Abstract
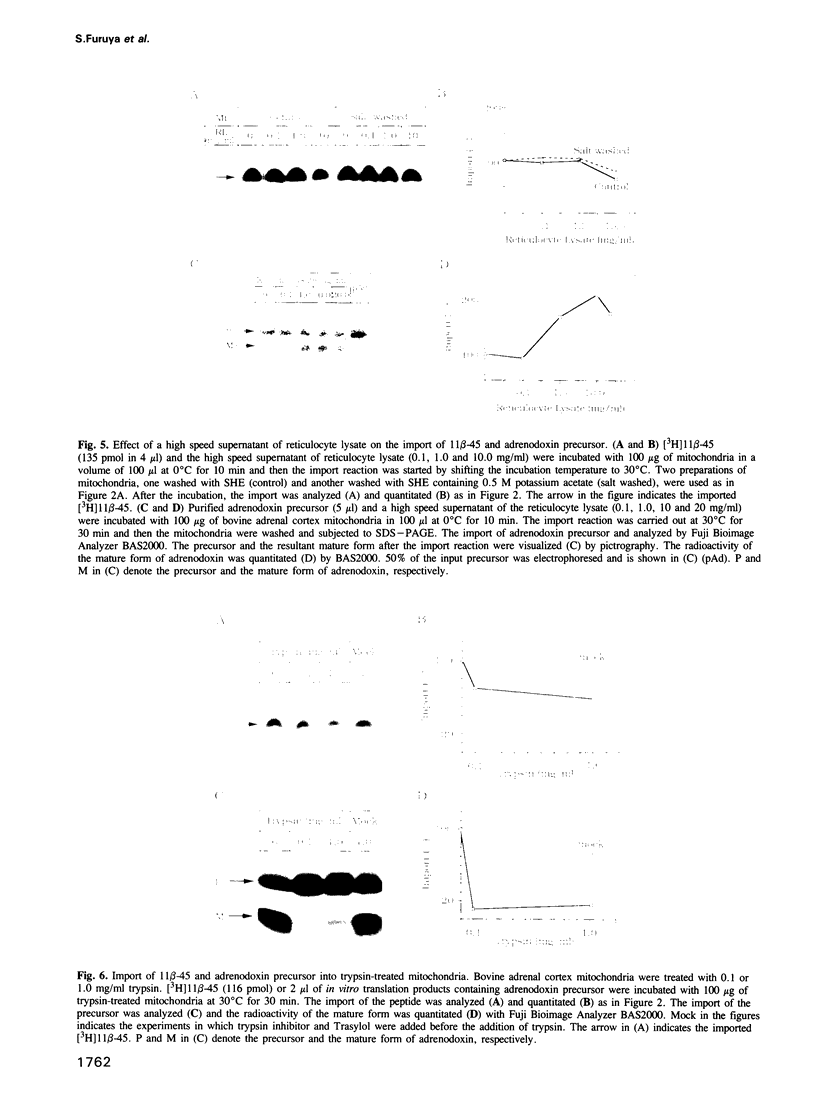
We chemically synthesized a peptide, 11 beta-45, which was composed of 45 amino acid residues including the whole extension peptide and some of the mature portion of bovine cytochrome P-450(11 beta) precursor. 11 beta-45 was imported into mitochondria in vitro depending on the mitochondrial membrane potential, but its import did not require extramitochondrial ATP. Although cytosolic protein factors in the high speed supernatant of reticulocyte lysate are known to stimulate the import of various precursor proteins into mitochondria, the import of 11 beta-45 was not stimulated by cytosolic factors in reticulocyte lysate. The import of the peptide did not require mitochondrial surface protein components because its import was not affected by trypsin treatment of mitochondria. On the other hand, trypsin treatment of mitoplasts resulted in a great reduction in the import of the peptide, indicating that 11 beta-45 interacts during the import process with some protein components located inside mitochondria. These observations indicated that the peptide 11 beta-45 was imported via the potential-dependent pathway as in the case of precursor proteins, but skipped the interactions with cytosolic factors and mitochondrial surface components normally required for the import of precursor proteins.
