Abstract
The ecological and physiological significance of body size is well recognized. However, key macroevolutionary questions regarding the dependency of body size trends on the taxonomic scale of analysis and the role of environment in controlling long-term evolution of body size are largely unknown. Here, we evaluate these issues for decapod crustaceans, a group that diversified in the Mesozoic. A compilation of body size data for 792 brachyuran crab and lobster species reveals that their maximum, mean and median body size increased, but no increase in minimum size was observed. This increase is not expressed within lineages, but is rather a product of the appearance and/or diversification of new clades of larger, primarily burrowing to shelter-seeking decapods. This argues against directional selective pressures within lineages. Rather, the trend is a macroevolutionary consequence of species sorting: preferential origination of new decapod clades with intrinsically larger body sizes. Furthermore, body size evolution appears to have been habitat-controlled. In the Cretaceous, reef-associated crabs became markedly smaller than those in other habitats, a pattern that persists today. The long-term increase in body size of crabs and lobsters, coupled with their increased diversity and abundance, suggests that their ecological impact may have increased over evolutionary time.
Keywords: body size, Crustacea, Decapoda, environment, habitat, Mesozoic
1. Introduction
Body size is a key biological trait that affects the physiology and ecology of organisms (e.g. [1–4]). On evolutionary time scales, body size often displays non-random trends in central tendency and dispersion, including multiple variants of ‘Cope's rule’, a hypothesis that, in its strict form, postulates that monophyletic clades of organisms increase in body size over geological time [5–8]. Trends in body size may also correlate with clade diversity [9,10] (but see [11]). Evolution of body size is potentially influenced by a variety of factors that operate over a range of spatial and temporal scales. These include, among others, long-term changes linked to oxygen levels (e.g. [12,13]), spatial gradients (‘Bergman's rule’) across latitudes (e.g. [14]), dwarfing (‘Lilliput effect’) following mass extinctions (e.g. [15,16]; but see [17]), changes in body size as a function of niche availability [18], long-term trends driven by climate change (e.g. [19]) and taxon-specific intrinsic constraints [20]. Despite extensive literature on body size evolution, several key macroevolutionary questions remain poorly understood, including the dependency of body size trends on the taxonomic scale of analysis [8,21] and the role of environment in controlling long-term body size evolution.
Here, the issues of taxonomic scaling and environmental controls are evaluated for Mesozoic (252–66 Ma) marine decapod crustaceans, arthropods assigned to the Modern Evolutionary Fauna [22]. Palaeontological bio-inventorying of Mesozoic decapods has been advancing rapidly in recent decades [23–25], providing an opportunity to assess body size for numerous fossil species from various habitats. In addition, Mesozoic decapods offer a particularly attractive target for macroevolutionary research. Several decapod clades, including lobsters and Brachyura (crabs), evolved and/or radiated during the ‘Mesozoic decapod revolution’ [24,26], an important component of the ‘Mesozoic marine revolution’ [27]. The dramatic diversification of decapods also appears to be linked to the waxing and waning of reefs, as suggested qualitatively [28–30] and quantitatively [24]. However, our current knowledge of decapod body size is limited to a few local to regional palaeoecological studies with a limited temporal scope [31,32]. Using a comprehensive and internally consistent database of Mesozoic decapod sizes compiled at five taxonomic levels (from species to order), we assess whether (i) any significant body size trends are observed through evolutionary time, (ii) body size patterns depend on the taxonomic scale of observation, and (iii) body size patterns differ when data are partitioned by habitat.
2. Material and methods
Species-level body size measurements were assembled for Mesozoic marine lobsters (infraorders Polychelida, Glypheidea, Astacidea and Achelata) and crabs (infraorder Brachyura), the two dominant groups that represent 75% of documented decapod diversity during the Mesozoic [24]. Anomurans and shrimp were excluded here because of their low documented diversity compared with crabs and lobsters, and their patchy fossil record in part due to preservation in Lagerstätten. Furthermore, species-rich clades including paguroids and callianassoids are described primarily using appendage fragments or partial carapaces only, making them unsuitable for analyses herein. Decapod sizes were based on measurements reported in the literature or published figured specimens. For lobsters, per-species body size was estimated separately for each stage using maximum carapace length along the axial part of the carapace (excluding rostrum). The carapace width was not incorporated because it is infrequently measurable due to partial preservation and unsuitable orientation of many fossil lobster remains. For crabs, both maximum carapace length (excluding rostrum) and width were obtained. Because species in lobster and crab clades are predominantly defined based on carapaces (with exception of the uncommon aethrid crabs), both decapod clades are well represented in our data. The resulting database includes 792 species (337 lobsters and 455 crabs) with 888 per-species per-stage body size measurements.
