, & Swartzwelder, 2006). Although women on average drink fewer drinks at a time, they attain higher blood ethanol concentrations (BECs) for a given dose of alcohol even when dose is adjusted for body weight (Frezza et al., 1990; Mumenthaler, Taylor, O'Hara, & Yesavage, 1999; Whitfield & Martin, 1994). In the few studies which have evaluated BEC in people who are drinking voluntarily, women and men drank to comparable BECs (York, Welte, & Hirsch, 2003; York & Welte, 1994). Although more men than women smoke cigarettes, women on average smoke more cigarettes than men, have more trouble quitting and experience more intense withdrawal symptoms (O'Dell & Torres, 2014)
These drug use statistics parallel reports of sex/gender differences observed in laboratory studies of self-administration and subjective effects of drugs in humans. In laboratory studies, men reported more positive subjective effects of psychostimulants, cannabinoids and narcotics than women (Anker & Carroll, 2011; Becker & Hu, 2008; Carroll & Anker, 2010; Carroll, Lynch, Roth, Morgan, & Cosgrove, 2004; Evans & Foltin, 2010; Evans, Haney, Fischman, & Foltin, 1999; Fattore, Altea, & Fratta, 2008; Fattore, Fadda, & Fratta, 2009; Lynch et al., 2008; Lynch, Roth, & Carroll, 2002; Roth, Cosgrove, & Carroll, 2004; Vansickel, Stoops, & Rush, 2010). Reports of sex/gender differences in the reinforcing effects of nicotine and its self-administration in laboratory settings are mixed (O'Dell & Torres, 2014; Perkins, Donny, & Caggiula, 1999) and both men and women with family histories of alcohol dependence or current alcohol dependence report lower sensitivity to alcohol than non-users (Nolen-Hoeksema & Hilt, 2006; Schuckit, Smith, & Kalmijn, 2004). Menstrual cycle influences the subjective effects of psychostimulants (lower responses during the luteal phase) but such effects are not reported for nicotine, narcotics, marijuana or alcohol (Evans & Foltin, 2010; Fattore et al., 2008; Fattore et al., 2009; Mumenthaler, O'Hara, Taylor, Friedman, & Yesavage, 2001; Niaura, Nathan, Frankenstein, Shapiro, & Brick, 1987; Terner & de Wit, 2006).
Trajectories of drug use and alcoholism may be different in men and women. The phenomenon of “telescoping” of the time from initiation to problematic use and entrance into treatment in women has been reported for numerous drugs including alcohol, psychostimulants, narcotics and cannabis (Brady & Randall, 1999; Ehlers et al., 2010; Greenfield, Back, Lawson, & Brady, 2010; Haas & Peters, 2000; Hernandez-Avila, Rounsaville, & Kranzler, 2004; Khan et al., 2013; Lynch et al., 2002; Mann et al., 2005; Piazza, Vrbka, & Yeager, 1989; Randall et al., 1999; Schuckit, Daeppen, Tipp, Hesselbrock, & Bucholz, 1998). This phenomenon may not be present in younger age cohorts (Alvanzo et al., 2011; Johnson, Richter, Kleber, McLellan, & Carise, 2005; Keyes, Martins, Blanco, & Hasin, 2010; Zilberman, Tavares, & el-Guebaly, 2003) and may reflect early transition to treatment seeking but not necessarily early escalation of use (Lewis & Nixon, 2014). Age of onset of drinking alcohol and perhaps other drug use has shifted earlier in recent years in women but not men (Grucza, Norberg, Bucholz, & Bierut, 2008). Even among women who started later, some studies show that they accelerate drinking more rapidly than men (Bohman, Cloninger, Sigvardsson, & von Knorring, 1987; Cloninger, Sigvardsson, & Bohman, 1988; Gilligan, Reich, & Cloninger, 1987; Lovallo, Yechiam, Sorocco, Vincent, & Collins, 2006; Piazza et al., 1989; Randall et al., 1999; Schuckit et al., 1998; Schuckit et al., 2001; Tarter et al., 1999) and may show more persistence once they are diagnosed (Edens, Glowinski, Grazier, & Bucholz, 2008).
In summary, in human populations, men use addictive drugs more than women in adulthood, and the sex difference is greatest at the highest levels of consumption. However, these differences vary by age cohort: the differences are smallest at the youngest ages, and greatest from young adulthood into old age. One question that is undergoing active study in this field is whether these age differences represent “cohort effects” with younger users reflecting changing cultural attitudes about drug use, especially in women, or stable differences across different stages of life. This will be described in more detail below.
3. Sex Differences in Drug Use in Animal Models
Studies in animal models can evaluate sex differences in psychoactive drug consumption in a context that is not affected by the many social and environmental factors that influence human drug and alcohol consumption. Studies in non-human primates are perhaps most relevant to humans, but not abundant and contradictory. Several studies involving multiple primate species (cynomolgous macaque, rhesus, chimpanzee, orangutan) reported males drank more ethanol than females in a free access setting (Fahlke et al., 2000; Fitzgerald, Barfield, & Warrington, 1968; Vivian et al., 2001) but in another species (vervet), greater consumption by adolescent females than adolescent males was observed (Ervin, Palmour, Young, Guzman-Flores, & Juarez, 1990). However, other studies reported only marginal sex differences in alcohol consumption by non-human primates (Fahlke et al., 2000; Lorenz et al., 2006; Pakarinen, Williams, & Woods, 1999; Vivian, Higley, Linnoila, & Woods, 1999). In most non-human primate studies, individual differences in ethanol consumption and cohort effects were substantial and the subject numbers were small, and so the ability to study endocrine and sex differences was limited. Furthermore, alcohol consumption in non-human primates is also sensitive to social influences like dominance (McKenzie-Quirk & Miczek, 2008).
There have been only a few studies of sex differences in psychostimulant self-administration in non-human primates, but these have shown that females will attain higher progressive ratios (work harder for) cocaine than males (Carroll, Batulis, Landry, & Morgan, 2005; Mello, Knudson, & Mendelson, 2007). Sex differences in cannabinoid and nicotine self-administration in non-human primates have not been studied.
Studies of sex differences in drug-self administration in rodents are more abundant and consistent in the finding that females acquire self-administration faster, escalate use more during extended access and will work harder under a progressive ratio to obtain psychostimulants, nicotine, opioids and tetrahydrocannabinol (Anker & Carroll, 2011; Becker & Hu, 2008; Carroll & Anker, 2010; Carroll et al., 2004; Fattore et al., 2008; Fattore et al., 2009; Feltenstein, Ghee, & See, 2012; Levin et al., 2011; Lynch, 2006; Lynch et al., 2002). Most rodent studies have detected robust sex differences in alcohol consumption (females consume more, especially in mice) although the greater fluid consumption by females must be considered in evaluating reported results. Female mice consistently consume more alcohol than males under a variety of paradigms (Middaugh & Kelley, 1999; Rhodes et al., 2007; Tambour, Brown, & Crabbe, 2008) Most studies of rats report more ethanol intake in females than males, although a number of these studies were confounded by greater overall fluid intake (Blanchard & Glick, 1995; Juarez & Barrios de Tomasi, 1999; Lancaster & Spiegel, 1992; Piano, Carrigan, & Schwertz, 2005; Sluyter, Hof, Ellenbroek, Degen, & Cools, 2000). In rodents, female rats will self-administer lower doses of nicotine and respond more during progressive ratio responding, but as in humans, baseline levels of self-administration are roughly comparable in males and females (Donny et al., 2000; Feltenstein, Ghee, & See, 2012; Lynch et al., 2002; Perkins et al., 2009; Perkins et al., 1999).
In adult rats, the presence of ovarian but not testicular hormones influences self-administration. Numerous studies have shown that estradiol augments and progesterone inhibits self-administration and the reinforcing effects of psychostimulants, ethanol, nicotine and opioids (Anker & Carroll, 2011; Becker & Hu, 2008; Becker, Perry, & Westenbroek, 2012; Carroll & Anker, 2010; Carroll et al., 2004; Donny et al., 2000; Feltenstein et al., 2012; Levin et al., 2011; Lynch, 2009; O'Dell & Torres, 2014; Perkins et al., 1999). In contrast, the presence of testosterone was reported to have no effect on cocaine self-administration (Caine et al., 2004; Mello, Knudson, Kelly, Fivel, & Mendelson, 2011) self-administration of morphine (Cooper & Wood, 2014). However, testosterone could contribute to drug taking through actions on impulsive or risk-taking behavior rather than directly affecting the reinforcing properties of the drugs. There is evidence in rats that testosterone does not directly influence impulsivity in go/nogo tasks or preference for immediate vs. delayed reward, but that it does increase responding for a “risky” (large but punished) reward (Cooper, Goings, Kim, & Wood, 2014; Kritzer, Brewer, Montalmant, Davenport, & Robinson, 2007).
4. Emergence of Sex/Gender Differences in Drug Use during Development in Humans
The average age of initiation of tobacco use is 17.3 years, of alcohol is 17.8 years, of marijuana is 18, use of MDMA, stimulants and heroin between 20–22 ("National Household Survey on Drug Use and Health," 2013) Ages of initiation of tobacco use, cannabis and alcohol are fairly similar in males and females, with “early initiators” beginning ages 12–14, with a continuous rise in numbers of users to the early 20’s. No invariant sequence of initiation exists currently, in contrast to earlier generations in which tobacco and alcohol use typically preceded cannabis use. The rate of alcohol consumption among young adults from 18–25 is about 60%, and young adults constitute the highest proportion of heavy drinkers (Greenfield & Rogers, 1999). Similarly, young adults constitute the highest percentage of tobacco users, with about 37% reporting some use of tobacco products.
Sex differences in initiation ages and use are small to negligible in the youngest adolescents in both cross sectional and longitudinal studies of drug use initiation in humans. In the rather broad 12–17 age group, NHSDUH data show that smoking, alcohol use and cannabis use are about comparable in males and females in the United States. The youngest adolescent girls (age 14–15) drink as frequently and as much as boys (McPherson, Casswell, & Pledger, 2004). Sex differences in cannabis initiation are small at the youngest ages (13–14) and become greater as youth approach the end of high school and boys begin to use more than girls (Schepis et al., 2011). In two large nationwide samples, the National Longitudinal Study of Adolescent Health (AddHealth) and the Adolescent Health Risk Study (AHRS), levels of smoking rose from the earliest age in the study (13–14) until 18, but levels of alcohol consumption increased more rapidly in males than females (Jackson, Sher, Cooper, & Wood, 2002). A community based study in Colorado showed rates of tobacco and alcohol use in males and females was similar up to age 18 (Young et al., 2002). Studies of middle- and high-school youth show that nonmedical use of prescription narcotic use is one of the few categories in which adolescent females may exceed males (Cranford, McCabe, & Boyd, 2013; McCabe, Boyd, & Teter, 2009; McCabe, West, & Boyd, 2013). By late adolescence (over 18), most studies show that men drink more frequently, and drink more in a given drinking episode than women, and male dominance in alcohol abuse is emerging (Palmer et al., 2009; White et al., 2006). Similarly, male rates of smoking, as well as use of and dependence on alcohol and marijuana use begin to exceed female rates in older age cohorts (over 17) (Hicks et al., 2007; Palmer et al., 2009).
Pubertal development represents a significant risk factor in the initiation and progression of substance use in adolescent humans, especially females. Early pubertal development is associated with early initiation of tobacco, alcohol and marijuana use (Cance, Ennett, Morgan-Lopez, Foshee, & Talley, 2013; Copeland et al., 2010; Patton et al., 2004; Windle et al., 2008) A recent study reported that early pubertal maturation predicts alcohol use in both boys and girls, and also predicts alcohol use disorders specifically in girls (Costello, 2007). Multiple factors likely contribute to this association, including biologic, social and environmental factors that will be discussed below.
5, Emergence of Sex/Gender Differences in Drug Use during Development in Animal Models
Animal studies can elucidate the important roles of biologic factors like pubertal hormone changes, brain maturation and pharmacokinetic and pharmacodynamic effects of drugs in the emergence of sex differences in addictive drug consumption. The vast majority of such studies address issues related to biological sex, not gender. However, there is some interesting emerging evidence about the influence of social environment on drug taking in males and females that may constitute a modest inroad in understanding drug taking and non-human “gender.” A small but growing number of animal studies address social and environmental factors like previous stress, the presence of peers and availability of alternative behaviors like the opportunity to exercise. However, such studies are limited. In most studies, drug taking occurs in a highly structured and socially impoverished environment in comparison to human life, and such studies provide less insight about more complex human social and environmental factors that contribute to drug taking.
The vast majority of animal studies which have investigated the establishment of sex differences in addictive drug vulnerability have studied either rates of drug self-administration (the most direct analogy to the human drug taking), measures of the reinforcing or aversive effects of drugs using methods like conditioned place preference and conditioned taste or place aversion, or the sensitivity to behavioral effects of administered drug., There are numerous studies with psychostimulants and nicotine, but many fewer with ethanol, marijuana or narcotics.
Nicotine self-administration in rats during adolescence is greater than in adulthood in both sexes, but females maintain drug taking into adulthood while males decrease consumption (Levin et al., 2007; Levin, Rezvani, Montoya, Rose, & Swartzwelder, 2003; Levin et al., 2011). Adolescent but post-pubertal female rats (postnatal age 40–45) acquired nicotine self-administration at lower doses and attained higher break points than males of comparable age (Lynch, 2009). Adolescent female mice consumed more nicotine on a mg/kg basis in two bottle choice paradigms, although pharmacokinetic factors may have influenced drug consumption as plasma cotinine (the primary nicotine metabolite) levels were comparable in males and females (Klein, Stine, Vandenbergh, Whetzel, & Kamens, 2004; Nesil, Kanit, Collins, & Pogun, 2011). The emergence of sex differences in cocaine self-administration in rats showed a pattern similar to that of nicotine during adolescence: postpubertal females acquired at lower doses, exhibited higher breakpoints and escalated use more in one study, (Lynch, 2008) although another reported minimal sex differences in adolescents (Kantak, Goodrich, & Uribe, 2007). Female adolescent rats self-administered more amphetamine than adolescent males, a sex difference which persisted into adulthood (Shahbazi, Moffett, Williams, & Frantz, 2008).
While adult female rodents in most reports consume more alcohol on a mg/kg basis than males, studies in adolescents are inconsistent. The Spear group found that adolescent males drank more ethanol than adolescent females while adult females drank more than adult males (Vetter-O'Hagen, Varlinskaya, & Spear, 2009; Vetter-O'Hagen & Spear, 2011). A study from our lab using every other day drinking did not detect the emergence of a significant sex difference (Schramm-Sapyta et al., 2014). Female adolescent mice consumed more ethanol than males at the earliest age tested (PN 28) and the difference increased in age (Tambour et al., 2008). However, a similar pattern observed in adults in this study led authors to conclude that adolescence/puberty may not have been the key influence, but that females increased consumption more over time. A similar pattern of gradually exaggerating sex differences during adolescence was reported in rats (Lancaster, Brown, Coker, Elliott, & Wren, 1996), although no adult control was conducted in this study. There are virtually no studies exploring the emergence of sex differences in self-administration of cannabinoids, narcotics or sedative-hypnotics, a significant gap in the field.
Reinforcing effects of psychostimulants, nicotine and alcohol as assessed with conditioned place preference exhibit a similar development of sexual dimorphism during puberty in both mice (Balda et al., 2006; Roger-Sanchez et al., 2012) and rats (Balda et al., 2006; Edwards et al., 2014; O'Dell & Torres, 2014; Zakharova, Wade, et al., 2009). In general, no sex differences are observed pre-pubertally, but greater CPP (or CPP at lower doses) emerge in females relative to males after puberty.
A growing literature investigating the effects of drugs of abuse on social behavior in rodents and the much smaller literature about the modulation of drug-taking by peers demonstrates that drugs influence social behavior and that peers influence drug taking, especially in adolescence. The most evidence exists for alcohol. Numerous studies by the Spear lab and others demonstrate that ethanol has developmentally specific effects on social behavior in rats: specifically that ethanol facilitates social interactions more in adolescents and cause social inhibition less in adolescents than adults (see recent review in (Varlinskaya & Spear, 2014)). These effects occur roughly comparable in males and females at the young ages at which most studies were conducted. Sex differences in these effects are not marked. Alcohol not only influences social behavior, but experience with alcohol-intoxicated peers influences alcohol consumption in a sex and developmentally specific way in rats. Experience with an alcohol-intoxicated familiar rats leads to greater alcohol consumption by both adult males and females and adolescent females, but adolescent males avoid alcohol after such experience.
Peers also affect nicotine self-administration in adolescence. In a study of nicotine self-administration in the presence of olfacto-gustatory clues, both males and females only self-administered nicotine when partnered with a peer that consumed the cue (but not the nicotine) (Chen, Sharp, Matta, & Wu, 2011). Both nicotine and cocaine also can enhance the rewarding value of peers in adolescents rats (Thiel, Okun, & Neisewander, 2008; Thiel, Sanabria, & Neisewander, 2009), although the influence of sex was not considered in these studies.
This emerging literature is at least exploring how psychoactive drugs influence adolescent rodents in social settings, and the evidence indicates that at least alcohol, nicotine and cocaine enhance social interactions or social reward, and can promote drug consumption by peers. In situations in which this has been studies, effects were comparable in males and females, but all rodent studies have employed only same-sex social partners.
In summary, the greater consumption of nicotine, cocaine, amphetamine and alcohol by female rodents has been observed most frequently after females have completed puberty, although some studies report sex differences even before puberty, during early adolescence. One critical control missing from some studies was a comparable adult control group. However, two studies with ethanol suggested that some of the acceleration in drug consumption in adolescent females represents progression of consumption that can be observed at any age, raising the possibility that sex-specific neuroadaptations play a role in the emergence of sex differences at any age. This factor could play a role in the “telescoping” effect noted above even in adult populations.
5. Emergence of Sex/Gender Differences in Drug Taking: Summary of epidemiologic and animal studies
In summary, both animal and human studies describe some common phenomena: a similarity of drug action and drug taking in males and females before puberty with divergence in drug taking post-pubertally for some but not all drugs. One startling contrast between human and animal studies is that adult female rodents consistently acquire drug self-administration sooner and escalate drug use more than males, while in human populations, use is about equal in males and females in early adolescence, but more males than females use addictive drugs as they progress from adolescence to adulthood. These differences are not perhaps as stark as they seem, nor do they invalidate the animal models. Some of the characteristics exhibited by mature female rodents that appear during adolescence (rapid acquisition) are mirrored in a number of human studies showing faster escalation in women users once they start Second, drug taking in animal studies is dominated by biologic factors like the rewarding effects of drugs which are likely exaggerated in the typical impoverished environment of rodent studies. As we will review below, strong social and environmental factors exert sex/gender-specific effects in human populations. Investigation of social factors influencing drug taking in animal studies are sparse, and cannot replicate human culture.
