Abstract
In plants, environmental adversity often leads to the formation of highly reactive oxygen radicals. Since resistance to such conditions may be correlated with the activity of enzymes involved in oxygen detoxification, we have generated transgenic tobacco plants which express elevated levels of manganese superoxide dismutase (MnSOD) within their chloroplasts or mitochondria. Leaf discs of these plants have been analyzed in conditions in which oxidative stress was generated preferentially within one or the other organelle. It was found that high level overproduction of MnSOD in the corresponding subcellular location could significantly reduce the amount of cellular damage which would normally occur. In contrast, small increases in MnSOD activity were deleterious under some conditions. A generally applicable model correlating the consequences of SOD with the magnitude of its expression is presented.
Full text
PDF

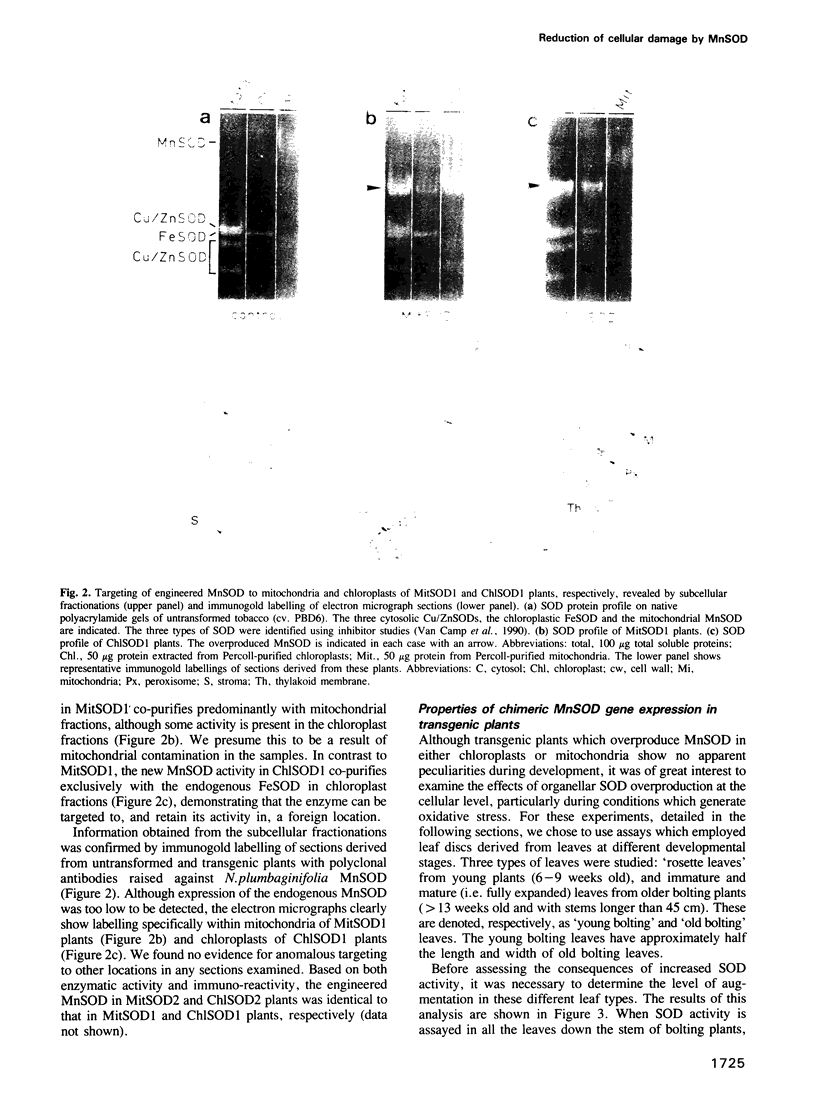
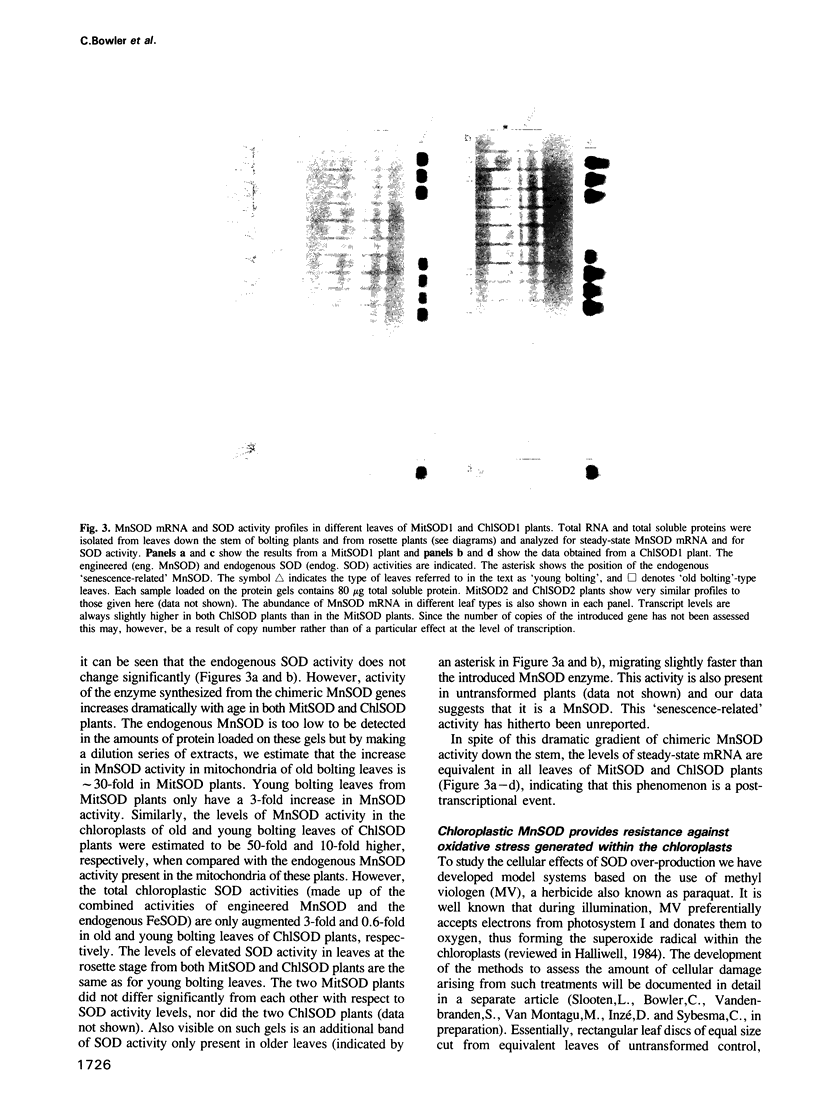
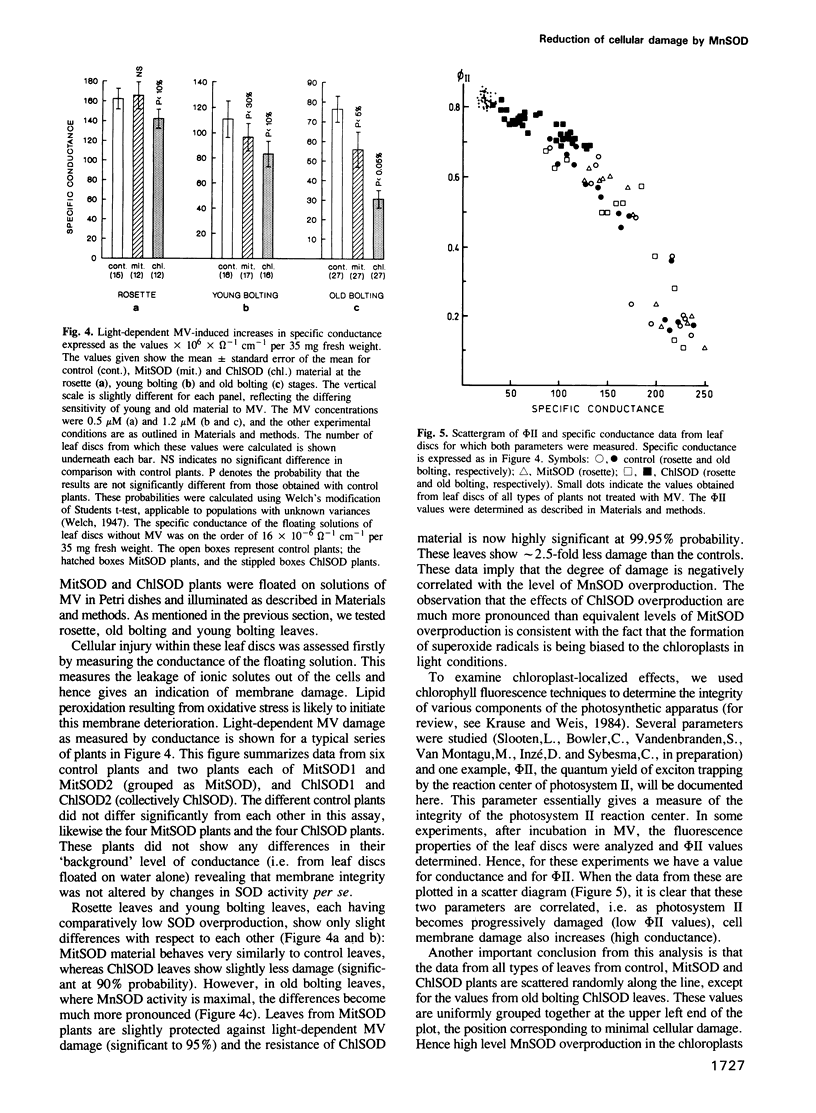
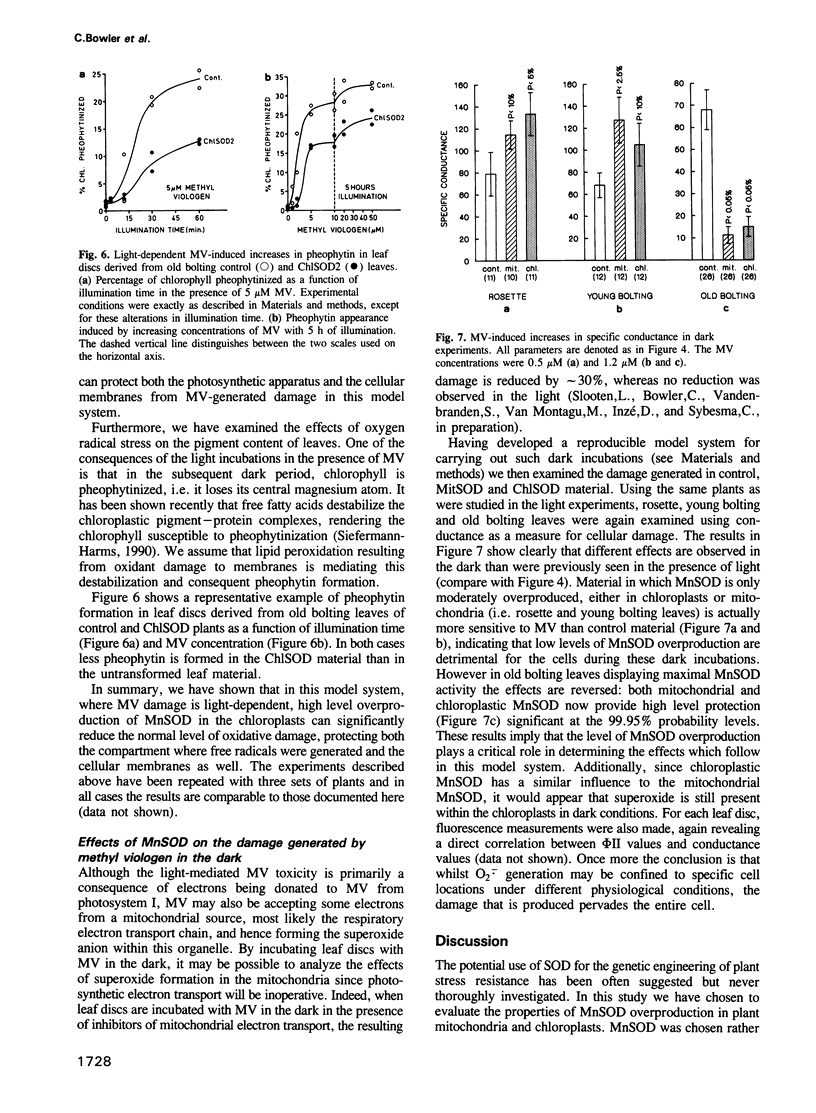