Although large specimens are more likely to be found and reported in the palaeontological literature (e.g. [33]), no association between the number of measured specimens and the maximum body size was observed. Families represented by large numbers of occurrences (e.g. Dakoticancridae) do not dominate the largest body size classes, and the largest specimens (e.g. Avitelmessus grapsoideus) do not represent families with large sample sizes. Moreover, no significant correlation was found between maximum body size and the number of specimens collected for the most diverse Cretaceous decapod locality for which specimen-level size measurements are available (ESM, figure S1). Per-taxon maximum body size was used previously for other invertebrate groups (e.g. [6,11,34–36]) as a viable estimate for adult specimen size. Log-transformed body size measurements were used to quantify patterns in mean, median, maximum and minimum size for both lobsters and crabs.
Additionally, sedimentary facies were recorded for each species per stratigraphic stage using the following tripartite classification: (1) ‘reef-associated limestones’—defined [37]; (2) ‘siliciclastics’—dominated by terrigenous sediments; and (3) ‘other limestones and marls’—predominantly marine non-siliciclastic sediments. A more detailed categorization for decapod-bearing sedimentary facies is possible (see [25], for lobsters), but such fine-scale partitioning of the data is analytically prohibitive given the resulting low sample sizes. Consequently, following Klompmaker et al. [24], we use the above classification to ensure adequate sample size per category for broader-scale analyses comparing fewer categories. A species was assigned to one of the three categories when it was found in one category only. Species for which the sedimentary facies was unknown were excluded (approx. 8% of data).
To assess body size trends through time, decapod occurrences were assigned to stratigraphic stages using the January 2015 ICS time scale. Additionally, taxa with occurrences that could not be ascribed to a single stage (approx. 10% of the data) were treated in three different ways: (i) these taxa were excluded, (ii) size data were assigned to the first stage of the age range, and (iii) size data were assigned to the last stage of the age range. Due to the limited sample size in certain stages for both groups, multiple adjacent stages were binned when necessary until a minimum acceptable number of occurrences was reached. This number was varied to investigate the influence of binning decisions on the estimated trends. A threshold of n = 20 was used in the final analyses to maximize the number of time bins without excessively compromising the per-bin sample sizes. Binning was done separately for crabs, lobsters and pooled data (decapods). For binned stages, average body size was computed using both median and weighted mean, with stage means weighted by per-stage numbers of occurrences. For binned stages, geological age was estimated as the midpoint of this time interval. The confidence bars around per-bin body size statistics were estimated separately for each time bin by uniform bootstrapping (resampling with replacement). Standard errors (standard deviations of bootstrap distributions), rather than 95% confidence intervals, were used here. Standard errors are thought to yield more realistic estimates of analytical errors when taxa are phylogenetically related and thus not independent [38]. In addition, for each bootstrap iteration Spearman's rank correlation (ρ) between geological age and body size was computed. The significance of ρ was estimated using the percentile approach [39]. Finally, Monte Carlo randomizations were performed by randomly reassigning observations (body size values with time bins) across all time bins, while maintaining the original sample sizes for each bin. This simulation produces a null model under the hypothesis that body size patterns are random through time and any observed changes reflect variation in sampling intensity. Confidence bands, based on standard errors, were computed using the percentile approach. All bootstrap and randomization simulations were based on 1000 iterations per bin (repeated simulations indicate that this is an adequate number of iterations to ensure stable parameter estimates here).
Trends within lineages were assessed at the family, genus and species levels. All occurrences without a confident stage-level age assignment were removed. At each taxonomic level, changes in body size through time were computed using two strategies: (i) total net change, based on a body size difference between the first and last occurrence of a given lineage; and (ii) incremental net changes, based on each successive body size change between consecutive stratigraphic stages in which a given lineage was recorded (e.g. a lineage known from four stages would yield three incremental body size changes and one total net change). Neither metric could be computed for lineages occurring in one stage only (90% of the species, 53% of genera and 22% of families). The net changes were expressed as a deviation in log-transformed size through time. If a directional body size trend is present, the distribution of deviations should be significantly different from 0, a hypothesis that is evaluated here using a t-test and a sign-based exact binomial test. In addition, a maximum-likelihood approach was employed to evaluate the observed trends in terms of directional change, random walk and stasis [40]. The models were assessed separately for 10 lower lobster and crab taxa (ESM, table S1) that can be reasonably assumed to have represented monophyletic clades and that are adequately represented for multiple time bins. To investigate the relative size of speciose families, a deviation between the median body size of a given family and the median body size of the higher-level taxon to which that family belongs was computed. The median for lobsters was based on carapace length measurements, whereas the median for crabs was based on the geometric mean size (√(length × width)). Deviations were also computed for lobster infraorders. This is because the monophyly of lobsters is controversial, whereas that of infraorders is better supported (e.g. [41]; but see [42,43]). Deviations for crabs were estimated relative to the median value for the entire group because Brachyura have been interpreted to be monophyletic (e.g. [44,45]). Only families with at least 10 species were included for this particular part as they have most effect on overall size trends (81.1% of data). Isolated occurrences of species (more than three stages apart from any other data point) were excluded.