Finally, pregnancy exerts a strong influence on drug taking in human females. Alcohol, cigarette and marijuana use are dramatically lower in pregnant humans than non-pregnant humans, and after childbirth, substance use does not reach previous levels (binge alcohol and marijuana use remain less than half that of women with no children) (SAMSHA, 2009). Unfortunately, similar data are not available for men. A provocative animal study showing that cocaine self-administration diminished dramatically in pregnant rodents, only to resume after pregnancy, suggests biological factors contribute to decreased consumption by women during pregnancy (Hecht, Spear, & Spear, 1999). However, mixed data on consumption of alcohol by high-drinking rat and mouse strains during pregnancy has been published with some studies showing no change and some studies showing decreases (Brady, Allan, & Caldwell, 2012; Kleiber, Wright, & Singh, 2011; Wolfe, Means, & McMillen, 2000). As pregnancy/onset of family responsibilities occurs only after puberty, this could contribute to the emergence of sex/gender differences in drug taking that will be explored below.
6. Sex/Gender Differences in the Neurobiology of Addiction
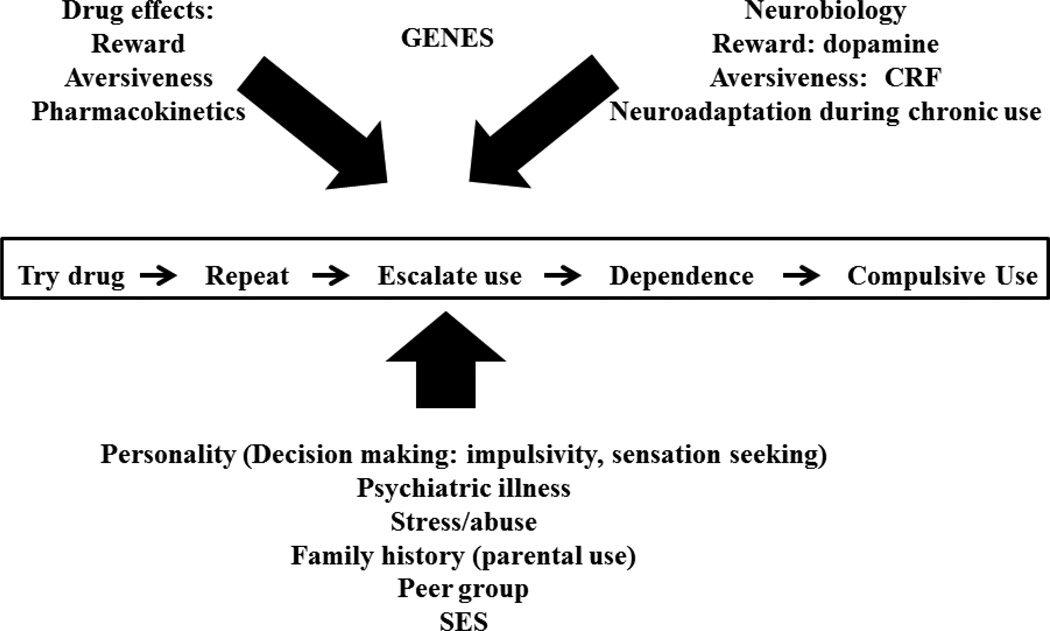
In order to explain the role of neurodevelopmental events in the initiation and progression of substance use and abuse during adolescence, it is necessary to provide a brief overview of the different stages of addiction, and what is known about sex/gender differences in these factors. As the literature in this area is vast, this review has relied on a number of outstanding, recent reviews in this area for the sake of brevity. Figure 1 shows a simplified view of the sequence of substance use which progresses to compulsive use and the many intrinsic and extrinsic factors that affect this progression. An individual must first experiment and then repeat use of drug. A subset of individuals will gradually escalate use to the point that the underlying neuroadaptations induced by drug exposure will manifest as dependence and compulsive use.
Figure 1.
Factors In Addiction
Biologic factors which influence the progression of drug use include the extent to which an individual finds drugs reinforcing or aversive and the contribution that pharmacokinetics/pharmacodynamics influence drug effects within an individual. The neurobiology of the reward system including dopamine and opioid systems that mediate the reinforcing effects of addictive drugs, and the importance of the CRF/dynorphin systems that signal increasing drug aversiveness during escalated drug taking are prominent neurobiologic adaptations that contribute to the progression of drug use (Koob, 2010; Koob et al., 2014; Koob & Le Moal, 2001; Taber, Black, Porrino, & Hurley, 2012; Zorrilla, Logrip, & Koob, 2014).
Genetic vulnerability clearly plays a role in addiction, although the ideal placement of this factor in this figure would be orthogonal to the figure, as genetics certainly affects both biologic and non-biologic issues in substance abuse. This is a small but growing area. Numerous genes including cytochrome 2A6, which degrades nicotine, genes related to dopamine function (especially monoamine oxidase which is X-linked), as well as opiate GABA and glutamate receptors are involved (Ahijevych, 1999; Becker & Hu, 2008; Bobzean, DeNobrega, & Perrotti, 2014; Enoch, 2003; Enoch & Goldman, 2001; Kreek et al., 2012; Nielsen & Kreek, 2012; Reed, Butelman, Yuferov, Randesi, & Kreek, 2014; Satarug, Tassaneeyakul, Na-Bangchang, Cashman, & Moore, 2006; Seeman, 2009; Tsankova, Renthal, Kumar, & Nestler, 2007). As our understanding of how these genetic influences contribute to the emergence of sex differences in substance use and abuse is marginal, we will not consider this important topic further.
Many intrinsic and extrinsic factors influence whether an individual progresses along this continuum, as shown in the bottom panel. Intrinsic factors include the personality/neurobiology of the individual which influences the likelihood of initiation and progression of substance use, as well as psychiatric comorbidities. Extrinsic factors include social environment (family and peers), previous stress or abuse and engagement in “protective” activities like religious groups. Sex/gender differences in all of these factors have been identified in substance-using populations.
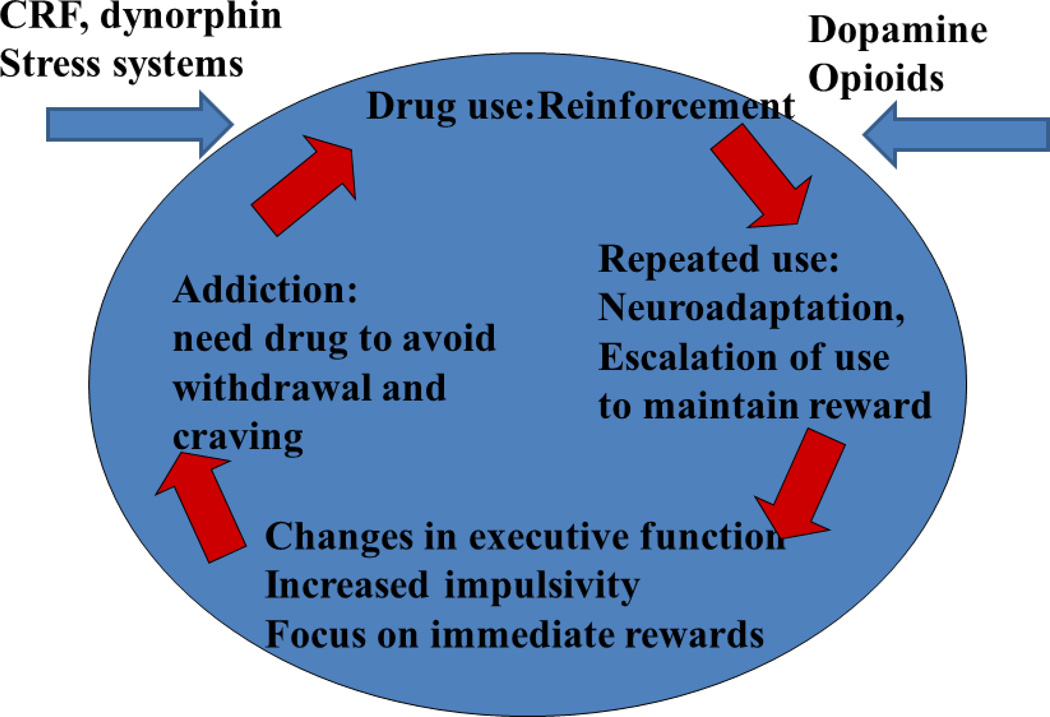
Figure 2 depicts the key neurobiologic components of the cycle of addiction. The cycle begins at the top with initiation of drug use, followed (in a proportion of users) by repeated use and gradual escalation of use. During this phase of escalated use, executive function (which may be deficient before initiation) becomes further biased toward immediate rather than delayed rewards and impulsive choice rather than appropriate self-regulation. As use escalates and tolerance develops, withdrawal symptoms begin to emerge. During the final and potentially most intractable stage of addition, drug use is likely driven by the desire to avoid the negative affect and symptoms of withdrawal and to satisfy the craving for drug use more than reinforcement.
Figure 2.
Neurobiology Factors in Addiction
Four neurobiologic systems are particularly important in the evolution of this cycle. Dopamine systems are integral to the reward system, and likely attach salience to reinforcement-predictive stimuli including drugs. Endogenous opioid systems also play a significant role in the reinforcing effects of drugs of abuse. While dopamine may signal “salience” of drug rewards, opioid systems in dorsal and ventral striatum as well as the ventral tegmental area contribute to the “consummatory” qualities or satisfaction provided by drug consumption (Becker et al., 2012; Gianoulakis, 2009; Le Merrer, Becker, Befort, & Kieffer, 2009). The corticotropin releasing factor (CRF) and dynorphin systems drive the negative affect and anhedonic state which significantly control drug taking at later stages (Bari & Robbins, 2013; Bickel & Yi, 2008; George, Le Moal, & Koob, 2012; Koob, 2010; Koob et al., 2014; Koob & Volkow, 2010; Trifilieff & Martinez, 2013; Zorrilla et al., 2014). While some users successfully exit the cycle and maintain prolonged abstinence, stress or repeat drug exposure often trigger relapse and re-initiation of drug use.
Sex/gender differences exist in all four of these key substrates for the addiction cycle. The existence of sex differences in dopamine systems are the best described: both genetic sex and ovarian hormones augment dopamine function in dorsal and ventral striatum and enhance self-administration of psychomotor stimulants, narcotics and alcohol at least in rodents (Becker, 2009; Carroll & Anker, 2010; Di Paolo, 1994; Dluzen & McDermott, 2008; Dow-Edwards, 2010; Gillies & McArthur, 2010; O'Dell & Torres, 2014). Estradiol promotes while progesterone inhibits dopamine release and the reinforcing effects of most addictive drugs (Anker & Carroll, 2011; Becker & Beer, 1986; Becker & Cha, 1989; Becker et al., 2012; Becker & Ramirez, 1981; Becker & Rudick, 1999; Castner, Xiao, & Becker, 1993; Di Paolo, 1994; Walker et al., 2012; Walker, Ray, & Kuhn, 2006; Walker, Rooney, Wightman, & Kuhn, 2000). Estradiol also alters firing rate of dopamine neurons (Chiodo & Caggiula, 1983; Zhang, Yang, Yang, Jin, & Zhen, 2008). The anatomy of dopamine neurons also differs in males and females: female rats are reported to have more dopamine neurons than adults in most (but not all) studies (Johnson, Day, et al., 2010; Johnson, Ho, et al., 2010; McArthur, McHale, & Gillies, 2007). Results in mice differ, with more dopamine neurons reported in a study using quantitative stereology, and no sex differences reported in a study using somewhat less quantitative methods (Johnson, Day, et al., 2010; Johnson, Ho, et al., 2010; Levin et al., 2006; Lieb et al., 1996; McArthur et al., 2007; Sibug et al., 1996) Dopamine receptors have been studied less, and sex differences may be less robust than those observed for presynaptic functions. However, evidence suggests that sex/gender may also influence dopamine receptor number and function. D2 dopamine receptors vary over the menstrual/estrous cycle in both humans and rodents (Levesque & Di Paolo, 1990). Estradiol perhaps through ERβ, augments D2 receptor number (Morissette et al., 2008).
The existence of sex differences in dopamine function in non-human primates and humans is much less studied, contradictory data exist and interpretation of PET studies always are complicated by the ambiguity of whether results reflect higher binding to dopamine-relevant proteins because expression is higher or endogenous release of competing dopamine is lower. However, several studies conclude that basal dopamine production is greater in females than males (Evans & Foltin, 2010; Laakso et al., 2002; Laasko et al., 2002; Martin-Soelch et al., 2011; Pohjalainen, Rinne, Nagren, Syvalahti, & Hietala, 1998; Riccardi et al., 2011; Urban et al., 2010) Like rodents, non-human primates have more dopamine neurons in the ventral tegmental area, (Leranth et al., 2000). Finally, D2 receptors may vary over the menstrual cycle in humans (Munro et al., 2006; Wong et al., 1988) but see (Kaasinen, Nagren, Hietala, Farde, & Rinne, 2001). This enhanced dopamine neuron number and/or function is widely speculated to confer the protection against the development of Parkinson ’s disease in humans (Gillies, Murray, Dexter, & McArthur, 2004; Gillies, Pienaar, Vohra, & Qamhawi, 2014; Smith & Dahodwala, 2014). Similar cyclic differences in D2 receptor occupancy also have been reported in nonhuman primates (Gould, Duke, & Nader, 2014)
Sex differences in dopaminergic function in the prefrontal cortex which is important for executive function are much less studied. The elegant work of Mary Kritzer in rats has shown that sex differences here reflect a significant role of androgen in enhancing dopaminergic innervation and working memory (Kritzer, 2003; Kritzer, 1997, 2000; Kritzer, Adler, & Bethea, 2003; Kritzer, Adler, Marotta, & Smirlis, 1999; Kritzer et al., 2007; Kritzer & Creutz, 2008; Kritzer & Kohama, 1998).
There has been considerably less investigation of underlying sex/gender differences in opioid mediation of reward, although a dominance of dynorphin expression in striatonigral neurons exist in females, and a dominance of enkephalin in striatopallidal neurons in males: how these relate to the role of opioids in drug reinforcement is not at all understood (see review in Becker (Becker et al., 2012). If dynorphin function is eventually shown to be enhanced in females, it could contribute significantly to sex differences in dynorphin-mediated anxiety during withdrawal.
Sexual dimorphism in CRF mechanisms exist across mammalian species including rodents, and humans. Marked sexual dimorphism exists in CRF regulation of HPA axis function and in CRF receptor function both in the brain and pituitary of rats, with females exhibiting greater CRF mediated responses (Bangasser et al., 2010; Bangasser et al., 2013; Bangasser & Valentino, 2012; Dalla et al., 2005; Handa, Burgess, Kerr, & O'Keefe, 1994; Ogilvie & Rivier, 1997; Rivier, 1993, 1999; Valentino, Bangasser, & Van Bockstaele, 2013; Walker, Francis, Cabassa, & Kuhn, 2001). Stress reactivity of the HPA axis is markedly greater in female mice and rats, an effect mediated both by estradiol enhancement and androgen inhibition of HPA axis function (Bale, 2006; Bangasser & Valentino, 2012; Dalla, Pitychoutis, Kokras, & Papadopoulou-Daifoti, 2010; Fernandez-Guasti, Fiedler, Herrera, & Handa, 2012; Fox & Sinha, 2009; Handa et al., 1994; Shansky, 2009; Valentino, Reyes, Van Bockstaele, & Bangasser, 2012; Veldhuis, Sharma, & Roelfsema, 2013). Such sex differences exist but they are smaller and less consistently observed in humans (Veldhuis et al., 2013; Young & Korszun, 2010; Young, 1998). In any species, sex differences at multiple levels including release of CRF, release of ACTH, adrenal sensitivity to ACTH, plasma binding of corticosteroids and corticosteroid action all must be considered (Chrousos, Torpy, & Gold, 1998; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Quinn, Ramamoorthy, & Cidlowski, 2014). Sex differences in the activation of CRF systems during drug withdrawal are poorly studied although some results support an enhanced role of CRF in reinstatement in females (Buffalari, Baldwin, Feltenstein, & See, 2012).
Finally, interest in the role of norepinephrine in both rewarding effects of drugs of abuse and stress-reactivity and relapse is growing (Becker et al., 2012; Curtis, Bethea, & Valentino, 2006), and in rats at least, the noradrenergic neurons in the locus coeruleus exhibit marked sexual dimorphism with females demonstrating extensive dendritic arborization and more NE neurons than males (Bangasser & Valentino, 2012; Bangasser, Zhang, Garachh, Hanhauser, & Valentino, 2011; Curtis et al., 2006; Valentino et al., 2012). Finally, notable sex differences exist in certain aspects of GABA and glutamate function. While differences in GABA and glutamate function may be important for the intoxicating effects of ethanol, studies relating sex differences in GABA and glutamate function to substance use and abuse are sparse. Given the special importance of glutamate adaptations to later stages of addiction, this represents an important gap in the field.
7. Sex Differences in Intrinsic and Extrinsic Factors Contributing to Addiction
The pharmacologic effects of drugs on specific neurotransmitter systems are not the only factors which influence the development of substances abuse. There are significant intrinsic and extrinsic risk factors that men and women share. Behavioral or personality characteristics including high sensation seeking, impulsivity and poor self-regulation are significant risk factors for substance use and abuse in men and women which precede the development of substance use (Bari & Robbins, 2013; Fineberg et al., 2014; Koob et al., 2014; Koob & Volkow, 2010; Schumann et al., 2010; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). Impulsivity/novelty seeking also predict drug self-administration in animal models (Anker, Perry, Gliddon, & Carroll, 2009; Broos, Diergaarde, Schoffelmeer, Pattij, & De Vries, 2012; Carroll, Anker, & Perry, 2009; Dalley et al., 2007; Poulos, Le, & Parker, 1995). Impulsivity not only predicts the subsequent development of substance abuse, but extensive drug exposure also increases impulsivity as shown in rats and non-human primates (Carroll, Mach, La Nasa, & Newman, 2009; Winstanley et al., 2009). While high impulsivity is frequently identified in addict populations, relative normal inhibitory function in long-term abstinent addicts has been interpreted as recovery from drug-induced impulsivity (Bell, Foxe, Ross, & Garavan, 2014; Connolly, Foxe, Nierenberg, Shpaner, & Garavan, 2012; Morie et al., 2014). Although longitudinal studies of recovery would provide more rigorous evidence, these studies suggest that drug-induced impulsivity also occurs in human addict populations.