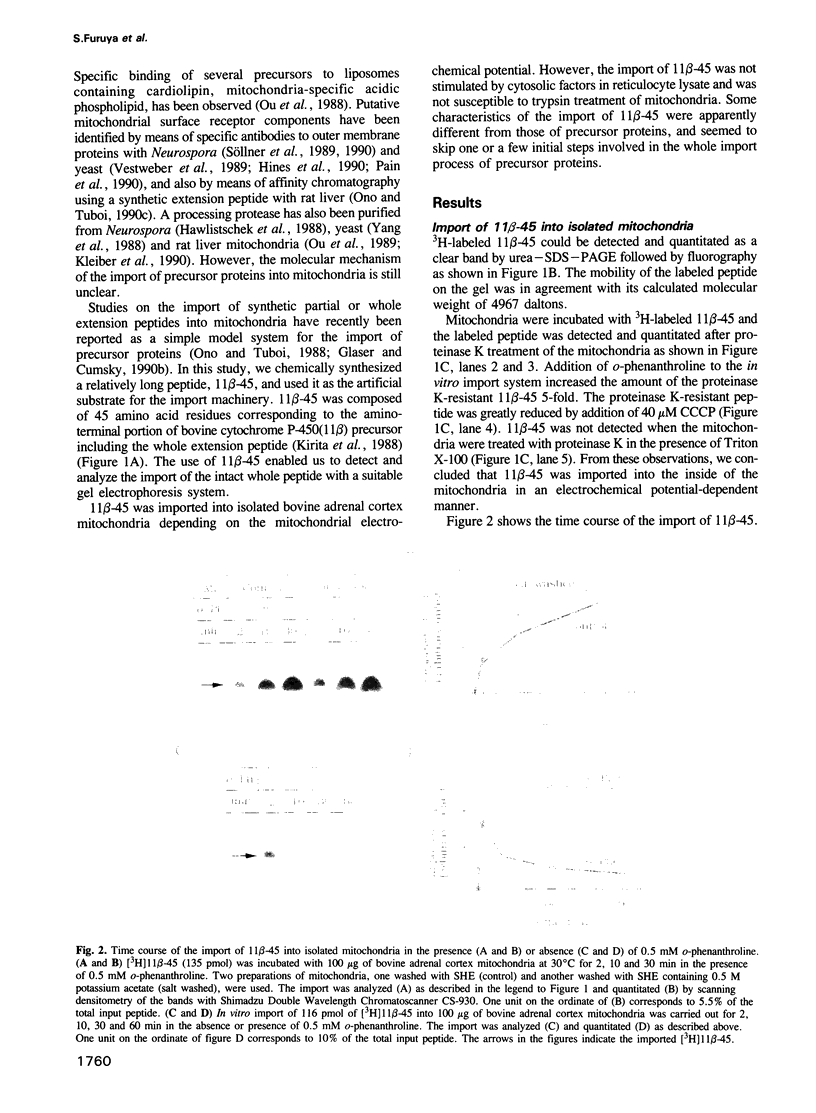
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi H., Lee S., Kanmera T., Mihara H., Kato T. Interaction of synthetic fragments of the extension peptide of cytochrome P-450(SCC) precursor with phospholipid bilayer. J Biochem. 1987 Oct;102(4):813–820. doi: 10.1093/oxfordjournals.jbchem.a122120. [DOI] [PubMed] [Google Scholar]
- Burr F. A., Burr B. Slab gel system for the resolution of oligopeptides below molecular weight of 10,000. Methods Enzymol. 1983;96:239–244. doi: 10.1016/s0076-6879(83)96022-6. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Oppliger W., Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987 Apr;6(4):1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S., Okada M., Ito A., Aoyagi H., Kanmera T., Kato T., Sagara Y., Horiuchi T., Omura T. Synthetic partial extension peptides of P-450(SCC) and adrenodoxin precursors: effects on the import of mitochondrial enzyme precursors. J Biochem. 1987 Oct;102(4):821–832. doi: 10.1093/oxfordjournals.jbchem.a122121. [DOI] [PubMed] [Google Scholar]
- Gillespie L. L., Argan C., Taneja A. T., Hodges R. S., Freeman K. B., Shore G. C. A synthetic signal peptide blocks import of precursor proteins destined for the mitochondrial inner membrane or matrix. J Biol Chem. 1985 Dec 25;260(30):16045–16048. [PubMed] [Google Scholar]
- Glaser S. M., Cumsky M. G. A synthetic presequence reversibly inhibits protein import into yeast mitochondria. J Biol Chem. 1990 May 25;265(15):8808–8816. [PubMed] [Google Scholar]
- Glaser S. M., Cumsky M. G. Localization of a synthetic presequence that blocks protein import into mitochondria. J Biol Chem. 1990 May 25;265(15):8817–8822. [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Hines V., Brandt A., Griffiths G., Horstmann H., Brütsch H., Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J. 1990 Oct;9(10):3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Rosenberg L. E. Arginine in the leader peptide is required for both import and proteolytic cleavage of a mitochondrial precursor. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4930–4933. doi: 10.1073/pnas.82.15.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius R., Lambrechts H., Nicolay K., de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990 Jan 29;1021(2):217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Hwang S. T., Schatz G. Translocation of proteins across the mitochondrial inner membrane, but not into the outer membrane, requires nucleoside triphosphates in the matrix. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8432–8436. doi: 10.1073/pnas.86.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally K. W., Tedeschi H., Maloff B. L. Use of dyes to estimate the electrical potential of the mitochondrial membrane. Biochemistry. 1978 Aug 8;17(16):3419–3428. doi: 10.1021/bi00609a036. [DOI] [PubMed] [Google Scholar]
- Kirita S., Morohashi K., Hashimoto T., Yoshioka H., Fujii-Kuriyama Y., Omura T. Expression of two kinds of cytochrome P-450(11 beta) mRNA in bovine adrenal cortex. J Biochem. 1988 Nov;104(5):683–686. doi: 10.1093/oxfordjournals.jbchem.a122533. [DOI] [PubMed] [Google Scholar]
- Kleiber J., Kalousek F., Swaroop M., Rosenberg L. E. The general mitochondrial matrix processing protease from rat liver: structural characterization of the catalytic subunit. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7978–7982. doi: 10.1073/pnas.87.20.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto T., Morohashi K., Ito A., Omura T. Site-directed mutagenesis of basic amino acid residues in the extension peptide of P-450(SCC) precursor: effects on the import of the precursor into mitochondria. J Biochem. 1987 Oct;102(4):833–838. doi: 10.1093/oxfordjournals.jbchem.a122122. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Mori M. Purified presequence binding factor (PBF) forms an import-competent complex with a purified mitochondrial precursor protein. EMBO J. 1990 Oct;9(10):3201–3208. doi: 10.1002/j.1460-2075.1990.tb07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogishima T., Okada Y., Omura T. Import and processing of the precursor of cytochrome P-450(SCC) by bovine adrenal cortex mitochondria. J Biochem. 1985 Sep;98(3):781–791. doi: 10.1093/oxfordjournals.jbchem.a135335. [DOI] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Protein import into yeast mitochondria is inhibited by antibodies raised against 45-kd proteins of the outer membrane. EMBO J. 1987 Jul;6(7):2109–2115. doi: 10.1002/j.1460-2075.1987.tb02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Tuboi S. Presence of the cytosolic factor stimulating the import of precursor of mitochondrial proteins in rabbit reticulocytes and rat liver cells. Arch Biochem Biophys. 1990 Mar;277(2):368–373. doi: 10.1016/0003-9861(90)90592-m. [DOI] [PubMed] [Google Scholar]
- Ono H., Tuboi S. Purification and identification of a cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. Arch Biochem Biophys. 1990 Aug 1;280(2):299–304. doi: 10.1016/0003-9861(90)90333-t. [DOI] [PubMed] [Google Scholar]
- Ono H., Tuboi S. Purification of the putative import-receptor for the precursor of the mitochondrial protein. J Biochem. 1990 Jun;107(6):840–845. doi: 10.1093/oxfordjournals.jbchem.a123135. [DOI] [PubMed] [Google Scholar]
- Ono H., Tuboi S. The cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. J Biol Chem. 1988 Mar 5;263(7):3188–3193. [PubMed] [Google Scholar]
- Ou W. J., Ito A., Okazaki H., Omura T. Purification and characterization of a processing protease from rat liver mitochondria. EMBO J. 1989 Sep;8(9):2605–2612. doi: 10.1002/j.1460-2075.1989.tb08400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou W. J., Ito A., Umeda M., Inoue K., Omura T. Specific binding of mitochondrial protein precursors to liposomes containing cardiolipin. J Biochem. 1988 Apr;103(4):589–595. doi: 10.1093/oxfordjournals.jbchem.a122312. [DOI] [PubMed] [Google Scholar]
- Pain D., Murakami H., Blobel G. Identification of a receptor for protein import into mitochondria. Nature. 1990 Oct 4;347(6292):444–449. doi: 10.1038/347444a0. [DOI] [PubMed] [Google Scholar]
- Pak Y. K., Weiner H. Import of chemically synthesized signal peptides into rat liver mitochondria. J Biol Chem. 1990 Aug 25;265(24):14298–14307. [PubMed] [Google Scholar]
- Pfaller R., Pfanner N., Neupert W. Mitochondrial protein import. Bypass of proteinaceous surface receptors can occur with low specificity and efficiency. J Biol Chem. 1989 Jan 5;264(1):34–39. [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Randall S. K., Shore G. C. Import of a mutant mitochondrial precursor fails to respond to stimulation by a cytosolic factor. FEBS Lett. 1989 Jul 3;250(2):561–564. doi: 10.1016/0014-5793(89)80796-3. [DOI] [PubMed] [Google Scholar]
- Sheffield W. P., Shore G. C., Randall S. K. Mitochondrial precursor protein. Effects of 70-kilodalton heat shock protein on polypeptide folding, aggregation, and import competence. J Biol Chem. 1990 Jul 5;265(19):11069–11076. [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Pfaller R., Griffiths G., Pfanner N., Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990 Jul 13;62(1):107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Incorporation of a synthetic mitochondrial signal peptide into charged and uncharged phospholipid monolayers. Biochemistry. 1986 Nov 18;25(23):7470–7476. doi: 10.1021/bi00371a032. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Neupert W. Precursor proteins are transported into mitochondria in the absence of proteolytic cleavage of the additional sequences. J Biol Chem. 1983 Nov 10;258(21):13340–13346. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Proteinaceous receptors for the import of mitochondrial precursor proteins. J Biol Chem. 1984 Jun 25;259(12):7850–7856. [PubMed] [Google Scholar]