Size trends per sedimentary facies were investigated for lobsters and crabs separately using two different strategies. First, the median size was determined per period (Triassic, Jurassic and Cretaceous) per facies. Second, the proportion of species per facies per 10 mm size class was computed for pre-Cretaceous and Cretaceous time intervals for both taxa separately.
3. Results
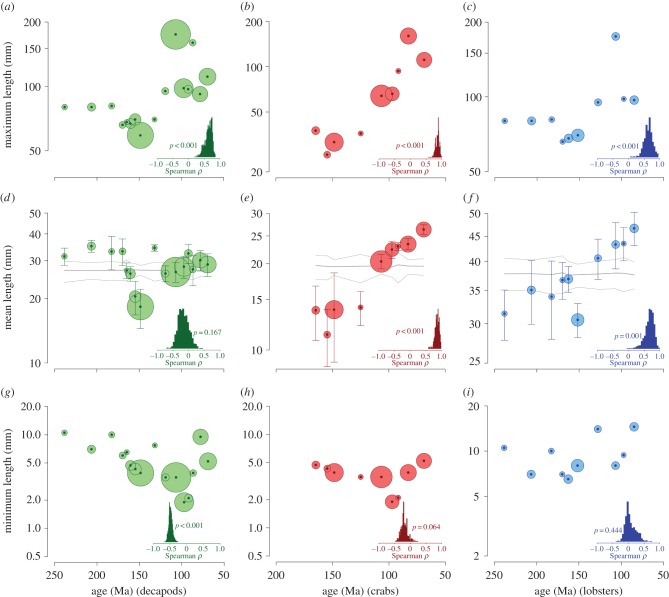
We compiled and analysed a new database (available on Dryad) of Mesozoic crustaceans totalling 888 per-species per-stage body size measurements for 792 species (337 lobsters and 455 crabs). At higher taxonomic scales, regardless of the sample binning and stage assignment strategies, log-transformed mean and median body size remain stable, maximum size increases, and minimum size decreases when crab and lobster data are combined (figure 1a,d,g; ESM, figures S2–S8). By contrast, log-transformed mean, median and maximum body length of lobsters and crabs analysed separately show an increase throughout the Mesozoic (figure 1b,c,e,f). The same results were obtained for crab body width and the geometric mean of crab width and length (ESM, figure S9). Minimum body size does not increase or decrease for either group (figure 1h,i). These visually obvious patterns are supported by bootstrap simulations (inset charts and p-values in figure 1). Monte Carlo randomizations consistently indicate that the trends in mean body size for crabs and lobsters cannot be attributed to variable sampling of randomly distributed data (grey lines in figure 1e,f). For pooled data (figure 1d), many of the bin values fall within the predictions of the null model.
Figure 1.
Maximum (upper row), weighted mean (middle row) and minimum lengths for (a,d,g) decapods, (b,e,h) crabs and (c,f,i) lobsters during the Mesozoic. Confidence bars are ±1 s.e. based on standard deviations of bootstrap-resampled weighted means (1000 iterations per time bin). p-values report significance for the null hypothesis that ρ = 0. Grey lines represent randomization estimates (1000 iteration each) for temporal trends in the weighted mean expected under the null model of body size measurements randomly distributed across means. Thick middle lines represent the mean of the simulation; thin lines represent confidence bands (±1 s.e.). Inset charts (lower right of each panel) depict bootstrap distributions (1000 iterations each) of the Spearman's rank statistic (ρ) for correlation between geological age and weighted mean size. Area of dots represents relative sample size (lowest: 20; highest: 115 for Decapoda, 84 for crabs, 49 for lobsters). Vertical axis with logarithmic scale (base 2). (Online version in colour.)