Sex/gender differences in the behavioral characteristics described above have been observed in healthy populations, even though mediating neural mechanisms are poorly understood Sensation seeking is consistently reported to be greater in males than females (Cross, Copping, & Campbell, 2011; Zuckerman & Kuhlman, 2000). However, sex differences in measures of impulsive choice or impulsive action are more subtle. Both human and rodent females typically show steeper discounting (bias toward immediate not delayed rewards) in the delayed discounting task, a widely used measured of impulsive choice, although some studies indicate human males discount more steeply for actual rewards (Cross et al., 2011; McClure, Podos, & Richardson, 2014; Perry, Nelson, Anderson, Morgan, & Carroll, 2007; Weafer & de Wit, 2014). Female mice also exhibit steeper delay discounting under conditions of mild food deprivation (Koot, van den Bos, Adriani, & Laviola, 2009), In studies of behavioral control or impulsive action, results depend upon the test (go/no vs. stop signal) but on average sex differences in adult humans are modest and task-specific (Garavan, Hester, Murphy, Fassbender, & Kelly, 2006; Weafer & de Wit, 2014). Similarly, in rat studies, females make more premature errors with long intervals in the 5 choice serial reaction time task and more errors in a go/no paradigm, but males show more motor impulsivity in an attentional task (Anker, Gliddon, & Carroll, 2008; Burton & Fletcher, 2012; Jentsch & Taylor, 2003). Several studies document the ability of women to exhibit better inhibitory control than men in the presence of reward. In a study of “desire vs. reason” women exhibited more self-control in the presence of reward (Diekhof et al., 2012)and women show faster inhibitory responses to a rare event (Yuan, He, Qinglin, Chen, & Li, 2008). While performance in individual tasks varies, the trend in studies of inhibitory function show that men are more sensation seeking while women are more punishment sensitive (Cross et al., 2011). Hosseini-Kamkar and Morton (Hosseini-Kamkar & Morton, 2014) reviewed evidence that women are least impulsive during the follicular phase of their menstrual cycle when they are fertile, and suggested that these differences might explain inconsistent findings across studies.
The complex neural circuits encompassing cortical and subcortical regions through which humans and animals select and implement behaviors circumstances are increasingly well understood (Bari & Robbins, 2013; Fineberg et al., 2014; Pattij & De Vries, 2013; Perry et al., 2011), but studies of sex differences in these circuits are rare.
A history of physical and sexual abuse and resultant PTSD is a risk factor for substance abuse (Cottler, Compton, Mager, Spitznagel, & Janca, 1992; Greenfield et al., 2010; Lawson, Back, Hartwell, Moran-Santa Maria, & Brady, 2013). In addition, co-morbidity of substance abuse and other psychiatric disorders including depression, anxiety, conduct disorder or ADHD is common (Charach, Yeung, Climans, & Lillie, 2011; Compton, Thomas, Stinson, & Grant, 2007; Flory & Lynam, 2003; Goldstein et al., 2007; Hasin, Stinson, Ogburn, & Grant, 2007). Significant sex/gender differences in all of these factors exist. Childhood sexual abuse is reported more frequently in female than male substance-abusing populations and is associated with more drug use and higher rates of relapse (Afifi, Henriksen, Asmundson, & Sareen, 2012; Brady & Randall, 1999; Clark et al., 2012; Hyman, Garcia, & Sinha, 2006; Hyman et al., 2008). The incidence of depression, anxiety and bipolar disorder is greater among female than male substance abusers, while the incidence of conduct disorder and ADHD are higher among male than female substance abusers in most studies (although sex/gender neutral studies with ADHD also exist (Compton et al., 2000; Compton, Dawson, Conway, Brodsky, & Grant, 2013; Compton et al., 2007; Zilberman, Tavares, Blume, & el-Guebaly, 2003).
8. Adolescence: The Critical Period for Emergence of Drug Use
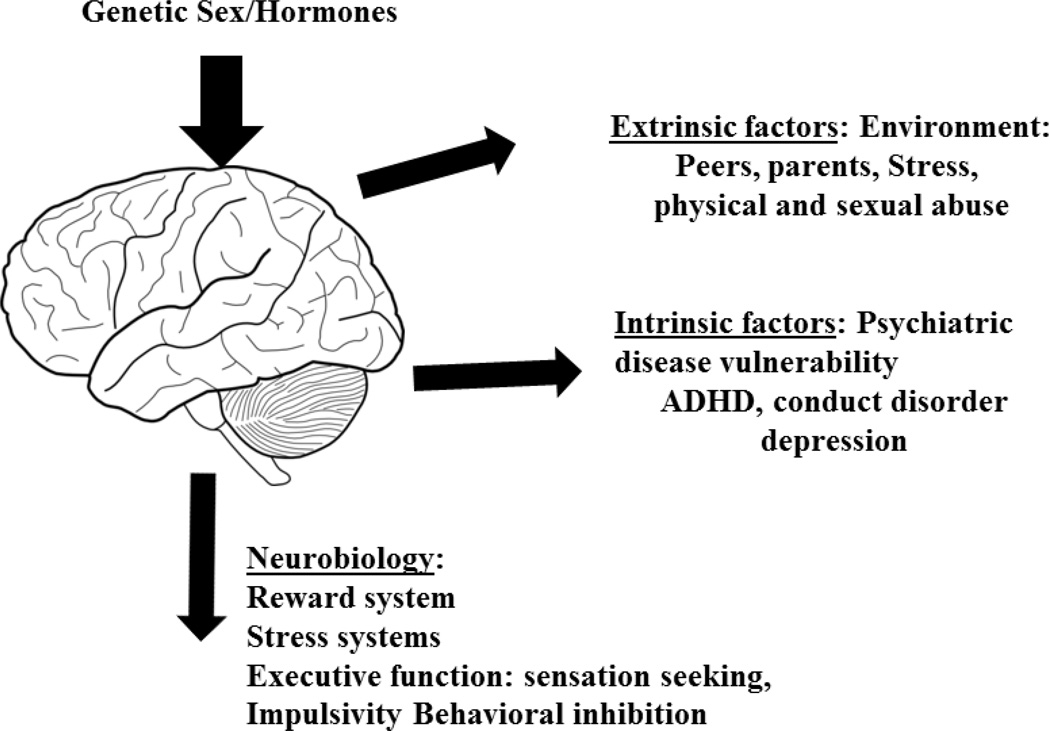
Experimentation with drugs during adolescence is virtually normative (Schramm-Sapyta, Walker, Caster, Levin, & Kuhn, 2009). Nevertheless, most individuals do not become drug-dependent. The characteristics which increase vulnerability to or protect males and females from substance abuse during this developmental transition can differ. For the purposes of understanding the latter, we will focus on events related specifically to substance abuse which exhibit sex/gender differences. The influences on adolescents which impact the initiation of substance use are shown in Figure 3. During this critical developmental epoch, genetic sex and hormones continue to sculpt the final maturation of the brain, leading to the emergence of sex differences in critical behavioral variables including function of the brain areas involved in initiation and progression of substance abuse (the reward system, threat system and executive function), extrinsic variables including peers and family and stress intrinsic variables including the emergence of sex differences in psychiatric co-morbidities including attention deficit hyperactivity disorder (ADHD), conduct disorder and depression. These will be discussed in the following sections.
Figure 3.
Factors Influencing Initiation of Substance Use during Adolescence
The normal neurobehavioral state of the brain which prepares the adolescent to leave the natal family predisposes vulnerable individuals to experiment with and repeat drug use. Adolescents are sensation seeking and impulsive relative to younger and older individuals (Steinberg, 2008; Steinberg et al., 2008; Steinberg et al., 2009), and are converting their social network from being family-centric to peer-centric (RJ., 2005; Smith, Steinberg, Strang, & Chein, 2014; Steinberg, 2008).
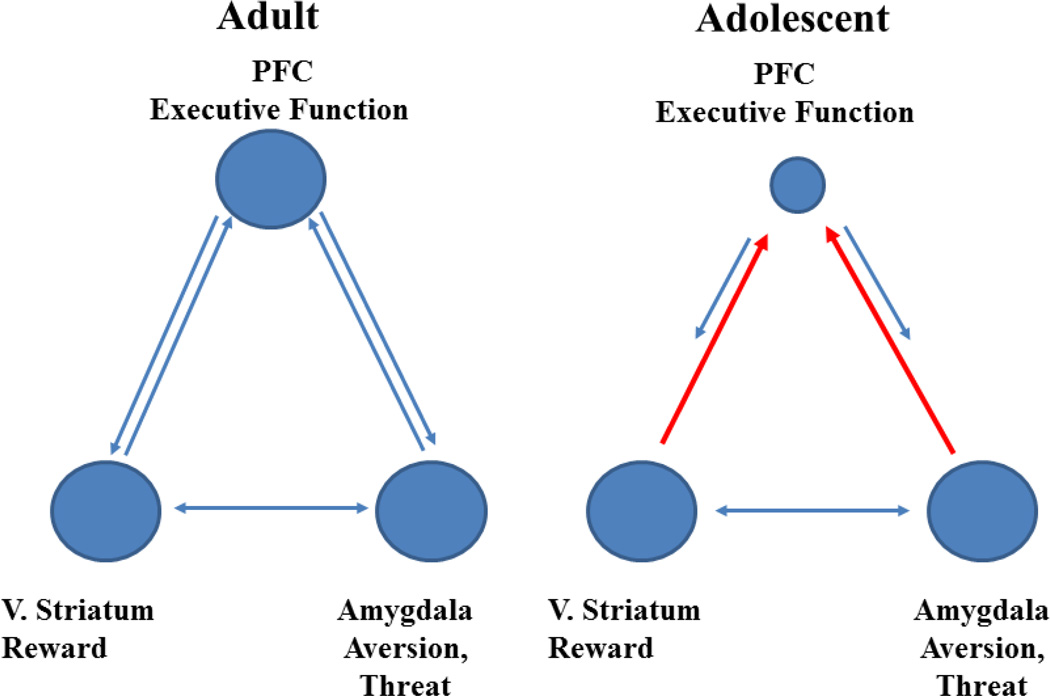
To understand the neural substrates of these developmentally-specific behavior patterns and how they influence experimentation with drugs, Monique Ernst and colleagues have introduced the “triad of motivated behavior” as a model. As shown in Figure 4, prefrontal cortex executive function provides balance for and control over two subcortical influences on behavior: the reward system which drives motivated behavior, and the extended amygdala, which evaluates affective valence and may signal especially information about threat to the cortex (Eldreth, Hardin, Pavletic, & Ernst, 2013; Ernst & Fudge, 2009; Ernst & Korelitz, 2009; Ernst, Pine, & Hardin, 2006; Ernst, Romeo, & Andersen, 2009; Richards, Plate, & Ernst, 2012). All three points of this triangle function differently in adolescents and adults.
Figure 4.
The triadic model of executive function (adapted from (Richards et al., 2012)
The bottom left point of the triangle, reward-related areas, seems to play a more dominant role in decision making in adolescents than adults. B.J Casey has shown that reward-dominated subcortical areas drive decision making during adolescence (Casey, Jones, & Somerville, 2011; Casey, Duhoux, & Malter Cohen, 2010; Casey, Getz, & Galvan, 2008; Casey, Jones, et al., 2010; Chambers, Taylor, & Potenza, 2003). This state may increase the reinforcing efficacy of drugs which activate reward systems. Similarly, the bottom right, the amygdala which responds to threat responds more powerfully to emotional content like fearful faces during adolescence than adulthood, although this response is not consistently accompanied by the behavioral inhibition/avoidance that occurs in adults (Guyer et al., 2008; Hare et al., 2008; Killgore, Oki, & Yurgelun-Todd, 2001). Finally and most importantly, cortical control, the peak of the pyramid, is weaker during adolescence. One reason for this may be that adolescents respond more to salient reward cues relative to adults, and may also respond more to any stimulus with strong emotional content due to weaker control over amygdala processing of environmental stimuli (Ernst, Daniele, & Frantz, 2011; Richards et al., 2012). The development of response inhibition by the cortex exhibits a gradual ontogeny in contrast to the earlier appearance of responses driven by emotional factors, which may explain this developmental trajectory that contributes to addiction risk during adolescence (Blakemore & Robbins, 2012; Brenhouse & Andersen, 2011; Ernst & Fudge, 2009).
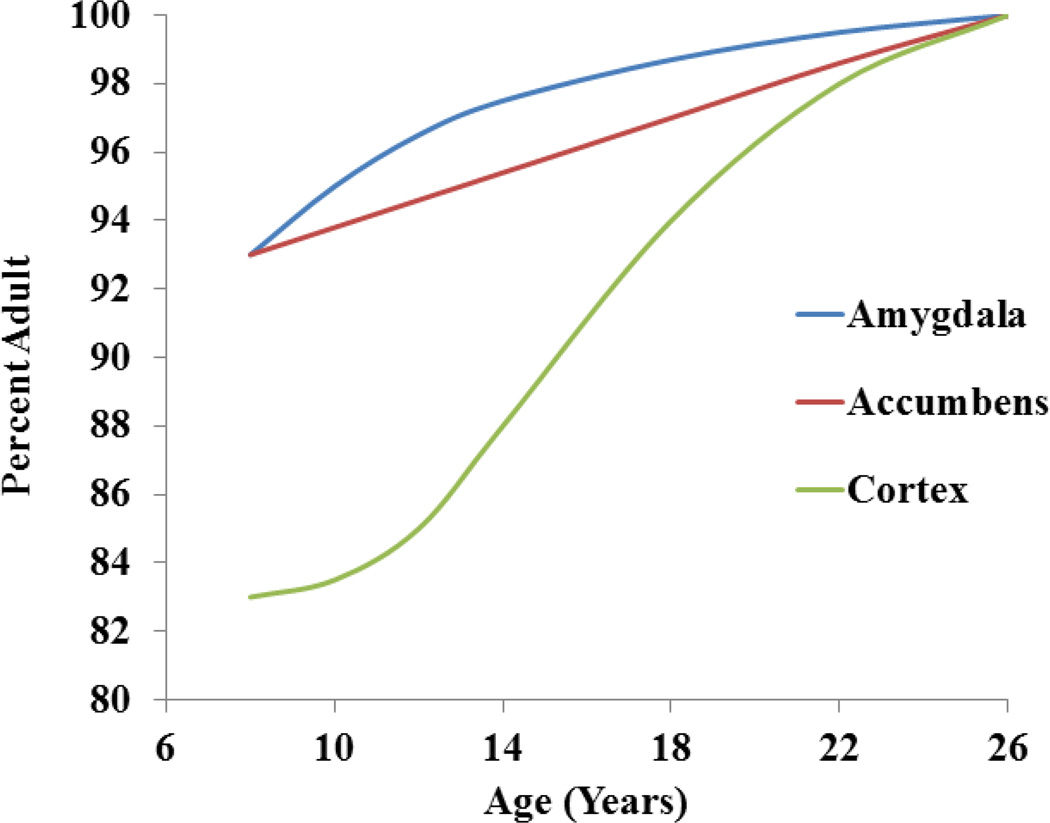
The structural and functional changes which mediate these important neurobehavioral characteristics are poorly understood even though the structural development of the brain during adolescence is increasingly well characterized. Several excellent recent reviews profile the most important changes that are relevant to addiction (Brenhouse & Andersen, 2011; Giedd et al., 1999; Laviola, Adriani, Terranova, & Gerra, 1999; Spear, 2000). The elegant longitudinal studies of human brain structure by Giedd and colleagues have shown that maximal brain volume is attained during childhood followed by a period of loss which proceeds caudally to rostrally (Giedd, Raznahan, Mills, & Lenroot, 2012). Gray matter in the frontal cortex falls during adolescence while myelination continues linearly, and synaptic pruning is an active process. However, two specific changes relate in an intriguing way to the triadic model described above: structural maturation of the caudate and amygdala precede that of the cortex, while cortical: amygdala connectivity is a relatively late phenomenon (Cressman et al., 2010; Cunningham, Bhattacharyya, & Benes, 2008). However each area has its own trajectory, and none are exactly linear (Mills, Goddings, Clasen, Giedd, & Blakemore, 2014). Accumbens volume falls starting early during adolescence, amygdala volume in males continues reaches an asymptote during late adolescence while cortical thinning, a marker for one aspect of cortical maturation continues into the early 20’s, Finally, gray matter volume is at best an indirect measure of maturation that may reflect total neuronal volume (cell bodies and processes) but does not capture many functionally important but subtle events. Figure 5 provides a relative graphic of the structural development of accumbens, amygdala and cortex, based on the aforementioned study. By replotting the data on an increasing trajectory toward adult values, regardless of whether volume is increasing or decreasing, the later attainment of adult structure in the cortex is clear.
Figure 5.