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988 Sep 9;54(6):823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Ausubel F. M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol. 1986 Nov;168(2):795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., De Loose M., Van Montagu M., Inzé D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1989 Jan;8(1):31–38. doi: 10.1002/j.1460-2075.1989.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., Van den Bulcke M., Bauw G., Vandekerckhove J., Van Montagu M., Inzé D. A plant manganese superoxide dismutase is efficiently imported and correctly processed by yeast mitochondria. Proc Natl Acad Sci U S A. 1989 May;86(9):3237–3241. doi: 10.1073/pnas.86.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare D. A., Rabinowitch H. D., Fridovich I. Superoxide dismutase and chilling injury in Chlorella ellipsoidea. Arch Biochem Biophys. 1984 May 15;231(1):158–163. doi: 10.1016/0003-9861(84)90372-2. [DOI] [PubMed] [Google Scholar]
- Cornelissen M., Vandewiele M. Nuclear transcriptional activity of the tobacco plastid psbA promoter. Nucleic Acids Res. 1989 Jan 11;17(1):19–29. doi: 10.1093/nar/17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M., De Brouwer D., Tenning P. Transformation of Brassica napus and Brassica oleracea Using Agrobacterium tumefaciens and the Expression of the bar and neo Genes in the Transgenic Plants. Plant Physiol. 