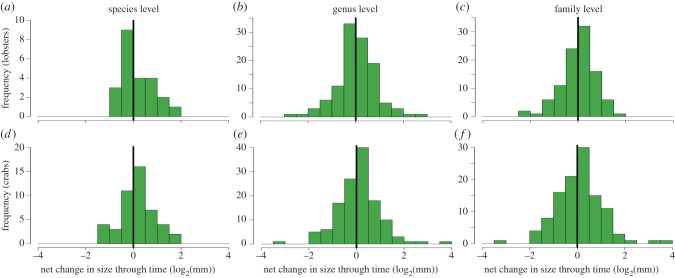
The increase in body size is not reflected within lineages of lower taxonomic units (family, genus and species) as a comparable frequency of lineages increased and decreased in body size through time (p > 0.05 in all cases; figure 2; ESM, figure S10). Consistently, none of 10 adequately sampled decapod lineages (genera or families) offered support for a directional trend in body size through time (ESM, table S1). Species-rich families that have a particularly large body size and appeared and/or became diverse in the mid-to-late Mesozoic are the Palinuridae, Erymidae and Nephropidae for lobsters, and the raninoidan families (Cenomanocarcinidae, Raninidae and Palaeocorystidae) for crabs (figure 3; ESM, figure S11). At the infraorder level for lobsters, the Achelata (mainly palinurids) and Astacidea (mainly nephropids) are relatively large (ESM, figure S12).
Figure 2.
Trends within lineages for lobsters and crabs based on incremental net changes. p-values (t-test and sign-based exact binomial test, respectively): (a) 0.18, 1; (b) 0.58, 0.92; (c) 0.52, 0.27; (d) 0.10, 0.14; (e) 0.25, 0.14; (f) 0.24, 0.30. (Online version in colour.)
Figure 3.
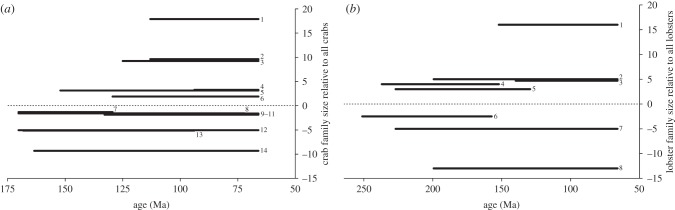
Size of Mesozoic crab and lobster families, where the medians are applied to their respective stratigraphic range. (a) Bar graph of crab family sizes (medians of geometric means) relative to median of geometric means for all crabs. (1) Cenomanocarcinidae, 19 measured species in different stages; (2) Raninidae, 19 sp.; (3) Palaeocorystidae, 35 sp.; (4) Retroplumidae, 14 sp.; (5) Dynomenidae, 15 sp.; (6) Etyidae, 30 sp.; (7) Tanidromitidae, 15 sp.; (8) Goniodromitidae, 69 sp.; (9) Homolidae, 27 sp.; (10) Lyreididae, 15 sp.; (11) Necrocarcinidae, 38 sp.; (12) Longodromitidae, 40 sp.; (13) Prosopidae, 29 sp.; (14) Glaessneropsidae, 13 sp. (b) Bar graph of lobster family sizes (medians of length in mm) relative to median of length for all lobsters. (1) Palinuridae, 25 sp.; (2) Erymidae, 83 sp.; (3) Nephropidae, 58 sp.; (4) Coleiidae, 27 sp.; (5) Eryonidae, 12 sp.; (6) Litogastroidae, 30 sp.; (7) Glypheidae, 62 sp.; (8) Mecochiridae, 29 sp.
The lobster and crab size increase throughout the Mesozoic is observed regardless of the type of environment they inhabited (figure 4). However, the magnitude of increase differs across environments. In the Jurassic, crab body sizes are statistically indistinguishable (Kruskal–Wallis: p = 0.29) for the three habitat types (figure 4). The size increase from the Jurassic to the Cretaceous is notably smaller for reef-associated crabs and, consequently, Cretaceous crabs are significantly smaller than those in other habitats (Mann–Whitney: p = 0.0001; ESM, figure S13a). By contrast, the habitat-dependent divergence in body size is not observed for Cretaceous lobsters (ESM, figure S13b).
Figure 4.
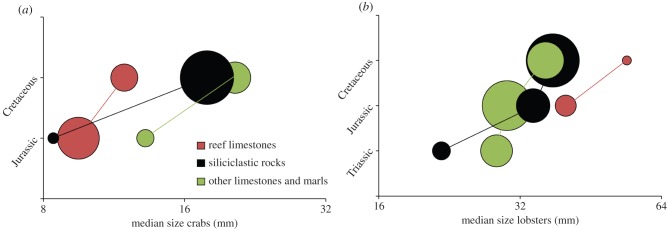
(a) Crab and (b) lobster species size per Mesozoic period across reef limestones, siliciclastic rocks, and other limestones and marls. Area of dots represents relative sample size (highest: 144 for crabs, 70 for lobsters; lowest: 6 for crabs, 2 for lobsters). Horizontal axis with logarithmic scale (base 2). (Online version in colour.)