Trajectory of accumbens, amygdala and cortical development (modified from (Mills et al., 2014)
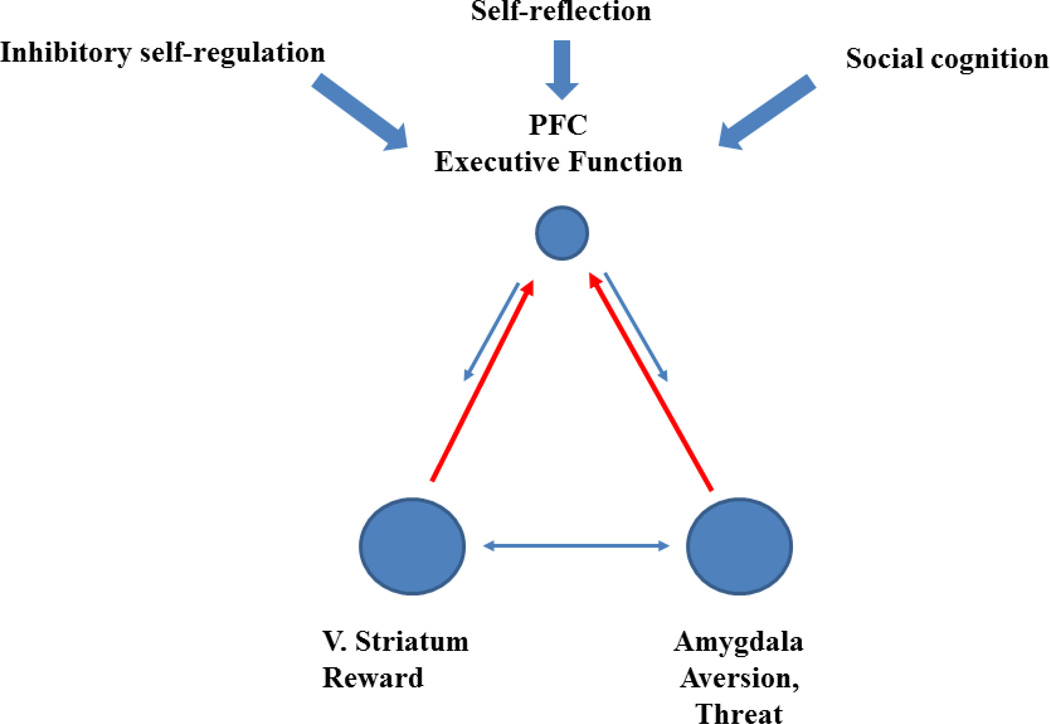
Functional studies using fMRI imaging during task performance have provided better insight into at least which brain areas are active when adolescents and adults are performing comparable tasks. Many of these are cited to support the triadic model. However, there are several caveats to this simple model which have been summarized in two recent reviews (Crone & Dahl, 2012; Pfeifer & Allen, 2012). First, the exact test situations vary widely in different studies, and especially the degree of motivation and use of social or non-social cues can yield quite different results. The robust activation of reward networks in adolescents by salient reinforcers can generate quite different results from a less engaging task. In addition the maturation of cortical areas involved in social-affective perceptions and behaviors (Crone & Dahl, 2012) which allows adolescents to understand and value the perspective of other people is ongoing and a key contributor to experimental results which involve social cues. Indeed, the rising importance of peers is a key characteristic of adolescent social behavior. Finally, increased activity in fMRI is often interpreted oppositely by different groups: it is interpreted by some to mean an area is “online” and functional, and by another to indicate inefficient activation of an immature network. Figure 6 provides a revised “triad” which accommodates multiple elements of executive function during adolescence (social cognition, the ability for self-reflection and self-regulation), each of which likely exhibits its gradually increasing but unique developmental trajectory across adolescence.
Figure 6.
The modified triadic model of executive function
9. Factors Governing Emergence of Drug Use During Adolescence
Neurobiologic factors influencing Drug Action during Adolescence
Neurobiologic factors influence the pharmacologic effects of addictive drugs in adolescents. In general, the rewarding effects of drugs of abuse are greater and aversive effects of drugs of abuse are less in adolescents. Both characteristics create a positive bias in drug experience.
Studies in experimental animals support the greater reinforcing effects of drugs in adolescents. Most data which directly address age differences in reinforcing effects derive from animal studies, as ethical constraints limit such studies in humans. Both self-administration and conditioned place preference studies in rodents show that nicotine, alcohol and psychomotor stimulants are more reinforcing in adolescents than adults in most (Badanich, Adler, & Kirstein, 2006; Balda et al., 2006; Edwards et al., 2014; Natarajan, Wright, & Harding, 2011; Natividad, Torres, Friedman, & O'Dell, 2013; O'Dell, 2009; Philpot, Badanich, & Kirstein, 2003; Wong, Ford, Pagels, McCutcheon, & Marinelli, 2013) but not all (Adriani & Laviola, 2003; Dickinson, Kashawny, Thiebes, & Charles, 2009; Frantz, O'Dell, & Parsons, 2007; Holtz & Carroll, 2013) studies. Narcotics provide an exception, in which self-administration in rodents has been reported to be greater in adolescent than adult rats (Doherty & Frantz, 2012) but conditioned place preference in mice less in adolescents than adults (Niikura, Ho, Kreek, & Zhang, 2013; Zhang et al., 2009)
The lower aversiveness of drugs of abuse in adolescents is an even more consistent finding than that of enhanced reward in experimental studies in rodents. Multiple laboratories have used conditioned taste aversion or conditioned place aversion to show that adolescent rodents are less likely to avoid a taste or place paired with a dose of drug than adults. This is true for every drug that has been tested including alcohol, nicotine, THC, cocaine, amphetamine, narcotics and even LiCl, the prototype GI irritant that is used as a control in these studies (Acevedo, Molina, Nizhnikov, Spear, & Pautassi, 2010; Anderson, Agoglia, Morales, Varlinskaya, & Spear, 2012; Anderson, Morales, Spear, & Varlinskaya, 2013; Anderson, Varlinskaya, & Spear, 2010; Carvalho, Reyes, Ramalhosa, Sousa, & Van Bockstaele, 2014; Drescher, Foscue, Kuhn, & Schramm-Sapyta, 2011; Hurwitz, Merluzzi, & Riley, 2013; Natarajan et al., 2011; Pandolfo, Vendruscolo, Sordi, & Takahashi, 2009; Philpot et al., 2003; Schramm-Sapyta et al., 2007; Schramm-Sapyta et al., 2010; Schramm-Sapyta et al., 2014; Schramm-Sapyta, Morris, & Kuhn, 2006; Sherrill, Berthold, Koss, Juraska, & Gulley, 2011; Shram, Siu, Li, Tyndale, & Le, 2008; Vetter-O'Hagen et al., 2009). The latter finding suggests that the lack of conditioned taste aversion reflects a fundamental aspect of how the adolescent brain processes aversive input rather than a characteristic of any particular drug. At first, these studies seem to contradict the human imaging studies showing enhanced amygdala reactivity to aversive stimuli. However, this may reflect the difference in experimental approach: rodent studies all require that animals process the aversive stimulus, remember it, recall it at a later time and behave accordingly. All that we can measure in rodents is how they behave at a later time. As pointed out in the earlier section, high amygdala reactivity is not necessarily accompanied by increased behavioral inhibition, a finding generally compatible with the “triadic” hypothesis that what is lacking is inhibitory control facilitated by the cortex. Recent studies showing that adolescents are less likely to recall contextual fear memories than either juvenile or adult animals (Pattwell, Bath, Casey, Ninan, & Lee, 2011), but can even recall an experience in early adulthood that they did not recall earlier support this interpretation. Future research is required to understand whether memory recall, or behavioral response to the memory is different in adolescents than adults. Several studies with nicotine and one with heroin showing that adolescent animals experience fewer unconditioned negative effects of withdrawal however, support the more general hypothesis that aversive stimuli have less weight than reinforcing stimuli in adolescents (Doherty & Frantz, 2013; O'Dell, Bruijnzeel, Ghozland, Markou, & Koob, 2004; O'Dell et al., 2006; O'Dell, Torres, Natividad, & Tejeda, 2007; Shram et al., 2008).
The dopamine system which mediates reward merits particular attention as it may play a significant role in the initiation of drug use in general and the emergence of sex-specific addiction vulnerabilities. The ontogeny of dopamine systems during adolescence surprisingly does not point to the state of obvious hyperfunction that might be expected, but a situation of immature presynaptic stores and exaggerated postsynaptic reactivity. Dopaminergic innervation of dorsal, ventral striatum and prefrontal cortex reaches a peak during adolescence and then declines to adult levels in rodents and humans (Andersen & Gazzara, 1993, 1994, 1996; Haycock et al., 2003). Basal dopamine is lower in adolescent rat striatum, and studies of psychostimulant-induced changes in dopamine release are conflicting, with reports of responses both higher (for cocaine) and lower (for amphetamine) than are observed in adults (Camarini, Griffin, Yanke, Rosalina dos Santos, & Olive, 2008; Cao, Lotfipour, Loughlin, & Leslie, 2007; Kuczenski & Segal, 2002; Matthews, Bondi, Torres, & Moghaddam, 2013; Stansfield & Kirstein, 2005; Walker, Francis, Caster, & Kuhn, 2007; Walker et al., 2010). There is a hyperproduction of D1 and D2 receptors during adolescence followed by a pruning which could play a significant role in modulating dopamine function during adolescence (Andersen, Thompson, Rutstein, Hostetter, & Teicher, 2000; Meng, Ozawa, Itoh, & Takashima, 1999; Teicher, Andersen, & Hostetter, 1995). C-fos responses to dopaminergic agents in rodents which reflect the integration of pre- and postsynaptic responses reflect this pattern of lower presynaptic function but greater postsynaptic function, showing lower responses to agents that must mobilize stores but higher responses to agents like cocaine that are not limited by immature stores (Andersen, LeBlanc, & Lyss, 2001; Cao et al., 2007; Caster & Kuhn, 2009).
More dramatic discontinuities in dopamine receptor function during adolescence occur in cortex. Transient expression of D1 receptors on cortical afferents to the nucleus accumbens occurs during adolescence (Brenhouse, Sonntag, & Andersen, 2008). Furthermore, a dramatic change in cortical D2 function occurs during adolescence, with a switch from inhibitory to excitatory action (O'Donnell, 2010). The late cortical changes in D2 receptor function are particularly intriguing given the importance of D2 receptors for response inhibition, a late-appearing phenomenon (Ghahremani et al., 2012).
Behavioral effects of investigator-administered addictive drugs have been studied extensively, but their findings are contradictory across laboratories and hard to correlate with addiction risk. For example, both enhanced and decreased locomotor responses to psychomotor stimulants, nicotine and narcotics are reported in adolescents compared to adults although reported increases outnumber decreases (Adriani & Laviola, 2000; Adriani, Macri, Pacifici, & Laviola, 2002; Cao et al., 2010; Caster, Walker, & Kuhn, 2005; Faraday, Elliott, Phillips, & Grunberg, 2003; Koek, 2014; Koek, France, & Javors, 2012; McQuown, Dao, Belluzzi, & Leslie, 2009; Zombeck, Lewicki, Patel, Gupta, & Rhodes, 2010)
Decreased sedative:hypnotic effects of ethanol, have been observed consistently in rodent models (Hefner & Holmes, 2007; Little, Kuhn, Wilson, & Swartzwelder, 1996; Silveri & Spear, 1998, 1999). Decreased sensitivity to ethanol is the variable which best predicts ethanol intake in animals (Bell, Rodd, Lumeng, Murphy, & McBride, 2006) as well as humans (Schuckit, 1992, 1994), suggesting that adolescent insensitivity could be a factor that drives high alcohol consumption during this developmental epoch.
Studies of neuroadaptation during exposure to addictive drugs during adolescence are surprisingly sparse, but some behavioral studies provide information about the relative behavioral plasticity of adolescents and adults after repeated drug administration. Behavioral sensitization after repeated treatment with psychostimulants is widely used as a behavioral “surrogate” for neuroplasticity in the ascending dopamine system and its targets, although its relevance to addiction has been debated (Robinson & Berridge, 2008; Vanderschuren & Pierce, 2010). Studies of sensitization in adolescents and adults have yielded surprisingly contradictory results. The psychomotor stimulants amphetamine, methylphenidate and cocaine have been studied the most. Some studies report less sensitization in adolescents than adults after repeated treatment with amphetamine or cocaine (Laviola, Wood, Kuhn, Francis, & Spear, 1995; Torres-Reveron & Dow-Edwards, 2005; Zakharova, Leoni, Kichko, & Izenwasser, 2009), others report more sensitization to the challenge drug and/or cross-sensitization to another psychomotor stimulant (Adriani, Chiarotti, & Laviola, 1998; Brandon, Marinelli, Baker, & White, 2001; Caster et al., 2005; Caster, Walker, & Kuhn, 2007; Guerriero, Hayes, Dhaliwal, Ren, & Kosofsky, 2006). One factor might be that drugs which rely on dopamine release like amphetamine and methylphenidate may be less effective than drugs that inhibit uptake like cocaine due to the relative lack of stores to mobilize in adolescents compared to adults (Walker et al., 2010). However, similarly disparate findings are reported for nicotine, with one study reporting a sensitization that perseveres to adulthood (Faraday et al., 2003), another which reported sensitization in adults but not adolescents (Zago et al., 2012), and a third which reported comparable sensitization following adolescent or adult treatment (Adriani, Deroche-Gamonet, Le Moal, Laviola, & Piazza, 2006) Two studies reported increased cross-sensitization to cocaine (Santos, Marin, Cruz, Delucia, & Planeta, 2009) or amphetamine (McQuown et al., 2009) after adolescent nicotine. Similarly, one study reported comparable sensitization to heroin in adolescents and adults (Doherty & Frantz, 2013), while another reported more persevering sensitization in adolescents than adults (Koek, 2014). Overall, results of sensitization experiments are equivocal about whether sensitization is more, less or comparable in adolescents and adults. Different treatment ages, treatment paradigms, post-treatment challenge timing (immediately after treatments vs. in adulthood), different species (mice vs. rats) all could contribute to these disparate findings.
Sensitization is only a surrogate for the effects of early initiation on neuroadapation and vulnerability to escalation of drug intake during repeated intake or later vulnerability to initiation and progression in the use of other drugs. Such experiments are very difficult to accomplish because it is difficult to start self-administration studies during the brief window of rodent adolescence. While there are many studies of how investigator-administered drugs during adolescence influence subsequent self-administration, results are conflicting, drug-specific and plagued by the same differences in protocols described above for sensitization. The small number of self-administration studies in adolescents which have followed extinction, withdrawal and/or relapse point to persevering neural adaptations in adolescents. Some studies with psychomotor stimulants and ethanol indicate that self-administration is higher, escalation greater, extinction of drug-taking is slower and drug and stress-induced relapse more likely, a finding suggestive of more persevering neuroadaptations after adolescent use (Anker & Carroll, 2010; Brenhouse & Andersen, 2008; Schramm-Sapyta et al., 2008; Siegmund, Vengeliene, Singer, & Spanagel, 2005; Wong et al., 2013). The slower extinction may well reflect a general characteristic of adolescent brain function, as extinction of fear-based memories is slower in adolescent brain, a characteristic that is thought to reflect failure of neuroadaptation in specific cortical areas (prelimbic and infralimbic) of adolescent brain (Pattwell et al., 2012; Pattwell, Lee, & Casey, 2013). However, this is not a universal finding, as another study reported less cue-induced reinstatement and comparable cocaine-induced reinstatement in adolescents compared to adults (Li & Frantz, 2009).
In summary, biologic variables that influence drug taking on average promote more drug use in adolescents than adults. Human imaging and cognitive studies as well as self-administration studies in animal models suggest that enhanced behavioral control by the reinforcing properties of additive drugs plays a role in this vulnerability. The enhanced sensitivity to emotional cues combined with a relative lack of aversive input/behavioral inhibition in response to such input minimizes restraints on drug taking by aversive effects of drug taking or withdrawal.
10. Intrinsic and Extrinsic Factors that Influence Initiation of Addiction during Adolescence
The important intrinsic factors which enhance or protect against initiation and progression of drug use in adolescents are personality characteristics and the emergence during adolescence of psychiatric illnesses which influence the progression of substance use. “Novelty seeking” and/or sensation seeking (these are behavioral constructs with somewhat overlapping definitions) are characteristic of adolescents in general, and those who rank at extreme levels of high novelty seeking are at special risk for initiation of substance abuse (see above). Tarter’s group has developed a metric they characterize as “neurobehavioral disinhibition” which includes similar characteristics, and includes impulsivity/difficulty in response inhibition, and high ranking in this characteristic during early adolescence is strongly predictive of the later development of dependence on tobacco, marijuana, alcohol and cocaine over the next 7–9 years (Clark, Cornelius, Kirisci, & Tarter, 2005; Kirisci, Tarter, Mezzich, & Vanyukov, 2007; Tarter et al., 1999). In contrast, the personality trait of conscientiousness, which reflects the capacity for self-regulation, is highly protective against the development of substance abuse (Whelan et al., 2014).
Anxiety, depression, bipolar disorder conduct disorder and ADHD enhance the progression of substance use (Charach et al., 2011; Deas, 2006; Matthys, Vanderschuren, & Schutter, 2013; Simkin, 2002; Zulauf, Sprich, Safren, & Wilens, 2014). While anxiety disorders tend to first appear during childhood, levels of this disorder rise during adolescence, as does depression. Bipolar disorder typically appears during late adolescence and early adulthood, and is associated with increased abuse of both alcohol (to dampen manic symptoms) and psychomotor stimulants (to relieve depressive symptoms) (Blumberg, 2007; Deas, 2006; Goldstein & Bukstein, 2010).
Extrinsic factors both contribute to and protect from initiation and progression of substance use during adolescence. A childhood history of physical or sexual abuse (Downs & Harrison, 1998; Nomura, Hurd, & Pilowsky, 2012) or profound stress (Enoch, 2011) are associated with development of substance abuse during adolescence. Peers play a dominant role in initiation of tobacco and alcohol use, and association with deviant peers is a very important predictor of the initiation and progression especially of smoking during adolescence (Haas & Schaefer, 2014; Kobus, 2003; Pollard, Tucker, Green, Kennedy, & Go, 2010). Family environment is also crucial: high levels of parental use and low parental supervision are associated with higher rates of initial use and progression (Clark et al., 2005; Kirisci et al., 2007). Overall, parental supervision and involvement is a protective factor which retards initiation and progression of tobacco and alcohol use during adolescence (Mahabee-Gittens, Xiao, Gordon, & Khoury, 2013; Morin, Rodriguez, Fallu, Maiano, & Janosz, 2012; Ryan, Jorm, & Lubman, 2010; Van Der Vorst, Engels, Dekovic, Meeus, & Vermulst, 2007; van der Zwaluw et al., 2010). However, the match between personality and parenting style has a strong impact on initiation and progression of substance use during adolescence. While effective, “authoritative” parenting is effective overall, the pairing of an impulsive, acting out adolescent and authoritarian rather than authoritative parent is a risk factor which increases the risk of substance abuse by adolescents (Armstrong et al., 2013). Finally, other activities in the life of adolescents have a protective effects, especially engagement in religious activities and sports (Agrawal & Lynskey, 2009; Metzger, Dawes, Mermelstein, & Wakschlag, 2011; Nasim, Belgrave, Jagers, Wilson, & Owens, 2007; Silins et al., 2013) Engagement is sports is especially protective against initiation of smoking (Adachi-Mejia, Gibson Chambers, Li, & Sargent, 2014).