1989 Oct;91(2):694–701. doi: 10.1104/pp.91.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq A., Vandewiele M., De Rycke R., Van Damme J., Van Montagu M., Krebbers E., Vandekerckhove J. Expression and Processing of an Arabidopsis 2S Albumin in Transgenic Tobacco. Plant Physiol. 1990 Apr;92(4):899–907. doi: 10.1104/pp.92.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Gielen J., Seurinck L., Van Montagu M., Schell J. Identification of sequences involved in the polyadenylation of higher plant nuclear transcripts using Agrobacterium T-DNA genes as models. EMBO J. 1983;2(3):419–426. doi: 10.1002/j.1460-2075.1983.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase--implication for gene dosage effects in Down syndrome. Cell. 1988 Jan 29;52(2):259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Gruber M. Y., Glick B. R., Thompson J. E. Cloned manganese superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2608–2612. doi: 10.1073/pnas.87.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Harbour J. R., Bolton J. R. Superoxide formation in spinach chloroplasts: electron spin resonance detection by spin trapping. Biochem Biophys Res Commun. 1975 Jan 2;64(3):803–807. doi: 10.1016/0006-291x(75)90118-7. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Jones G. R. Free radicals in immunological killing: the case of tumor necrotising factor (TNF). Med Hypotheses. 1986 Nov;21(3):267–271. doi: 10.1016/0306-9877(86)90019-8. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko M., Konstantinov A. A., Khomutov G. B., Tikhonov A. N., Ruuge E. K. Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain. FEBS Lett. 1983 May 2;155(1):19–24. doi: 10.1016/0014-5793(83)80200-2. [DOI] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H. Superoxide Dismutase: A POSSIBLE PROTECTIVE ENZYME AGAINST OZONE INJURY IN SNAP BEANS (PHASEOLUS VULGARIS L.). Plant Physiol. 1982 Jun;69(6):1444–1449. doi: 10.1104/pp.69.6.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Monk L. S., Fagerstedt K. V., Crawford R. M. Superoxide Dismutase as an Anaerobic Polypeptide : A Key Factor in Recovery from Oxygen Deprivation in Iris pseudacorus? Plant Physiol. 1987 Dec;85(4):1016–1020. doi: 10.1104/pp.85.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman J. M., Dunsmuir P. Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol. 1990 Apr;14(4):501–511. doi: 10.1007/BF00027496. [DOI] [PubMed] [Google Scholar]
- Van Camp W., Bowler C., Villarroel R., Tsang E. W., Van Montagu M., Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]