The duration of families and the associated median lengths do not correlate significantly for both lobsters and crabs (ESM, figure S14; Spearman's ρ: p = 0.22 for lobsters; p = 0.75 for crabs; first differences p = 0.88 and 0.44, respectively), indicating that body size and clade longevity do not influence each other substantially. Also, diversity and median lengths do not correlate for both groups (ESM, figure S15, Spearman's ρ: p = 0.93 for lobsters; p = 0.42 for crabs; first differences p = 0.74 and 0.47, respectively).
4. Discussion
Although Cope's rule in the strict sense is most appropriate to consider at the lineage level, positive directionality of body size trends has been tested at a variety of taxonomic scales (table 1). Empirical tests rarely included more than one taxonomic level (table 1). The results reported here highlight the critical importance of taxonomic scaling in studying evolutionary trends in body size. Different trends in the body size of Mesozoic decapods are visible depending on the taxonomic scale of observation. At the intermediate taxonomic scale (lobsters and crabs), both groups follow a trend that is transitional between the ‘Cope's rule sensu stricto’ and the ‘increased variance’ models [5,7]: a progressive increase in maximum, mean and median body size, but not minimum size. However, these trends disappear at the coarsest taxonomic level, when data are pooled for all analysed decapods. This outcome is caused by the differential diversification of the two analysed decapod groups. That is, the mean and median body size of decapods remained relatively stable through the Mesozoic because the body size trends observed within lobsters and crabs are offset by an increase in the proportion of the smaller crabs at the expense of the relatively larger lobsters [24].
Table 1.
Recent examples of the presence (Y) or absence (N) of Cope's rule expression or variants thereof in marine invertebrates; numbers inside parenthesis denote number of taxa used.
| phylum | class | subclass | order | infraorder | family | genus | species | |
|---|---|---|---|---|---|---|---|---|
| herein: Mesozoic decapods | N (1) | Y? (5) | N (70) | N (237) | N (792) | |||
| [21]: Palaeozoic brachiopods | Y (1) | Y (4) | Y (11) | N (10) | ||||
| [5,6]: Cretaceous bivalves + gastropods | N (approx. 300) | |||||||
| [46]: Cenozoic planktonic foraminifera | N (1) | |||||||
| [47]: Ordovician trilobites | N (1) | |||||||
| [48]: Early Jurassic ammonoids | N (1) | |||||||
| [19]: Cenozoic ostracods | Y (1) | Y (9) | ||||||
| [49]: Cenozoic ostracods | Y (19) |
Directional changes in size are also not manifested at the finer taxonomic scales of species-, genus- and family-level lineages. Thus, the body size trends within lobsters and crabs cannot be explained as a scaled-up product of directional trends occurring at finer taxonomic scales. Instead, the trends appear to have been driven by the appearance and radiation of new, relatively large-bodied decapod clades (figure 3). This process may represent an example of species sorting (e.g. [50]): preferential origination of new decapod clades with intrinsically larger body sizes. Clades that are largely responsible for the size increase are the frog crabs (Raninoida) for crabs, and palinurids (Achelata) and nephropids (Astacidea) for lobsters. Raninoids originated in the Cretaceous and have a burying lifestyle (e.g. [51–53]). A hiding lifestyle may apply also for the relatively large, species-rich lobster clades in the Cretaceous based on the sheltering and/or burrowing habit of their extant relatives [54–56]. This effective hiding behaviour may have enabled many species within those clades to obtain a larger maximum size, suggesting ecological determinants driving species sorting and the resulting evolutionary increase in body size.
Regardless of their environmental setting, lobsters and crabs increased in size throughout the Mesozoic (figure 4), demonstrating that these patterns are not due to uneven habitat sampling or long-term habitat shifts through time. However, the rate of increase varies, with reef-dwelling crabs increasing at a slower pace. The habitat-related differentiation of crab body size initiated in the late Mesozoic appears to have persisted to the present day, as extant decapods in reefs remain significantly smaller compared with those living in other habitats [57]. Habitat differentiation in body size was also reported for another clade of crustaceans, the stomatopods [58].