11. Summary: risk and protective factors influencing the emergence of substance use and abuse in adolescence
Numerous factors contribute to the initiation and progression of substances abuse including how an individual reacts to a particular drug, the neurobiology of the adolescent brain in general, and the brain of vulnerable individuals (novelty-seeking, disinhibited). However, the interactions of the individual with his or her environment are also crucial: families, peers, individual engagement in activities all influence the initiation and progression of substance use. These factors have been outlined in such detail because a considerable body of evidence suggests that gender/sex influences the weight of all of these key variables. The remainder of this chapter will aim to describe these interactions.
Biologic Factors that Influence the Emergence of Sex/Gender Differences in Substance Use and Abuse during Adolescence
12. Sexual Differentiation of the Brain: The role of genes and hormones
Many aspects of brain structure and function are sexually dimorphic. The most obvious of these are the functions and structures supporting reproduction and sexual behavior, but the reward system, executive function, aggression and stress sensitivity are different in males and females. Multiple phenomena contribute to these differences, as depicted in Figure 7. Males and females receive their complement of X and Y chromosomes at fertilization, which dictate whether a fetus will be male (XY) or female (XX). A rapidly expanding literature shows that sex-specific gene expression occurs in the brain even before ovaries and testes form (Viveros et al., 2012). XX genotype alone accelerates habit formation, a critical component in the transition from reinforced to compulsive responding (Quinn, Hitchcott, Umeda, Arnold, & Taylor, 2007), and also influences nociception, aggression and maternal behavior (Arnold & Chen, 2009) The testis-determining gene sry exerts direct effects on brain development independent of the production of testosterone (De Vries et al., 2002; Dewing et al., 2006; Gatewood et al., 2006; Quinn et al., 2007).
Figure 7.
Sexual differentiation of brain and behaviour.
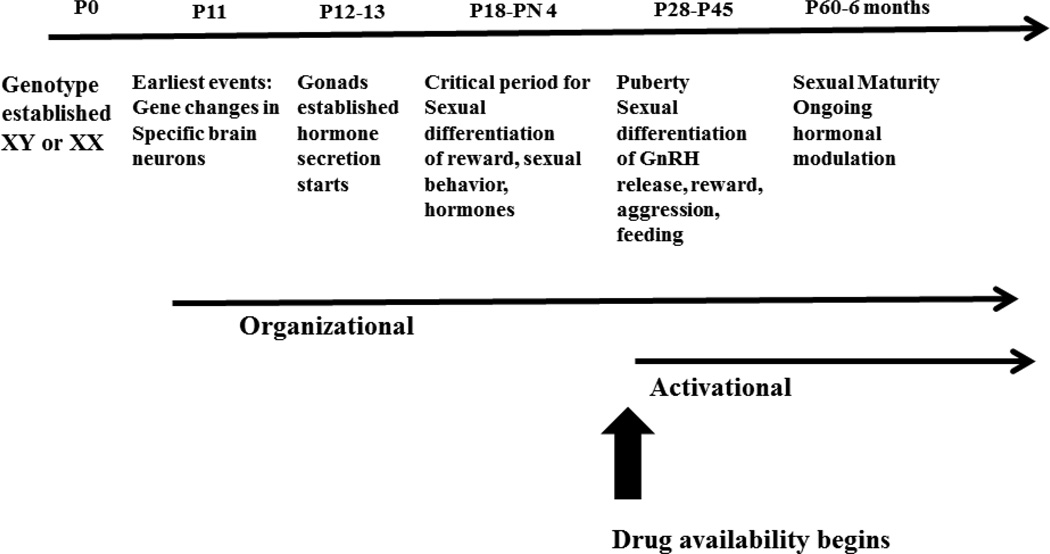
Once the gonads are established, hormonal factors begin to contribute to sexual differentiation of the brain. These changes take two forms which have been termed “organizational” and “activational” (Arnold, 2009). Organizational effects reflect irreversible effects on brain organization due to exposure to gonadal hormones during a critical period. Aromatization of testosterone to estradiol within the brain plays a critical role in this process in the male during fetal/early neonatal life as a fetal form of albumin prevents estradiol from entering the female brain (MacLusky, Lieberburg, & McEwen, 1979; MacLusky & Naftolin, 1981; McCall, Han, Millington, & Baum, 1981; Pardridge & Mietus, 1979). Rising testosterone and estradiol in males and females at puberty respectively causes further sexual differentiation of behaviors including aggression in males and feeding-related behaviors in females (Juraska, Sisk, & DonCarlos, 2013; Schulz, Molenda-Figueira, & Sisk, 2009; Schulz et al., 2004; Schulz & Sisk, 2006; Sisk & Foster, 2004; Sisk & Zehr, 2005). After puberty, gonadal steroid hormones exert ongoing “activational” effects that reverse when hormone is eliminated. The profound rise in gonadal hormones during puberty creates the endocrine environment of adulthood which contributes to many of these effects. Recent research also suggests a role for central production of estradiol by brain aromatase in sexual differentiation of the brain (Bakker, Honda, Harada, & Balthazart, 2002; Brock, Baum, & Bakker, 2011; Dugger, Morris, Jordan, & Breedlove, 2007; Garcia-Segura, 2008; Hill & Boon, 2009; Lephart, 1996).
All three major gonadal hormone receptors, ERα, ERβ and androgen receptor, contribute to sexual differentiation of the brain (Holterhus, 2011; Johansen, Jordan, & Breedlove, 2004; Kudwa, Michopoulos, Gatewood, & Rissman, 2006; Raskin et al., 2009; Sisk & Foster, 2004; Sisk & Zehr, 2005). Although work in this area is just beginning, epigenetic regulation of gonadal steroid hormone gene promoters is likely a mediating mechanism in the evolving role of these different receptors in sexual differentiation of specific brain regions/behavioral functions (Matsuda, 2014; Matsuda, Mori, & Kawata, 2012). Finally, as Figure 7 depicts, adolescence, the developmental epoch when most individuals begin to experiment with drugs, overlaps with puberty and the full expression of genomic and hormonal influences which contribute to the emergence of sex/gender specific behavior.
Sexually dimorphic changes in several key brain structures relevant to the triadic model finalize during adolescence in both humans and rodents. First, structures of reward-relevant areas like the caudate nucleus change differentially in adolescent males and females. Basal ganglia volume attains peak volume and then prunes to adult size earlier in girls than boys (Giedd et al., 2012). There are conflicting data, but some studies suggest that females attain higher final volume than males (reviewed in Giedd (Giedd et al., 2012). In amygdala, changes are nucleus specific, but size of the important basolateral amygdala that is crucial for stress systems attains adult dimensions in childhood in girls, but it continues to grow during adolescence in boys to a greater final volume (Juraska et al., 2013; Viveros et al., 2012). Finally, while thinning of the cortex occurs in both boys and girls, the sex difference (thickness greater in boys than girls) diminishes in critical prefrontal areas during adolescence, and girls attain adult thickness in these areas related to executive function and behavioral inhibition before boys (Raznahan et al., 2010). Each of these anatomical changes correlates with developing sexual dimorphisms in the neurobiologic substrates of addiction including determination of reward system, executive function and neurobiologic determinants of key behaviors like sensation seeking, as well as environmental and cultural influences on gender-typical behavior/social roles. Several critical examples are described below.
The emergence of sexually dimorphic characteristics which contribute to addiction is best understood for the dopamine system involved in the initiation of addiction. Genotype, organizational and activational effects of gonadal hormones all contribute to the establishment of sex/gender differences in adulthood. The testis-determining gene, sry, affects expression of a number of genes in dopamine neurons (Czech et al., 2012; Tao et al., 2012). Greater dopamine neuron numbers occur in females even in DA cell culture, in the absence of any hormonal information, while in this context, male neurons are bigger and express higher levels of some dopamine markers (Beyer, Eusterschulte, Pilgrim, & Reisert, 1992; Beyer, Ivanova, Karolczak, & Kuppers, 2002; Beyer, Pilgrim, & Reisert, 1991; Engele, Pilgrim, & Reisert, 1989; Reisert, Engele, & Pilgrim, 1989; Reisert et al., 1987). Anatomic differences in organization of ascending dopamine systems can be detected well before the pre/postnatal “critical” period in rodents (Kolbinger, Trepel, Beyer, Pilgrim, & Reisert, 1991; Reisert, Schuster, Zienecker, & Pilgrim, 1990). Therefore, the brain structure underlying the enhanced dopamine response to females may be established early in gestation.
Events during this pre/postnatal “critical period” in rodents also contribute to the sculpting of the emerging reward system. Exposure to testosterone during this time frame “masculinizes” behavioral responses to amphetamine in females, an effect that is insensitive to hormonal manipulations later in life (Forgie & Stewart, 1993; Forgie, M. L. & J. Stewart, 1994; Forgie, M.L. & J. Stewart, 1994). Dopamine innervation of the cortex is blunted by testosterone aromatized to estradiol during the pre/postnatal critical period (Stewart & Rajabi, 1994). The final organizational events and onset of activational contributions of gonadal hormones at puberty complete the emergence of sexual dimorphisms in dopamine function. Several waves of dopamine cell loss in rats occur after birth, the final one at puberty, which occurs disproportionately for males (Kuhn et al., 2010). Adult rats, like non-human primates, exhibit greater numbers of dopamine neurons in the ventral tegmental area than males, although these findings are not universal in all rodent studies (McArthur, McHale, & Gillies, 2006; McArthur et al., 2007). The enhanced dopamine release observed consistently in females of rats and mice relative to males also emerges during puberty. The combination of an organizational effect of testicular hormones to suppress dopamine function and the onset of activational effects of estradiol to augment dopaminergic function lead to a divergence of dopaminergic function (Kuhn et al., 2010; Walker & Kuhn, 2008).
Dopamine receptor populations also exhibit marked sexually dimorphic changes in rodents during adolescence that are well described: D2 receptor numbers rise precipitously and then prune markedly in males, while changes in females are blunted in comparison (Andersen, Rutstein, Benzo, Hostetter, & Teicher, 1997; Andersen & Teicher, 2000; Andersen et al., 2000). These changes are independent of hormonal changes at puberty, but the role of earlier endocrine and genomic effects has not been explored (Andersen, Thompson, Krenzel, & Teicher, 2002).
Numerous pubertal changes both in addictive drug action and self-administration that reflect sex-specific development of dopamine systems have been reported in animal models. Enhanced self-administration of cocaine and nicotine in females relative to males emerges, although the role of gonadal hormones was not investigated in these studies (Levin et al., 2011; Lynch, 2008, 2009). However, a study of the effects of prepubertal gonadectomy on cocaine-stimulated locomotion in adulthood, a common surrogate for activation of dopamine reward systems, showed that gonadectomy lowered responses in females and elevated them in males compared to gonadally intact animals (Parylak, Caster, Walker, & Kuhn, 2008). One intriguing study comparing acquisition, intake and motivation to self-administer cocaine suggested that organizational effects of estradiol at puberty specifically organizes motivation but not the other parameters (Perry, Westenbroek, & Becker, 2013).
Studies of the emergence of behavioral sensitization tell a similar story about the importance of puberty and gonadal hormones on the appearance of elevated sensitization in females. An extensive literature shows that chronic exposure of rats to cocaine or nicotine causes enhanced behavioral responding to a test dose of cocaine (sensitization) that is greater in females than in males (Booze, Welch, et al., 1999; Booze, Wood, Welch, Berry, & Mactutus, 1999; Hu & Becker, 2003; Quinones-Jenab & Jenab, 2012; Yang, Zhao, Hu, & Becker, 2007).
A similar role for organizational/activational influences on alcohol consumption may occur during puberty. Prepubertal gonadectomy reversed sex-specific patterns of ethanol consumption in adult mice, as early gonadectomized males consumed more than early-gonadectomized females (Sherrill, Koss, Foreman, & Gulley, 2011). Sex differences in the use-limiting effects of ethanol may emerge during puberty. Sex differences in ethanol-induced sedation exist on postnatal day 60 but not on day 30 (Cha, Li, Wilson, & Swartzwelder, 2006). Similar findings have been reported by Silveri and Spear (Silveri & Spear, 1998) who showed that adult female rats are less sensitive to the sedating effects of ethanol but that no sex difference existed during juvenile or adolescent development. While sex differences in alcohol-related impairment also develop during puberty in non-human primates, the changes are more complex. Males and females both exhibit a decrease in ataxia, but females exhibit more locomotor stimulation and impaired jumping with age compared to males (Schwandt, Barr, Suomi, & Higley, 2007).
The emergence of sex differences in stress systems during puberty may also contribute significantly to the emergence of sex-specific trajectories into drug use and abuse through effects both on the protracted stage of CRF-mediated drug craving, and on the role of stress and emergence of psychiatric disorders during puberty (see below). In both humans and rodents, HPA reactivity to stress is high in adolescence in comparison to earlier life stages and this increase interacts with the hormonal changes of puberty. In humans, cortisol responses to stress are high in adolescents compared to children (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009). Basal cortisol, stress-induced cortisol and CRH-induced rises in cortisol are higher in human females than males after puberty, reflecting a more pronounced fall in males than females (Adam, 2006; Blumenthal, Leen-Feldner, Badour, Trainor, & Babson, 2014; Gunnar et al., 2009; Kiess et al., 1995; Netherton, Goodyer, Tamplin, & Herbert, 2004; Oskis, Loveday, Hucklebridge, Thorn, & Clow, 2009; Shirtcliff et al., 2012; Stroud, Papandonatos, Williamson, & Dahl, 2011). Rodents show a similar pattern: they attain maximal HPA axis reactivity just before puberty (Jankord et al., 2011). Corticosterone responses to stress are high due in part to sluggish glucocorticoid feedback (Sapolsky & Meaney, 1986) As male rats go through puberty, HPA axis responses fall (Klein & Romeo, 2013; Romeo, 2010). Organizational effects during the perinatal period but not adolescence contribute to this fall (McCormick, Furey, Child, Sawyer, & Donohue, 1998; Patchev, Schroeder, Goetz, Rohde, & Patchev, 2004; Seale, Wood, Atkinson, Lightman, & Harbuz, 2005). However, activational effects of androgen to suppress corticosterone secretion contribute to regulation in adulthood (McCormick & Mathews, 2007; McCormick, Mathews, Thomas, & Waters, 2010). In female rats, the onset of estrous cyclicity and estradiol secretion allow them to maintain high, adolescent- like stress responses through activational effects at multiple sites including CRF production, glucocorticoid feedback and others (Evuarherhe, Leggett, Waite, Kershaw, & Lightman, 2009; Veldhuis et al., 2013).
The development of enhanced HPA axis reactivity during puberty in vulnerable human females has the potential to contribute to sex/gender specific trajectories in substance use and misuse in two ways. First, rising HPA axis reactivity is thought during puberty is thought to be related to the emergence of the higher incidence of depression in females relative to males (Young & Korszun, 2010; Young, 1998; Young & Altemus, 2004). Second, it may also be related to enhanced withdrawal from nicotine and stress-induced relapse to psychostimulant self-administration observed in animal models (Feltenstein, Henderson, & See, 2011; Hudson & Stamp, 2011; O'Dell, 2009; O'Dell et al., 2004).
Much less is known about emergence of sex differences in opioid systems of reward or dynorphin mediated stress responses: these remain gaps in the neurobiology literature.
13. Intrinsic Factors in Sex/Gender Differences in Emergence of Substance Use and Abuse during Adolescence
The appearance of sex differences in and hormonal influences on critical personality/neurobehavioral domains influence substance use and abuse risk play a crucial role in the patterns of substance use and abuse in developing males and females. This is true for the risk factors of sensation seeking and impulsivity and for the protective factors of behavioral inhibition and self-regulation.
In both male and female humans, the personality constructs of neurobehavioral disinhibition/novelty seeking (Lovallo et al., 2006; Tarter et al., 1999) or high novelty seeking/low harm avoidance (Bohman et al., 1987; Cloninger et al., 1988; Gilligan et al., 1987) are highly predictive of the development of alcohol (and other substance abuse) problems. Pubertal development is a risk factor for early substance use and abuse in both males and females, and several studies suggest that sensation seeking is a behavioral mediator of this association (Castellanos-Ryan, Parent, Vitaro, Tremblay, & Seguin, 2013; Forbes & Dahl, 2010; Gunn & Smith, 2010; Kong et al., 2013; Martin et al., 2002; Steinberg et al., 2008). High testosterone predicts drug use in both sexes (Kerschbaum, Ruemer, Weisshuhn, & Klimesch, 2006; Reynolds et al., 2007). However, during adolescence, an interesting discrepancy begins to appear which differentiates drug using and non-drug using females. Levels of neurobehavioral disinhibition/sensation seeking/impulsivity are generally lower in adolescent females than males, a difference which becomes exaggerated after puberty (Shulman, Harden, Chein, & Steinberg, 2014). However, adolescent females with high scores in these domains are more likely to develop substance abuse during adolescence, like males, and they are generally correlated with pubertal development in both males and females (Castellanos-Ryan et al., 2013; Grano N, 2004; Kirisci, Mezzich, Reynolds, Tarter, & Aytaclar, 2009; Kong et al., 2013; Martin et al., 2002). Therefore, while slightly lower levels of impulsivity may protect females in general, those at the high end of the spectrum are still at significant risk.
These personality domains correlate well in brain imaging and psychophysiology studies with reward sensitivity and striatal activation, in keeping with the triadic model proposed earlier. During puberty, testosterone was correlated with striatal activation during a gambling task for both males and females, suggesting that androgen effects on reward sensitivity plays a role in both males and females (Op de Macks et al., 2011). Perhaps the most interesting study is one showing that threat cues evoked exaggerated responses in both threat-related areas (amygdala) and reward related areas (striatum) in males, and that these responses correlated with testosterone (Spielberg, Olino, Forbes, & Dahl, 2014). Startle amplitude, a measure of CNS arousal in response to emotionally arousing pictures, increased in midpuberty/late puberty in comparison to earlier ages in males and females (Quevedo, Benning, Gunnar, & Dahl, 2009). This small but intriguing literature links these “personality” constructs to neurobiologic changes which occur during adolescence, and may suggest that the emergence of exaggerated male responses in the domains of sensation/impulsivity are driven by gonadal steroid hormone effects influencing basic neurobiologic circuits involved in threat and reward.