The small size of crab carapaces and their reduced abdomen early in their evolutionary history [29,59] may have helped them to invade and radiate in Late Jurassic reef environments because the reef framework increased the number of places to hide from predators. Conversely, the relatively long tails and large size of lobsters may have limited early lobsters from invading and radiating in Late Jurassic reefs because lobsters are rare in Mesozoic reefs [24]. Given their occurrence in modern reefs (20% of lobster species [60], notably the Achelata), they probably invaded reefs during the Cenozoic [25]. The ongoing decline of modern reefs (e.g. [61,62]) may result in the preferential loss of small-sized decapods because reef decapods are a substantial part of marine decapod diversity [63,64].
The observed patterns are unlikely to have been influenced substantially by major biases related to sampling coverage and taphonomic processes. First, the database is dominated by occurrences (more than 80%) from Europe and North America that represent a comparable latitudinal range for all time intervals (i.e. the body size trends are not a product of shifts in the latitudinal coverage through time). Although modern decapods are smaller near the equator compared with those in higher latitudes [65], differences in size between equatorial and North American/European decapods may have been minimal in the Mesozoic. This is because of the substantially warmer climates then (e.g. [66,67]) in combination with the more southern palaeogeographic location of North America and Europe, especially in the early Mesozoic [68]. Second, all data are derived from the literature, suggesting that specimen selection size biases, if any, are likely to be consistent across the database [30], which should not influence overall patterns. Third, lithification-related biases, which may lead to preferential loss of small-sized specimens [69,70], are unlikely to have influenced the results because nearly all Mesozoic decapod occurrences originated from lithified rocks and are expected to have been comparably affected by this bias.
This study demonstrates that the ‘Mesozoic decapod revolution’ [24]—a dramatic increase in biodiversity, the appearance and increasing dominance of crabs and Anomura, habitat expansion, and widening of behavioural traits including burrowing and durophagous predation (e.g. [26,28,29,71])—was accompanied by a progressive increase (due, probably, to species sorting) in the average body size of lobsters and crabs. Decapods also became more common prey in the Mesozoic [72]. Given that decapod abundance and species richness tend to be positively correlated in modern and ancient ecosystems (e.g. [31,57,73–75]), the long-term increase in lobster and crab body size documented here, in combination with their increase in taxon richness and abundance, indicates that the impact of these crustaceans on marine ecosystems may have increased substantially during the ‘Mesozoic marine revolution’ [27]. Finally, this study highlights the importance of scale dependency on body size evolution and the value of investigating evolutionary trends in body size in a palaeo-environmental context.
Acknowledgements
We thank Andrzej Kaim and an anonymous reviewer for helpful comments. This is UF Contribution to Paleobiology 691.
Data accessibility
Proprietary decapod database on Dryad: http://dx.doi.org/10.5061/dryad.kt57q.
Authors' contributions
A.A.K., C.E.S. and R.M.F. collected data; A.A.K. and M.K. designed study, analysed data and wrote paper; C.E.S. and R.M.F. provided input on paper; all gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Jon L. and Beverly A. Thompson Endowment Fund to A.A.K. and M.K., and NSF grants EF-0531670 and EAR-1223206 to R.M.F. and C.E.S.
References
- 1.Peters R. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Calder WA. 1984. Size, function, and life history. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Martin AP, Palumbi SR. 1993. Body size, metabolic rate, generation time, and the molecular clock. Proc. Natl Acad. Sci. USA 90, 4087–4091. ( 10.1073/pnas.90.9.4087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner JT. 2006. Why size matters: from bacteria to blue whales. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Jablonski D. 1996. Body size and macroevolution. In Evolutionary paleobiology (eds Jablonski D, Erwin DH, Lipps JH.), pp. 256–289. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Jablonski D. 1997. Body-size evolution in Cretaceous molluscs and the status of Cope's rule. Nature 385, 250–252. ( 10.1038/385250a0) [DOI] [Google Scholar]
- 7.Trammer J, Kaim A. 1997. Body size and diversity exemplified by three trilobite clades. Acta Palaeontol. Pol. 42, 1–12. [Google Scholar]
- 8.Heim NA, Knope ML, Schaal EK, Wang SC, Payne JL. 2015. Cope's rule in the evolution of marine animals. Science 347, 867–870. ( 10.1126/science.1260065) [DOI] [PubMed] [Google Scholar]
- 9.Trammer J, Kaim A. 1999. Active trends, passive trends, Cope's rule and temporal scaling: new categorization of cladogenetic changes in body size. Hist. Biol. 13, 113–125. ( 10.1080/08912969909386577) [DOI] [Google Scholar]
- 10.Trammer J. 2002. Power formula for Cope's rule. Evol. Ecol. Res. 4, 147–153. [Google Scholar]
- 11.Roy K, Jablonski D, Martien KK. 2000. Invariant size–frequency distributions along a latitudinal gradient in marine bivalves. Proc. Natl Acad. Sci. USA 97, 13 150–13 155. ( 10.1073/pnas.97.24.13150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapelle G, Peck LS. 1999. Polar gigantism dictated by oxygen availability. Nature 399, 114–115. ( 10.1038/20099) [DOI] [Google Scholar]
- 13.Payne JL, et al. 2009. Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proc. Natl Acad. Sci. USA 106, 24–27. ( 10.1073/pnas.0806314106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meiri S, Dayan T. 2003. On the validity of Bergmann's rule. J. Biogeogr. 30, 331–351. ( 10.1046/j.1365-2699.2003.00837.x) [DOI] [Google Scholar]
- 15.Twitchett RJ. 2007. The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 132–144. ( 10.1016/j.palaeo.2006.11.038) [DOI] [Google Scholar]
- 16.Smith JJ, Hasiotis ST, Kraus MJ, Woody DT. 2009. Transient dwarfism of soil fauna during the Paleocene–Eocene thermal maximum. Proc. Natl Acad. Sci. USA 106, 17 655–17 660. ( 10.1073/pnas.0909674106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brayard A, Nützel A, Stephen DA, Bylund KG, Jenks J, Bucher H. 2010. Gastropod evidence against the Early Triassic Lilliput effect. Geology 38, 147–150. ( 10.1130/G30553.1) [DOI] [Google Scholar]
- 18.Smith FA, et al. 2010. The evolution of maximum body size of terrestrial mammals. Science 330, 1216–1219. ( 10.1126/science.1194830) [DOI] [PubMed] [Google Scholar]
- 19.Hunt G, Roy K. 2006. Climate change, body size evolution, and Cope's Rule in deep-sea ostracodes. Proc. Natl Acad. Sci. USA 103, 1347–1352. ( 10.1073/pnas.0510550103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer BA, Marquet PA. 2013. Processes responsible for patterns in body mass distributions. In Animal body size: linking pattern and process across space, time, and taxonomic group (eds Smith FA, Lyons K.), pp. 168–186. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Novack-Gottshall PM, Lanier MA. 2008. Scale-dependence of Cope's rule in body size evolution of Paleozoic brachiopods. Proc. Natl Acad. Sci. USA 105, 5430–5434. ( 10.1073/pnas.0709645105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepkoski JJ., Jr 1981. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 7, 36–53. [Google Scholar]
- 23.Schweitzer CE, Feldmann RM, Garassino A, Karasawa H, Schweigert G. 2010. Systematic list of fossil decapod crustaceans species. Crust. Monogr. 10, 1–222. [Google Scholar]
- 24.Klompmaker AA, Schweitzer CE, Feldmann RM, Kowalewski M. 2013. The influence of reefs on the rise of Mesozoic marine crustaceans. Geology 41, 1179–1182. ( 10.1130/G34768.1) [DOI] [Google Scholar]
- 25.Schweitzer CE, Feldmann RM. 2014. Lobster (Decapoda) diversity and evolutionary patterns through time. J. Crust. Biol. 34, 820–847. ( 10.1163/1937240X-00002288) [DOI] [Google Scholar]
- 26.Schweitzer CE, Feldmann RM. 2010. The Decapoda (Crustacea) as predators on Mollusca through geologic time. Palaios 25, 167–182. ( 10.2110/palo.2009.p09-054r) [DOI] [Google Scholar]
- 27.Vermeij GJ. 1977. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology 3, 245–258. [Google Scholar]
- 28.Glaessner MF. 1969. Decapoda. In Treatise on invertebrate paleontology, part R4 (2) (ed. Moore RC.), pp. R400–R533, R626–R628 Boulder, CO: Geological Society of America; Lawrence, University of Kansas Press. [Google Scholar]
- 29.Förster R. 1985. Evolutionary trends and ecology of Mesozoic decapod crustaceans. Trans. R. Soc. Edinb. Earth Sci. 76, 299–304. ( 10.1017/S0263593300010518) [DOI] [Google Scholar]