Emergence of sex differences in protective factors including capacity for self-regulation may also contribute to the development of male dominance in substance use and abuse in adulthood. While adult male and female humans perform similarly in tasks related to behavioral inhibition in adulthood (Garavan et al., 2006; Li, Huang, Constable, & Sinha, 2006), adolescent females perform better than boys in tasks of motor inhibition (Aarnoudse-Moens, Duivenvoorden, Weisglas-Kuperus, Van Goudoever, & Oosterlaan, 2012; Bezdjian, Baker, Lozano, & Raine, 2009) and delay of gratification (Hosseini-Kamkar & Morton, 2014). In fact, most studies show that these sex/gender differences are established well before puberty and perhaps even more prominent in children than in adolescents (Hosseini-Kamkar & Morton, 2014). The early emergence of this sexual dimorphism could reflect early sexual differentiation of these areas. Imaging studies further suggest that adolescent males and females use slightly different strategies in selecting risk vs. reward: during a gambling task, adolescent females preferentially activated left inferior, frontal and striatal areas in contrast to males, with dominant right inferior parietal activation (Rubia et al., 2013), a pattern that has been linked to “top-down,” habit-learning dominance in females compared to visuospatial “bottom up” processing in males.
Co-morbid psychiatric illness is a factor in the progression of addiction in men and women, and sex/gender differences in the most frequently observed co-morbidities emerge during adolescence. In adolescent males, the psychiatric disorders most frequently cited as increasing the risk of substance abuse are conduct disorder and attention deficit hyperactivity disorder (ADHD) (Andersen & Teicher, 2000; Dakof, 2000; Deas, 2006; Latimer, Stone, Voight, Winters, & August, 2002), while depression is the most common diagnosis in females (Latimer et al., 2002; Simkin, 2002). After puberty sex/gender differences in depression change dramatically to being female-dominated (Angold & Costello, 2006; Angold, Costello, Erkanli, & Worthman, 1999; Angold, Costello, & Worthman, 1998), and depression is more frequently a factor in substance abuse in adolescent females than adolescent males (Latimer et al., 2002). On average girls more frequently endorse coping as a rationale for drinking alcohol while males more frequently cite getting high/having fun (Kuntsche & Muller, 2012). Similarly, teenage girls more frequently cite desire to reduce anxiety as a reason to smoke cigarettes than boys (O'Dell & Torres, 2014).
The emergence of sex-differences in executive function, reward sensitivity and behavioral inhibition discussed above as well as the different pattern of association between psychiatric diagnosis and sex during adolescence all converge to generate different trajectories into substance use by adolescent males and females. While rates of initiation and use may be similar, the factors driving use diverge rapidly during adolescence for males and females. Nevertheless, individual differences in all of these domains also contribute to vulnerability: females with conduct disorder and males with depression are at increased risk of substance abuse despite not being gender-typical.
14. Extrinsic Factors in Sex/Gender Differences in Emergence of Substance Use and Abuse during Adolescence
The intersection of sex/gender and environment play a significant role in the emergence of gender-specific patterns of substance abuse. Early adversity, especially physical or sexual abuse or early emotional maltreatment, is a dramatic predictor of later substance abuse problems. Although early physical or sexual abuse are risk factors for the development of substance abuse in both men and women (Afifi et al., 2012; Banducci, Hoffman, Lejuez, & Koenen, 2014; Clark et al., 2012; Dube et al., 2005), some studies report that women experience more dramatic impact on substance abuse behaviors (Hyman et al., 2006; Hyman et al., 2008)
Cultural variations in gender roles contributes significantly to initiation of substance use in different countries but rates of initiation of men and women in the youngest cohorts are equalizing in developed countries (Degenhardt et al., 2008; Okoli, Greaves, & Fagyas, 2013; Piko, Wills, & Walker, 2007; Zilberman, M. et al., 2003). Environmental factors including parenting style, the impact of peers, education and socioeconomic status all influence substance use initiation in all adolescents, but also have gender specific effects in humans. Overall, the impact of deviant peers on substance use initiation may be less in girls than boys (Kirisci et al., 2009). However, consumption of alcohol, smoking and use of many drugs in females is enhanced/encouraged by romantic partners to a greater extent in females than males, a pattern that emerges during adolescence (Brady & Randall, 1999; Branstetter, Blosnich, Dino, Nolan, & Horn, 2012; Forbes & Dahl, 2010; Miller et al., 2009). Cohort studies showing that initiation of smoking, alcohol consumption and use of cannabis are occurring earlier and equalizing across gender in current adolescents compared to earlier generations suggest that lower stigmatization and changing roles for women are changing drug use patterns in adolescent females (Degenhardt et al., 2008; Geels et al., 2013; Johnson & Gerstein, 2000; Kerr et al., 2009; Keyes et al., 2008; Pitel et al., 2010).
15. Conclusions
Experimentation with psychoactive drugs and the initiation of substance abuse for most humans begins during adolescence, the developmental epoch when the genetic and hormonal processes which contribute to the emergence of adult sex/gender-specific behaviors emerge. In addition, the social environment of this and earlier developmental epochs shapes social roles which further influence sex/gender-specific behaviors including drug taking. Drug experimentation and progression to substance use is enhanced in adolescent humans as well as in animal models. Peak use of addictive drugs occurs during early adulthood in humans and then tapers off dramatically into adulthood. Drug use by the youngest adolescents is similar in males and females in humans and in animal models, but differences emerge with adulthood. More men than women use and become dependent upon most drugs, and drug use falls more in females than males during the transition to adulthood. However, females may progress more rapidly from initiation of use to problematic use to treatment. In rodent models, which provide a window into the role of biologic factors in the absence of cultural and environmental risks, enhanced drug taking in females emerges at puberty, due in large part to organizational effects of androgen and activational effects of estradiol on the dopamine system. In rodents, like humans, the enhanced stress reactivity of females which contributes to stress and cue-induced relapse also develops after puberty. The risks in the drug-using population and the protective factors in the larger population that never progresses beyond drug experimentation contribute to this developmental pattern of drug use.
Risk factors which predict the development of substance abuse are similar in adolescent males and females. These include the neurobiology of adolescence which favors selection of rewarding experiences without inhibition by aversive consequences, the novelty seeking/sensation seeking personality and psychiatric comorbidities like depression as well as environmental factors including drug-using family environment. However, sex differences in the relative importance of each of these factors emerge during puberty. These include enhanced novelty seeking/sensation seeking especially in males, depression/anxiety in females, and the emergence of stress-induced risk for relapse in females. These intrinsic factors lead to the development of a sex/gender specific trajectory from experimentation into substance abuse for males and females. These are mediated by biologic factors in part including sexual differentiation of cortical and subcortical brain areas responsible for reward, executive function and stress. The emergence of sex differences in factors which protect the larger population of females who do not progress or stop using drugs is rarely considered or studied. These include lower levels of impulsivity and sensation seeking, higher levels of self-regulation during adolescence specifically and greater impact of family environment which provide a window into the role of biologic factors in the absence of cultural and environmental risks. These may continue to protect a large population of females despite the gradual change in social roles which protected women historically. Many of these risk factors reflect characteristics that are difficult to model in animals, which may contribute to the apparent divergence in sex/gender specific drug taking in animal models and humans.
In summary, biologic, psychiatric co-morbidities as well as personality and environment present sex/gender-specific risks as adolescents begin to initiate substance use. In females, enhanced dopaminergic and CRF-related functions as well as psychiatric comorbidity (depression) and previous abuse represent vulnerability factors. In men, neural development during adolescence and the pubertal testosterone surge lead to a greater increase in impulsivity/sensation seeking and poorer self-regulation which represents risk factors for them.
Acknowledgments
This work has not been submitted for publication elsewhere in any form. The author cites support from the National Institute for Alcohol and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no other sources of funding or conflicts of interest.
References
- Aarnoudse-Moens CS, Duivenvoorden HJ, Weisglas-Kuperus N, Van Goudoever JB, Oosterlaan J. The profile of executive function in very preterm children at 4 to 12 years. Dev Med Child Neurol. 2012;54(3):247–253. doi: 10.1111/j.1469-8749.2011.04150.x. [DOI] [PubMed] [Google Scholar]
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. 2010;52(5):424–440. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi-Mejia AM, Gibson Chambers JJ, Li Z, Sargent JD. The relative roles of types of extracurricular activity on smoking and drinking initiation among tweens. Acad Pediatr. 2014;14(3):271–278. doi: 10.1016/j.acap.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184(3–4):382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39(2):334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117(4):695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Afifi TO, Henriksen CA, Asmundson GJ, Sareen J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can J Psychiatry. 2012;57(11):677–686. doi: 10.1177/070674371205701105. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Correlates of later-onset cannabis use in the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2009;105(1–2):71–75. doi: 10.1016/j.drugalcdep.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahijevych K. Nicotine metabolism variability and nicotine addiction. Nicotine Tob Res. 1999;1(Suppl 2):S59–S62. doi: 10.1080/14622299050011821. discussion S69–70. [DOI] [PubMed] [Google Scholar]
- Alvanzo AA, Storr CL, La Flair L, Green KM, Wagner FA, Crum RM. Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend. 2011;118(2–3):375–382. doi: 10.1016/j.drugalcdep.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release: effects on spontaneous release. J Neurochem. 1993;61(6):2247–2255. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA. The development of D2 autoreceptor-mediated modulation of K(+)-evoked dopamine release in the neostriatum. Brain Res Dev Brain Res. 1994;78(1):123–130. doi: 10.1016/0165-3806(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA. Effects of (−)-sulpiride on dopamine release in striatum of developing rats: degree of depolarization influences responsiveness. J Neurochem. 1996;67(5):1931–1937. doi: 10.1046/j.1471-4159.1996.67051931.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41(4):345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24(1):137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27(6):683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Morales M, Spear LP, Varlinskaya EI. Pharmacological activation of kappa opioid receptors: aversive effects in adolescent and adult male rats. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15(4):919–937. ix. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29(5):1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208(2):211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19(5–6):615–629. doi: 10.1097/FBP.0b013e32830dc0ae. doi: 10.1097/FBP.0b013e32830dc0ae 00008877-200809000-00020 [pii] [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013. doi: S0091-3057(09)00164-6 [pii] 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, Ruttle PL, Burk LR, Costanzo PR, Strauman TJ, Essex MJ. Early risk factors for alcohol use across high school and its covariation with deviant friends. J Stud Alcohol Drugs. 2013;74(5):746–756. doi: 10.15288/jsad.2013.74.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55(5):570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22(20):9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51(2):341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50(4):529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Banducci AN, Hoffman EM, Lejuez CW, Koenen KC. The impact of childhood abuse on inpatient substance users: specific links with risky sex, aggression, and emotion dysregulation. Child Abuse Negl. 2014;38(5):928–938. doi: 10.1016/j.chiabu.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15877(9):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18(2):166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32(5):709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103(3–4):342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009;55(5):646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19(1):27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35(2):117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204(2):361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64(1):53–57. doi: 10.1016/s0091-3057(99)00091-x. doi: S0091-3057(99)00091-X [pii] [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11(3–4):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): a functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2014;82:143–150. doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Eusterschulte B, Pilgrim C, Reisert I. Sex steroids do not alter sex differences in tyrosine hydroxylase activity of dopaminergic neurons in vitro. Cell Tissue Res. 1992;270(3):547–552. doi: 10.1007/BF00645057. [DOI] [PubMed] [Google Scholar]
- Beyer C, Ivanova T, Karolczak M, Kuppers E. Cell type-specificity of nonclassical estrogen signaling in the developing midbrain. J Steroid Biochem Mol Biol. 2002;81:319–325. doi: 10.1016/s0960-0760(02)00119-x. [DOI] [PubMed] [Google Scholar]
- Beyer C, Pilgrim C, Reisert I. Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: sex differences and effects of sex steroids. J Neurosci. 1991;11(5):1325–1333. doi: 10.1523/JNEUROSCI.11-05-01325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Lozano DI, Raine A. Assessing inattention and impulsivity in children during the Go/NoGo task. Br J Dev Psychol. 2009;27(Pt 2):365–383. doi: 10.1348/026151008X314919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R. Temporal discounting as a measure of executive function: insights from the competing neuro-behavioral decision system hypothesis of addiction. Adv Health Econ Health Serv Res. 2008;20:289–309. [PubMed] [Google Scholar]
- Bingham CR, Shope JT, Tang X. Drinking behavior from high school to young adulthood: differences by college education. Alcohol Clin Exp Res. 2005;29(12):2170–2180. doi: 10.1097/01.alc.0000191763.56873.c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nat Neurosci. 2012;15(9):1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Glick SD. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev Alcohol. 1995;12:231–241. doi: 10.1007/0-306-47138-8_15. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Dimensions in the development of bipolar disorder. Biol Psychiatry. 2007;62(2):104–106. doi: 10.1016/j.biopsych.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Badour CL, Trainor CD, Babson KA. Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. J Adolesc. 2014;37(6):893–900. doi: 10.1016/j.adolescence.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Bohman M, Cloninger R, Sigvardsson S, von Knorring AL. The genetics of alcoholisms and related disorders. J Psychiatr Res. 1987;21(4):447–452. doi: 10.1016/0022-3956(87)90092-6. [DOI] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999;64(4):827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wood ML, Welch MA, Berry S, Mactutus CF. Estrous cyclicity and behavioral sensitization in female rats following repeated intravenous cocaine administration. Pharmacol Biochem Behav. 1999;64(3):605–610. doi: 10.1016/s0091-3057(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res. 2012;36(3):457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25(5):651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Branstetter SA, Blosnich J, Dino G, Nolan J, Horn K. Gender differences in cigarette smoking, social correlates and cessation among adolescents. Addict Behav. 2012;37(6):739–742. doi: 10.1016/j.addbeh.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122(2):460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31(15):5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology. 2012;37(6):1377–1386. doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;105(2):209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230(1):21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Camarini R, Griffin WC, 3rd, Yanke AB, Rosalina dos Santos B, Olive MF. Effects of adolescent exposure to cocaine on locomotor activity and extracellular dopamine and glutamate levels in nucleus accumbens of DBA/2J mice. Brain Res. 2008;1193:34–42. doi: 10.1016/j.brainres.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance JD, Ennett ST, Morgan-Lopez AA, Foshee VA, Talley AE. Perceived pubertal timing and recent substance use among adolescents: a longitudinal perspective. Addiction. 2013;108(10):1845–1854. doi: 10.1111/add.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(1):82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32(11):2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. doi: S0018-506X(09)00221-9 [pii] 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. doi: S0376-8716(08)00396-7 [pii] 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 2005;180(3):414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Mach JL, La Nasa RM, Newman JL. Impulsivity as a behavioral measure of withdrawal of orally delivered PCP and nondrug rewards in male and female monkeys. Psychopharmacology (Berl) 2009;207(1):85–98. doi: 10.1007/s00213-009-1636-y. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Reyes BA, Ramalhosa F, Sousa N, Van Bockstaele EJ. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Jones RM, Somerville LH. Braking and Accelerating of the Adolescent Brain. J Res Adolesc. 2011;21(1):21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52(3):225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Seguin JR. Pubertal development, personality, and substance use: a 10-year longitudinal study from childhood to adolescence. J Abnorm Psychol. 2013;122(3):782–796. doi: 10.1037/a0033133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Kuhn CM. Maturation of coordinated immediate early gene expression by cocaine during adolescence. Neuroscience. 2009;160(1):13–31. doi: 10.1016/j.neuroscience.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183(2):218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 2007;193(2):247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610(1):127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30(1):113–118. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36(13):2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Caggiula AR. Substantia nigra dopamine neurons: alterations in basal discharge rates and autoreceptor sensitivity induced by estrogen. Neuropharmacology. 1983;22(5):593–599. doi: 10.1016/0028-3908(83)90150-8. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Clark CB, Perkins A, McCullumsmith CB, Islam MA, Hanover EE, Cropsey KL. Characteristics of victims of sexual abuse by gender and race in a community corrections population. J Interpers Violence. 2012;27(9):1844–1861. doi: 10.1177/0886260511430390. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77(1):13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1988;12(4):494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, 3rd, Cottler LB, Ben Abdallah A, Phelps DL, Spitznagel EL, Horton JC. Substance dependence and other psychiatric disorders among drug dependent subjects: race and gender correlates. Am J Addict. 2000;9(2):113–125. doi: 10.1080/10550490050173181. [DOI] [PubMed] [Google Scholar]
- Compton WM, Dawson DA, Conway KP, Brodsky M, Grant BF. Transitions in illicit drug use status over 3 years: a prospective analysis of a general population sample. Am J Psychiatry. 2013;170(6):660–670. doi: 10.1176/appi.ajp.2012.12060737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121(1–2):45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology. 2014;40:201–212. doi: 10.1016/j.psyneuen.2013.11.017. S0306-4530(13)00433-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Wood RI. Androgens and opiates: testosterone interaction with morphine self-administration in male rats. Neuroreport. 2014;25(7):521–526. doi: 10.1097/WNR.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. Am J Psychiatry. 2010;167(10):1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ. Psychiatric predictors of adolescent and young adult drug use and abuse. Drug Alcohol Depend. 2007;88(Suppl 1):S1–S3. doi: 10.1016/j.drugalcdep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149(5):664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Cranford JA, McCabe SE, Boyd CJ. Adolescents' nonmedical use and excessive medical use of prescription medications and the identification of substance use subgroups. Addict Behav. 2013;38(11):2768–2771. doi: 10.1016/j.addbeh.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137(1):97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18(7):1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Czech DP, Lee J, Sim H, Parish CL, Vilain E, Harley VR. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem. 2012;122(2):260–271. doi: 10.1111/j.1471-4159.2012.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakof GA. Understanding gender differences in adolescent drug abuse: issues of comorbidity and family functioning. J Psychoactive Drugs. 2000;32(1):25–32. doi: 10.1080/02791072.2000.10400209. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135(3):703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106(3):226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas D. Adolescent substance abuse and psychiatric comorbidities. J Clin Psychiatry. 2006;67(Suppl 7):18–23. [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Current Biology. 2006;16(4):415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5(1):27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased sensitivity to ethanol reward in adolescent mice as measured by conditioned place preference. Alcohol Clin Exp Res. 2009;33(7):1246–1251. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Keil M, Obst KU, Henseler I, Dechent P, Falkai P, Gruber O. A functional neuroimaging study assessing gender differences in the neural mechanisms underlying the ability to resist impulsive desires. Brain Res. 2012;1473:63–77. doi: 10.1016/j.brainres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Sex differences in dopamine- and vesicular monoamine-transporter functions. Ann N Y Acad Sci. 2008;1139:140–150. doi: 10.1196/annals.1432.010. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology (Berl) 2012;219(3):763–773. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Attenuated effects of experimenter-administered heroin in adolescent vs. adult male rats: physical withdrawal and locomotor sensitization. Psychopharmacology (Berl) 2013;225(3):595–604. doi: 10.1007/s00213-012-2847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. Sex differences in the effects of cocaine abuse across the life span. Physiol Behav. 2010;100(3):208–215. doi: 10.1016/j.physbeh.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs WR, Harrison L. Childhood maltreatment and the risk of substance problems in later life. Health Soc Care Community. 1998;6(1):35–46. doi: 10.1046/j.1365-2524.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- Drescher C, Foscue EP, Kuhn CM, Schramm-Sapyta NL. Individual differences in cocaine conditioned taste aversion are developmentally stable and independent of locomotor effects of cocaine. Dev Cogn Neurosci. 2011;1(4):600–605. doi: 10.1016/j.dcn.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, Giles WH. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28(5):430–438. doi: 10.1016/j.amepre.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51(2):195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens EL, Glowinski AL, Grazier KL, Bucholz KK. The 14-year course of alcoholism in a community sample: do men and women differ? Drug Alcohol Depend. 2008;93(1–2):1–11. doi: 10.1016/j.drugalcdep.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AW, Konz N, Hirsch Z, Weedon J, Dow-Edwards DL. Single trial nicotine conditioned place preference in pre-adolescent male and female rats. Pharmacol Biochem Behav. 2014;125:1–7. doi: 10.1016/j.pbb.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102–110. doi: 10.1016/j.addbeh.2009.09.009. S0306-4603(09)00238-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth D, Hardin MG, Pavletic N, Ernst M. Adolescent transformations of behavioral and neural processes as potential targets for prevention. Prev Sci. 2013;14(3):257–266. doi: 10.1007/s11121-012-0322-1. [DOI] [PubMed] [Google Scholar]
- Engele J, Pilgrim C, Reisert I. Sexual differentiation of mesencephalic neurons in vitro: effects of sex and gonadal hormones. Int J Dev Neurosci. 1989;7(6):603–611. doi: 10.1016/0736-5748(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Pharmacogenomics of alcohol response and addiction. Am J Pharmacogenomics. 2003;3(4):217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3(2):144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Daniele T, Frantz K. New perspectives on adolescent motivated behavior: attention and conditioning. Dev Cogn Neurosci. 2011;1(4):377–389. doi: 10.1016/j.dcn.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Korelitz KE. Cerebral maturation in adolescence: behavioral vulnerability. Encephale. 2009;35(Suppl 6):S182–S189. doi: 10.1016/S0013-7006(09)73469-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J. Voluntary consumption of beverage alcohol by vervet monkeys: population screening, descriptive behavior and biochemical measures. Pharmacol Biochem Behav. 1990;36(2):367–373. doi: 10.1016/0091-3057(90)90417-g. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58(1):13–21. doi: 10.1016/j.yhbeh.2009.08.010. doi: S0018-506X(09)00188-3 [pii] 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to"binge" smoked cocaine use in humans. Neuropsychopharmacology. 1999;21(3):445–454. doi: 10.1016/S0893-133X(98)00120-1. doi: S0893-133X(98)00120-1 [pii] 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Evuarherhe O, Leggett J, Waite E, Kershaw Y, Lightman S. Reversal of the hypothalamo-pituitary-adrenal response to oestrogens around puberty. J Endocrinol. 2009;202(2):279–285. doi: 10.1677/JOE-09-0175. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24(5):644–650. [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav. 2003;74(4):917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(Suppl 1):S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. doi: S0306-4530(09)00252-2 [pii] 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44(8):607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Potenza MN. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19(1):69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald FL, Barfield MA, Warrington RJ. Voluntary alcohol consumption in chimpanzees and orangutans. Q. J. Stud Alcohol. 1968;29(2):330–336. [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: what role does conduct disorder play? Clin Child Fam Psychol Rev. 2003;6(1):1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Sex differences in amphetamine-induced locomotor activity in adult rats: role of testosterone exposure in the neonatal period. Pharmacol Biochem Behav. 1993;46(3):637–645. doi: 10.1016/0091-3057(93)90555-8. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Effect of prepubertal ovariectomy on amphetamine-induced locomotor activity in adult female rats. Horm Behav. 1994;28(3):241–260. doi: 10.1006/hbeh.1994.1021. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Sex differences in the locomotor-activating effects of amphetamine: role of circulating testosterone in adulthood. Physiology and Behavior. 1994;55(4):639–644. doi: 10.1016/0031-9384(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17(2):103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32(3):625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322(2):95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105(1):130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20(6):705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26(8):2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geels LM, Vink JM, van Beek JH, Bartels M, Willemsen G, Boomsma DI. Increases in alcohol consumption in women and elderly groups: evidence from an epidemiological study. BMC Public Health. 2013;13(1):207. doi: 10.1186/1471-2458-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106(1):58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, London ED. Striatal Dopamine D2/D3 Receptors Mediate Response Inhibition and Related Activity in Frontostriatal Neural Circuitry in Humans. J Neurosci. 2012;32(21):7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9(11):999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3(1):19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson's disease: A contribution to sex-specific neuroprotection by estrogens. Horm Behav. 2010;57(1):23–34. doi: 10.1016/j.yhbeh.2009.06.002. doi: S0018-506X(09)00132-9 [pii] 10.1016/j.yhbeh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson's disease. Pharmacol Biochem Behav. 2004;78(3):513–522. doi: 10.1016/j.pbb.2004.04.022. S0091305704001479 [pii] [DOI] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson's disease. Front Neuroendocrinol. 2014;35(3):370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan SB, Reich T, Cloninger CR. Etiologic heterogeneity in alcoholism. Genet Epidemiol. 1987;4(6):395–414. doi: 10.1002/gepi.1370040602. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Bukstein OG. Comorbid substance use disorders among youth with bipolar disorder: opportunities for early identification and prevention. J Clin Psychiatry. 2010;71(3):348–358. doi: 10.4088/JCP.09r05222gry. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Saha TD, Ruan WJ, Compton WM, Grant BF. Antisocial behavioral syndromes and DSM-IV alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Clin Exp Res. 2007;31(5):814–828. doi: 10.1111/j.1530-0277.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Gould RW, Duke AN, Nader MA. PET studies in nonhuman primate models of cocaine abuse: translational research related to vulnerability and neuroadaptations. Neuropharmacology. 2014;84:138–151. doi: 10.1016/j.neuropharm.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano NVM, Vahtera J, Elovainio M, Kivimaki M. Impulsivity as a predictor of smoking and alcohol consumption. Personality and individual differences. 2004;37(8):1693–1700. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. doi: S0193-953X(10)00019-5 [pii] 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield TK, Rogers JD. Who drinks most of the alcohol in the US? The policy implications. J Stud Alcohol. 1999;60(1):78–89. doi: 10.15288/jsa.1999.60.78. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Norberg K, Bucholz KK, Bierut LJ. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcohol Clin Exp Res. 2008;32(8):1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero RM, Hayes MM, Dhaliwal SK, Ren JQ, Kosofsky BE. Preadolescent methylphenidate versus cocaine treatment differ in the expression of cocaine-induced locomotor sensitization during adolescence and adulthood. Biol Psychiatry. 2006;60(11):1171–1180. doi: 10.1016/j.biopsych.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Gunn RL, Smith GT. Risk factors for elementary school drinking: pubertal status, personality, and alcohol expectancies concurrently predict fifth grade alcohol consumption. Psychol Addict Behav. 2010;24(4):617–627. doi: 10.1037/a0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Peters RH. Development of substance abuse problems among drug-involved offenders. Evidence for the telescoping effect. J Subst Abuse. 2000;12(3):241–253. doi: 10.1016/s0899-3289(00)00053-5. doi: S0899-3289(00)00053-5 [pii] [DOI] [PubMed] [Google Scholar]
- Haas SA, Schaefer DR. With a Little Help from My Friends? Asymmetrical Social Influence on Adolescent Smoking Initiation and Cessation. J Health Soc Behav. 2014;55(2):126–143. doi: 10.1177/0022146514532817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87(3):574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Health, N. H. S. o. D. U. a. US Dept' of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2005 [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35(2):136–145. [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191(2):311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. doi: 10.1016/j.drugalcdep.2004.02.001 S0376871604000304 [pii] [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. J Abnorm Psychol. 2007;116(3):433–447. doi: 10.1037/0021-843X.116.3.433. doi: 2007-11737-001 [pii] 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Boon WC. Estrogens, brain, and behavior: lessons from knockout mouse models. Semin Reprod Med. 2009;27(3):218–228. doi: 10.1055/s-0029-1216275. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97(8):1025–1036. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Holterhus PM. Molecular androgen memory in sex development. Pediatr Endocrinol Rev. 2011;9(Suppl 1):515–518. [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake. Psychopharmacology (Berl) 2013;227(2):243–250. doi: 10.1007/s00213-012-2958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Kamkar N, Morton JB. Sex differences in self-regulation: an evolutionary perspective. Front Neurosci. 2014;8:233. doi: 10.3389/fnins.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Stamp JA. Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev. 2011;35(3):427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Hurwitz ZE, Merluzzi AP, Riley AL. Age-dependent differences in morphine-induced taste aversions. Dev Psychobiol. 2013;55(4):415–428. doi: 10.1002/dev.21046. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Sinha R. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Drug Alcohol Abuse. 2006;32(4):655–664. doi: 10.1080/10623320600919193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92(1𠄳):208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97(5):517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci. 2003;117(1):76–83. doi: 10.1037//0735-7044.117.1.76. [DOI] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83(2):271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22(4):238–247. doi: 10.1111/j.1365-2826.2010.01965.x. doi: JNE1965 [pii] 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22(4):226–237. doi: 10.1111/j.1365-2826.2010.01964.x. doi: JNE1964 [pii] 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Richter L, Kleber HD, McLellan AT, Carise D. Telescoping of drinking-related behaviors: gender, racial/ethnic, and age comparisons. Subst Use Misuse. 2005;40(8):1139–1151. doi: 10.1081/JA-200042281. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Gerstein DR. Age, period, and cohort effects in marijuana and alcohol incidence: United States females and males, 1961–1990. Subst Use Misuse. 2000;35(6–8):925–948. doi: 10.3109/10826080009148427. [DOI] [PubMed] [Google Scholar]
- Juarez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19(1):15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64(2):203–210. doi: 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry. 2001;158(2):308–311. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15(1):37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Bond J, Ye Y, Rehm J. Age-period-cohort modelling of alcohol volume and heavy drinking days in the US National Alcohol Surveys: divergence in younger and older adult trends. Addiction. 2009;104(1):27–37. doi: 10.1111/j.1360-0443.2008.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum HH, Ruemer M, Weisshuhn S, Klimesch W. Gender-dependent differences in sensation seeking and social interaction are correlated with saliva testosterone titre in adolescents. Neuro Endocrinol Lett. 2006;27(3):315–320. [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93(1–2):21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry. 2010;167(8):969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130(1–3):101–108. doi: 10.1016/j.drugalcdep.2012.10.015. doi: 10.1016/j.drugalcdep.2012.10.015 S0376-8716(12)00415-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Mezzich AC, Reynolds M, Tarter RE, Aytaclar S. Prospective study of the association between neurobehavior disinhibition and peer environment on illegal drug use in boys and girls. Am J Drug Alcohol Abuse. 2009;35(3):145–150. doi: 10.1080/00952990902825405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Vanyukov M. Developmental trajectory classes in substance use disorder etiology. Psychol Addict Behav. 2007;21(3):287–296. doi: 10.1037/0893-164X.21.3.287. [DOI] [PubMed] [Google Scholar]
- Kleiber ML, Wright E, Singh SM. Maternal voluntary drinking in C57BL/6J mice: advancing a model for fetal alcohol spectrum disorders. Behav Brain Res. 2011;223(2):376–387. doi: 10.1016/j.bbr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78(1):13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Horm Behav. 2013;64(2):357–363. doi: 10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kobus K. Peers and adolescent smoking. Addiction. 2003;98(Suppl 1):37–55. doi: 10.1046/j.1360-0443.98.s1.4.x. [DOI] [PubMed] [Google Scholar]
- Koek W. Effects of repeated exposure to morphine in adolescent and adult male C57BL/6J mice: age-dependent differences in locomotor stimulation, sensitization, and body weight loss. Psychopharmacology (Berl) 2014;231(8):1517–1529. doi: 10.1007/s00213-013-3298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology (Berl) 2012;219(4):1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbinger W, Trepel M, Beyer C, Pilgrim C, Reisert I. The influence of genetic sex on sexual differentiation of diencephalic dopaminergic neurons in vitro and in vivo. Brain Res. 1991;544(2):349–352. doi: 10.1016/0006-8993(91)90079-b. [DOI] [PubMed] [Google Scholar]
- Kong G, Smith AE, McMahon TJ, Cavallo DA, Schepis TS, Desai RA, Krishnan-Sarin S. Pubertal status, sensation-seeking, impulsivity, and substance use in high school-aged boys and girls. J Addict Med. 2013;7(2):116–121. doi: 10.1097/ADM.0b013e31828230ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76 Pt B:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G. Gender differences in delay-discounting under mild food restriction. Behav Brain Res. 2009;200(1):134–143. doi: 10.1016/j.bbr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122(10):3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer M. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cerebral Cortex. 2003;13(3):282–296. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Selective Colocalization of Immunoreactivity for Intracellular Gonadal Hormone Receptors and Tyrosine Hydroxylase in the Ventral Tegmental Area, Substantia Nigra, and Retrorubral Fields in the Rat. Journal of Comaprative Neurology. 1997;379:247–260. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427(4):617–633. [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Bethea CL. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience. 2003;122:757–772. doi: 10.1016/s0306-4522(03)00548-7. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervationin adult male rats are most disruptive to afferents in prefrontal cortex. Cerebral Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51(2):183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J. Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395(1):1–17. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22(16):7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138(3):921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Muller S. Why do young people start drinking? Motives for first-time alcohol consumption and links to risky drinking in early adolescence. Eur Addict Res. 2012;18(1):34–39. doi: 10.1159/000333036. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52(7):759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Laasko A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52:759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9(5):415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Latimer WW, Stone AL, Voight A, Winters KC, August GJ. Gender differences in psychiatric comorbidity among adolescents with substance use disorders. Exp Clin Psychopharmacol. 2002;10(3):310–315. doi: 10.1037//1064-1297.10.3.310. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275(1):345–357. [PubMed] [Google Scholar]
- Lawson KM, Back SE, Hartwell KJ, Moran-Santa Maria M, Brady KT. A comparison of trauma profiles among individuals with prescription opioid, nicotine, or cocaine dependence. Am J Addict. 2013;22(2):127–131. doi: 10.1111/j.1521-0391.2013.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci. 2000;20(23):8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque D, Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990;24(2):281–284. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85(3):669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Rezvani AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res. 2011;225(2):473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Nixon SJ. Characterizing gender differences in treatment seekers. Alcohol Clin Exp Res. 2014;38(1):275–284. doi: 10.1111/acer.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 2009;204(4):725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32(4):1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and Postnatal Development of Dopmaminergic Neuron Numbers in the Male and Female Mouse Midbrain. Developmental Brain Research. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Lorenz JG, Long JC, Linnoila M, Goldman D, Suomi SJ, Higley JD. Genetic and other contributions to alcohol intake in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2006;30(3):389–398. doi: 10.1111/j.1530-0277.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2006;30(5):763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol. 2008;13(3–4):403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Lieberburg I, McEwen BS. The development of estrogen receptor systems in the rat brain: perinatal development. Brain Res. 1979;178(1):129–142. doi: 10.1016/0006-8993(79)90093-3. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual Differentiation of the Central Nervous System. Science. 1981;211(4488):1294–1303. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Mahabee-Gittens EM, Xiao Y, Gordon JS, Khoury JC. The dynamic role of parental influences in preventing adolescent smoking initiation. Addict Behav. 2013;38(4):1905–1911. doi: 10.1016/j.addbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33(9):1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Matsuda KI. Epigenetic changes in the estrogen receptor alpha gene promoter: implications in sociosexual behaviors. Front Neurosci. 2014;8:344. doi: 10.3389/fnins.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Kawata M. Epigenetic mechanisms are involved in sexual differentiation of the brain. Rev Endocr Metab Disord. 2012;13(3):163–171. doi: 10.1007/s11154-012-9202-z. [DOI] [PubMed] [Google Scholar]
- Matthews M, Bondi C, Torres G, Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38(7):1344–1351. doi: 10.1038/npp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys W, Vanderschuren LJ, Schutter DJ. The neurobiology of oppositional defiant disorder and conduct disorder: altered functioning in three mental domains. Dev Psychopathol. 2013;25(1):193–207. doi: 10.1017/S0954579412000272. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The Size and Distribution of Midbrain Dopaminergic Populations are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner. Neuropsychopharmacology. 2006;32(7):1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The Size and Distribution of Midbrain Dopaminergic Populations are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner. Neuropsychopharmacology. 2007;32(7):1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Teter CJ. Subtypes of nonmedical prescription drug misuse. Drug Alcohol Depend. 2009;102(1–3):63–70. doi: 10.1016/j.drugalcdep.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Boyd CJ. Motives for medical misuse of prescription opioids among adolescents. J Pain. 2013;14(10):1208–1216. doi: 10.1016/j.jpain.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AL, Han SJ, Millington WR, Baum MJ. Non-saturable transport of [3H]oestradiol across the blood-brain barrier in female rats is reduced by neonatal serum. J Reprod Fertil. 1981;61(1):103–108. doi: 10.1530/jrf.0.0610103. [DOI] [PubMed] [Google Scholar]
- McClure J, Podos J, Richardson HN. Isolating the delay component of impulsive choice in adolescent rats. Front Integr Neurosci. 2014;8:3. doi: 10.3389/fnint.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have 'organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105(2):295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. doi: S0091-3057(06)00222-X [pii] 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72(1):73–85. doi: 10.1016/j.bandc.2009.06.003. doi: S0278-2626(09)00099-2 [pii] 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201(1):137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M, Casswell S, Pledger M. Gender convergence in alcohol consumption and related problems: issues and outcomes from comparisons of New Zealand survey data. Addiction. 2004;99(6):738–748. doi: 10.1111/j.1360-0443.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Dao JM, Belluzzi JD, Leslie FM. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology (Berl) 2009;207(1):143–152. doi: 10.1007/s00213-009-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH. Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2011;36(11):2187–2199. doi: 10.1038/npp.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32(9):1956–1966. doi: 10.1038/sj.npp.1301314. doi: 1301314 [pii] 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res. 1999;843(1–2):136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Metzger A, Dawes N, Mermelstein R, Wakschlag L. Longitudinal Modeling of Adolescents' Activity Involvement, Problem Peer Associations, and Youth Smoking. J Appl Dev Psychol. 2011;32(1):1–9. doi: 10.1016/j.appdev.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17(3):185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Miller S, Lansford JE, Costanzo P, Malone PS, Golonka M, Killeya-Jones LA. Early Adolescent Romantic Partner Status, Peer Standing, and Problem Behaviors. J Early Adolesc. 2009;29(6):839–861. doi: 10.1177/0272431609332665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36(3–4):147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): a high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2014;82:151–160. doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Morin AJ, Rodriguez D, Fallu JS, Maiano C, Janosz M. Academic achievement and smoking initiation in adolescence: a general growth mixture analysis. Addiction. 2012;107(4):819–828. doi: 10.1111/j.1360-0443.2011.03725.x. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108(3–5):327–338. doi: 10.1016/j.jsbmb.2007.09.011. doi: S0960-0760(07)00262-2 [pii] 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, O'Hara R, Taylor JL, Friedman L, Yesavage JA. Influence of the menstrual cycle on flight simulator performance after alcohol ingestion. J Stud Alcohol. 2001;62(4):422–433. doi: 10.15288/jsa.2001.62.422. [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O'Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Res Health. 1999;23(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nasim A, Belgrave FZ, Jagers RJ, Wilson KD, Owens K. The moderating effects of culture on peer deviance and alcohol use among high-risk African-American Adolescents. J Drug Educ. 2007;37(3):335–363. doi: 10.2190/DE.37.3.g. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Wright JW, Harding JW. Nicotine-induced conditioned place preference in adolescent rats. Pharmacol Biochem Behav. 2011;99(3):519–523. doi: 10.1016/j.pbb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- National Household Survey on Drug Use and Health. US Dept' of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2013 [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O'Dell LE. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav Brain Res. 2013;257:275–285. doi: 10.1016/j.bbr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1–2):189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29(2):125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Nathan PE, Frankenstein W, Shapiro AP, Brick J. Gender differences in acute psychomotor, cognitive, and pharmacokinetic response to alcohol. Addict Behav. 1987;12(4):345–356. doi: 10.1016/0306-4603(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Kreek MJ. Common and specific liability to addiction: approaches to association studies of opioid addiction. Drug Alcohol Depend. 2012;123(Suppl 1):S33–S41. doi: 10.1016/j.drugalcdep.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychol. 2006;133(4):357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Hurd YL, Pilowsky DJ. Life-time risk for substance use among offspring of abusive family environment from the community. Subst Use Misuse. 2012;47(12):1281–1292. doi: 10.3109/10826084.2012.695420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76(Pt B):566–580. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29(1):17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18(3–4):306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766(1–2):19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1(3):228–257. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- Okoli C, Greaves L, Fagyas V. Sex differences in smoking initiation among children and adolescents. Public Health. 2013;127(1):3–10. doi: 10.1016/j.puhe.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Gunther Moor B, Overgaauw S, Guroglu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev Cogn Neurosci. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34(3):307–316. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Pakarinen ED, Williams KL, Woods JH. Food restriction and sex differences on concurrent, oral ethanol and water reinforcers in juvenile rhesus monkeys. Alcohol. 1999;17(1):35–40. doi: 10.1016/s0741-8329(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102(1–3):78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat-an animal model of attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2009;205(2):319–326. doi: 10.1007/s00213-009-1542-3. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat bloodbrain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89(3):314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Schroeder J, Goetz F, Rohde W, Patchev AV. Neurotropic action of androgens: principles, mechanisms and novel targets. Exp Gerontol. 2004;39(11–12):1651–1660. doi: 10.1016/j.exger.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Pattij T, De Vries TJ. The role of impulsivity in relapse vulnerability. Curr Opin Neurobiol. 2013;23(4):700–705. doi: 10.1016/j.conb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114(3):e300–e306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A. 2011;108(3):1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Lee FS, Casey BJ. Fear learning and memory across adolescent development: Hormones and Behavior Special Issue: Puberty and Adolescence. Horm Behav. 2013;64(2):380–389. doi: 10.1016/j.yhbeh.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav. 2013;64(4):573–578. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65(2):124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86(4):822–837. doi: 10.1016/j.pbb.2007.03.012. doi: S0091-3057(07)00114-1 [pii] 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27(4):593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM, Schwertz DW. Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol. 2005;35(2):113–118. doi: 10.1016/j.alcohol.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics. Int J Addict. 1989;24(1):19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- Piko BF, Wills TA, Walker C. Motives for smoking and drinking: country and gender differences in samples of Hungarian and US high school students. Addict Behav. 2007;32(10):2087–2098. doi: 10.1016/j.addbeh.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Pitel L, Geckova AM, van Dijk JP, Reijneveld SA. Gender differences in adolescent health-related behaviour diminished between 1998 and 2006. Public Health. 2010;124(9):512–518. doi: 10.1016/j.puhe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155(6):768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Pollard MS, Tucker JS, Green HD, Kennedy D, Go MH. Friendship networks and trajectories of adolescent tobacco use. Addict Behav. 2010;35(7):678–685. doi: 10.1016/j.addbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6(8):810–814. [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev Psychopathol. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10(11):1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci. 2014;1317:1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Influence of sex differences and gonadal hormones on cocaine addiction. ILAR J. 2012;53(1):14–22. doi: 10.1093/ilar.53.1.14. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60(2):252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29(14):4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Yuferov V, Randesi M, Kreek MJ. Genetics of opiate addiction. Curr Psychiatry Rep. 2014;16(11):504. doi: 10.1007/s11920-014-0504-6. [DOI] [PubMed] [Google Scholar]
- Reisert I, Engele J, Pilgrim C. Early sexual differentiation of diencephalic dopaminergic neurons of the rat in vitro. Cell Tissue Res. 1989;255(2):411–417. doi: 10.1007/BF00224125. [DOI] [PubMed] [Google Scholar]
- Reisert I, Han V, Lieth E, Toran-Allerand D, Pilgrim C, Lauder J. Sex steroids promote neurite growth in mesencephalic tyrosine hydroxylase immunoreactive neurons in vitro. Int J Dev Neurosci. 1987;5(2):91–98. doi: 10.1016/0736-5748(87)90054-2. [DOI] [PubMed] [Google Scholar]
- Reisert I, Schuster R, Zienecker R, Pilgrim C. Prenatal development of mesencephalic and diencephalic dopaminergic systems in the male and female rat. Brain Res Dev Brain Res. 1990;53(2):222–229. doi: 10.1016/0165-3806(90)90010-v. [DOI] [PubMed] [Google Scholar]
- Reynolds MD, Tarter R, Kirisci L, Kirillova G, Brown S, Clark DB, Gavaler J. Testosterone levels and sexual maturation predict substance use disorders in adolescent boys: a prospective study. Biol Psychiatry. 2007;61(11):1223–1227. doi: 10.1016/j.biopsych.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, Baldwin R. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [(1)(8)F]fallypride. Synapse. 2011;65(2):99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. Neural systems underlying motivated behavior in adolescence: implications for preventive medicine. Prev Med. 2012;55(Suppl):S7–S16. doi: 10.1016/j.ypmed.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17(4):854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64(4):739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- RJ NAaN. Parent and peer relations in middle childhood and early adolescence. Journal of Early Adolescence. 2005;25(2):223–249. [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger-Sanchez C, Aguilar MA, Rodriguez-Arias M, Aragon CM, Minarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol Teratol. 2012;34(1):108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15(12):1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev. 2002;26(3):381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28(6):533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, Smith A. Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Jorm AF, Lubman DI. Parenting factors associated with reduced adolescent alcohol use: a systematic review of longitudinal studies. Aust N Z J Psychiatry. 2010;44(9):774–783. doi: 10.1080/00048674.2010.501759. [DOI] [PubMed] [Google Scholar]
- SAMSHA. The NSDUH Report: Substance Abuse during Pregnancy and after Childbirth. Office of Applied Studies. 2009 [Google Scholar]
- Santos GC, Marin MT, Cruz FC, Delucia R, Planeta CS. Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict Biol. 2009;14(3):270–275. doi: 10.1111/j.1369-1600.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Satarug S, Tassaneeyakul W, Na-Bangchang K, Cashman JR, Moore MR. Genetic and environmental influences on therapeutic and toxicity outcomes: studies with CYP2A6. Curr Clin Pharmacol. 2006;1(3):291–309. doi: 10.2174/157488406778249343. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Desai RA, Cavallo DA, Smith AE, McFetridge A, Liss TB, Krishnan-Sarin S. Gender differences in adolescent marijuana use and associated psychosocial characteristics. J Addict Med. 2011;5(1):65–73. doi: 10.1097/ADM.0b013e3181d8dc62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191(4):867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34(12):2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O'Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl) 2014;231(8):1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res. 2008;32(5):754–762. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84(2):344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206(1):1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Reaction to alcohol as a predictor of alcoholism. Clin Neuropharmacol. 1992;15(Suppl 1)(Pt A):305A–306A. doi: 10.1097/00002826-199201001-00158. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol sensitivity and dependence. Exs. 1994;71:341–348. doi: 10.1007/978-3-0348-7330-7_34. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Daeppen JB, Tipp JE, Hesselbrock M, Bucholz KK. The clinical course of alcohol-related problems in alcohol dependent and nonalcohol dependent drinking women and men. J Stud Alcohol. 1998;59(5):581–590. doi: 10.15288/jsa.1998.59.581. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Bucholz KK, Reich T, Bierut L. Five-year clinical course associated with DSM-IV alcohol abuse or dependence in a large group of men and women. Am J Psychiatry. 2001;158(7):1084–1090. doi: 10.1176/appi.ajp.158.7.1084. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. Findings across subgroups regarding the level of response to alcohol as a risk factor for alcohol use disorders: a college population of women and Latinos. Alcohol Clin Exp Res. 2004;28(10):1499–1508. doi: 10.1097/01.alc.0000141814.80716.32. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, consortium I. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Barr CS, Suomi SJ, Higley JD. Age-dependent variation in behavior following acute ethanol administration in male and female adolescent rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2007;31(2):228–237. doi: 10.1111/j.1530-0277.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology. 2005;146(4):1973–1982. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Mechanisms of sex difference: a historical perspective. J Womens Health (Larchmt) 2009;18(6):861–866. doi: 10.1089/jwh.2008.1208. [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196(1):71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Shansky RM. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert Rev Neurother. 2009;9(7):967–973. doi: 10.1586/ern.09.46. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225(1):104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216(2):569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2012;54(5):493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008;198(2):181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Shulman EP, Harden KP, Chein JM, Steinberg L. Sex Differences in the Developmental Trajectories of Impulse Control and Sensation-Seeking from Early Adolescence to Early Adulthood. J Youth Adolesc. 2014 doi: 10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- Sibug R, Kuppers E, Beyer C, Maxson SC, Pilgrim C, Reisert I. Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Brain Res Dev Brain Res. 1996;93(1–2):136–142. doi: 10.1016/0165-3806(96)00024-7. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29(7):1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silins E, Hutchinson D, Swift W, Slade T, Toson B, Rodgers B. Factors associated with variability and stability of cannabis use in young adulthood. Drug Alcohol Depend. 2013;133(2):452–458. doi: 10.1016/j.drugalcdep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23(7):1180–1184. [PubMed] [Google Scholar]
- Simkin DR. Adolescent substance use disorders and comorbidity. Pediatr Clin North Am. 2002;49(2):463–477. doi: 10.1016/s0031-3955(01)00014-1. [DOI] [PubMed] [Google Scholar]
- Simons-Morton BG, Farhat T, ter Bogt TF, Hublet A, Kuntsche E, Nic Gabhainn S, Group HRBF. Gender specific trends in alcohol use: cross-cultural comparisons from 1998 to 2006 in 24 countries and regions. Int J Public Health. 2009;54(Suppl 2):199–208. doi: 10.1007/s00038-009-5411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Hof M, Ellenbroek BA, Degen SB, Cools AR. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacol Biochem Behav. 2000;67(4):801–808. doi: 10.1016/s0091-3057(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents' and adults' neural response to reward. Dev Cogn Neurosci. 2014 doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Exp Neurol. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing? Dev Cogn Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Neurochemical effects of cocaine in adolescence compared to adulthood. Brain Res Dev Brain Res. 2005;159(2):119–125. doi: 10.1016/j.devbrainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O'Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Res. 1994;646(1):157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology. 2011;36(8):1226–1238. doi: 10.1016/j.psyneuen.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Black DN, Porrino LJ, Hurley RA. Neuroanatomy of dopamine: reward and addiction. J Neuropsychiatry Clin Neurosci. 2012;24(1):1–4. doi: 10.1176/appi.neuropsych.24.1.1. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32(12):2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tao Q, Fan X, Li T, Tang Y, Yang D, Le W. Gender segregation in gene expression and vulnerability to oxidative stress induced injury in ventral mesencephalic cultures of dopamine neurons. J Neurosci Res. 2012;90(1):167–178. doi: 10.1002/jnr.22729. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Dev Psychopathol. 1999;11(4):657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84(1):1–13. doi: 10.1016/j.drugalcdep.2005.12.007. doi: S0376-8716(05)00400-X [pii] 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96(3):202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181(1):38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Kappa-opioid receptor signaling in the striatum as a potential modulator of dopamine transmission in cocaine dependence. Front Psychiatry. 2013;4:44. doi: 10.3389/fpsyt.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Abi-Dargham A. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68(8):689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83(4):737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62(1):13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vorst H, Engels RC, Dekovic M, Meeus W, Vermulst AA. Alcohol-specific rules, personality and adolescents' alcohol use: a longitudinal person-environment study. Addiction. 2007;102(7):1064–1075. doi: 10.1111/j.1360-0443.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Vermulst AA, Franke B, Buitelaar J, Verkes RJ, Scholte RH. Interaction between dopamine D2 receptor genotype and parental rule-setting in adolescent alcohol use: evidence for a gene-parenting interaction. Mol Psychiatry. 2010;15(7):727–735. doi: 10.1038/mp.2009.4. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR. Human sex differences in d-amphetamine self-administration. Addiction. 2010;105(4):727–731. doi: 10.1111/j.1360-0443.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol Behav. 2014 doi: 10.1016/j.physbeh.2014.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am. 2013;42(2):201–225. doi: 10.1016/j.ecl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35(11):2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Mendrek A, Paus T, Lopez-Rodriguez AB, Marco EM, Yehuda R, Wagner EJ. A comparative, developmental, and clinical perspective of neurobehavioral sexual dimorphisms. Front Neurosci. 2012;6:84. doi: 10.3389/fnins.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25(8):1087–1097. [PubMed] [Google Scholar]
- Vivian JA, Higley JD, Linnoila M, Woods JH. Oral ethanol self-administration in rhesus monkeys: behavioral and neurochemical correlates. Alcohol Clin Exp Res. 1999;23(8):1352–1361. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Q, Francis R, Caster JM, Kuhn C. Cocaine increases stimulated dopamine efflux in dorsal striatum more in paeriadolescence than adult rats. Neurotoxicology and Teratology, in revision. 2007 doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. J Pharmacol Exp Ther. 2001;297(1):291–298. [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AE, Arrant AE, Caster JM, Kuhn CM. Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology. 2012;62(7):2267–2277. doi: 10.1016/j.neuropharm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol. 2008;30(5):412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther. 2010;335(1):124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31(6):1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine Release and Uptake are Greater in Female than Male Rat Striatum as Measured by Fast Scan Cyclic Voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Weafer J, de Wit H. Sex differences in impulsive action and impulsive choice. Addict Behav. 2014;39(11):1573–1579. doi: 10.1016/j.addbeh.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Consortium I. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;51(7513):185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Kraus CL, Swartzwelder H. Many college freshmen drink at levels far beyond the binge threshold. Alcohol Clin Exp Res. 2006;30(6):1006–1010. doi: 10.1111/j.1530-0277.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Martin NG. Alcohol consumption and alcohol pharmacokinetics: interactions within the normal population. Alcohol Clin Exp Res. 1994;18(2):238–243. doi: 10.1111/j.1530-0277.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlstrom S, Bondy S, Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95(2):251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Dahl RE. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, Nestler EJ. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex. 2009;19(2):435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JR, Means LW, McMillen BA. Effects of pregnancy and progesterone on the consumption of ethanol by the high ethanol preferring (HEP) rat. Alcohol Alcohol. 2000;35(4):344–350. doi: 10.1093/alcalc/35.4.344. [DOI] [PubMed] [Google Scholar]
- Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography. Age and sex differences. Ann N Y Acad Sci. 1988;515:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33(11):4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184(2):174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL, Welte J, Hirsch J. Gender comparison of alcohol exposure on drinking occasions. J Stud Alcohol. 2003;64(6):790–801. doi: 10.15288/jsa.2003.64.790. [DOI] [PubMed] [Google Scholar]
- York JL, Welte J, Hirsch J, Hoffman JH, Barnes G. Association of age at first drink with current alcohol drinking variables in a national general population sample. Alcohol Clin Exp Res. 2004;28(9):1379–1387. doi: 10.1097/01.alc.0000139812.98173.a4. [DOI] [PubMed] [Google Scholar]
- York JL, Welte JW. Gender comparisons of alcohol consumption in alcoholic and nonalcoholic populations. J Stud Alcohol. 1994;55(6):743–750. doi: 10.15288/jsa.1994.55.743. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15(1):23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1(1):21–27. [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68(3):309–322. doi: 10.1016/s0376-8716(02)00225-9. doi: S0376871602002259 [pii] [DOI] [PubMed] [Google Scholar]
- Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008;45(6):986–993. doi: 10.1111/j.1469-8986.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- Zago A, Leao RM, Carneiro-de-Oliveira PE, Marin MT, Cruz FC, Planeta CS. Effects of simultaneous exposure to stress and nicotine on nicotine-induced locomotor activation in adolescent and adult rats. Braz J Med Biol Res. 2012;45(1):33–37. doi: 10.1590/S0100-879X2011007500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198(1):45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 2008;199(4):625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34(4):912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22(4):61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]
- Zilberman ML, Tavares H, Blume SB, el-Guebaly N. Substance use disorders: sex differences and psychiatric comorbidities. Can J Psychiatry. 2003;48(1):5–13. doi: 10.1177/070674370304800103. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165(4):1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35(2):234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers. 2000;68(6):999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- Zulauf CA, Sprich SE, Safren SA, Wilens TE. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16(3):436. doi: 10.1007/s11920-013-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]