- 30.Krobicki M, Zatoń M. 2008. Middle and Late Jurassic roots of brachyuran crabs: palaeoenvironmental distribution during their early evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 263, 30–43. ( 10.1016/j.palaeo.2008.01.025) [DOI] [Google Scholar]
- 31.Klompmaker AA, Ortiz JD, Wells NA. 2013. How to explain a decapod crustacean diversity hotspot in a mid-Cretaceous coral reef. Palaeogeogr. Palaeoclimatol. Palaeoecol. 374, 256–273. ( 10.1016/j.palaeo.2013.01.024) [DOI] [Google Scholar]
- 32.Vega FJ, Phillips GE, Nyborg T, Flores-Ventura J, Clements D, Espinosa B, Solís-Pichardo G. 2013. Morphology and size variation of a portunoid crab from the Maastrichtian of the Americas. J. South Am. Earth Sci. 47, 116–135. ( 10.1016/j.jsames.2013.07.005) [DOI] [Google Scholar]
- 33.Krause RA, Stempien JA, Kowalewski M, Miller AI. 2007. Body size estimates from the literature: utility and potential for macroevolutionary studies. Palaios 22, 60–73. ( 10.2110/palo.2005.p05-122r) [DOI] [Google Scholar]
- 34.Stanley SM. 1973. An explanation for Cope's rule. Evolution 27, 1–26. ( 10.2307/2407115) [DOI] [PubMed] [Google Scholar]
- 35.Lockwood R. 2005. Body size, extinction events, and the early Cenozoic record of veneroid bivalves: a new role for recoveries? Paleobiology 31, 578–590. ( 10.1666/04070.1) [DOI] [Google Scholar]
- 36.Zhang Y, Payne JL. 2012. Size-frequency distributions along a latitudinal gradient in Middle Permian fusulinoideans. PLoS ONE 7, e38603 ( 10.1371/journal.pone.0038603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiessling W. 2002. Secular variations in Phanerozoic reef ecosystems. In Phanerozoic reef patterns, SEPM special publication, vol. 72 (eds Kiessling W, Flügel E, Golonka J.), pp. 625–690. Tulsa, OK: SEPM. [Google Scholar]
- 38.Foote M. 1994. Morphological disparity in Ordovician–Devonian crinoids and the early saturation of morphological space. Paleobiology 20, 320–344. [Google Scholar]
- 39.Efron B. 1979. Bootstrap methods: another look at the jackknife. Ann. Stat. 7, 1–26. ( 10.1214/aos/1176344552) [DOI] [Google Scholar]
- 40.Hunt G. 2007. The relative importance of directional change, random walks, and stasis in the evolution of fossil lineages. Proc. Natl Acad. Sci. USA 104, 18 404–18 408. ( 10.1073/pnas.0704088104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracken-Grissom HD, et al. 2014. The emergence of the lobsters: phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (Decapoda: Achelata, Astacidea, Glypheidea, Polychelida). Syst. Biol. 64, 457–479. ( 10.1093/sysbio/syu008) [DOI] [PubMed] [Google Scholar]
- 42.Tsang LM, Ma KY, Ahyong ST, Chan TY, Chu KH. 2008. Phylogeny of Decapoda using two nuclear protein-coding genes: origin and evolution of the Reptantia. Mol. Phylogenet. Evol. 48, 359–368. ( 10.1016/j.ympev.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 43.Toon A, Finley M, Staples J, Crandall KA. 2009. Decapod phylogenetics and molecular evolution. In Decapod Crustacean phylogenetics: crustacean issues, vol. 18 (eds Martin JW, Crandall KA, Felder DL.), pp. 14–28. Boca Raton, FL: CRC Press. [Google Scholar]
- 44.Brösing A, Richter S, Scholtz G. 2007. Phylogenetic analysis of the Brachyura (Crustacea, Decapoda) based on characters of the foregut with establishment of a new taxon. J. Zoolog. Syst. Evol. Res. 45, 20–32. ( 10.1111/j.1439-0469.2006.00367.x) [DOI] [Google Scholar]
- 45.Tsang LM, Schubart CD, Ahyong ST, Lai JC, Au EY, Chan TY, Ng PKL, Chu KH. 2014. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol. Biol. Evol. 31, 1173–1187. ( 10.1093/molbev/msu068) [DOI] [PubMed] [Google Scholar]
- 46.Arnold AJ, Kelly DC, Parker WC. 1995. Causality and Cope's rule: evidence from the planktonic foraminifera. J. Paleontol. 69, 203–210. [Google Scholar]
- 47.Bell MA, Braddy SJ. 2012. Cope's rule in the Ordovician trilobite family Asaphidae (order Asaphida): patterns across multiple most parsimonious trees. Hist. Biol. 24, 223–230. ( 10.1080/08912963.2011.616201) [DOI] [Google Scholar]
- 48.Dommergues JL, Montuire S, Neige P. 2002. Size patterns through time: the case of the Early Jurassic ammonite radiation. Paleobiology 28, 423–434. () [DOI] [Google Scholar]
- 49.Hunt G, Wicaksono SA, Brown JE, MacLeod KG. 2010. Climate-driven body-size trends in the ostracod fauna of the deep Indian Ocean. Palaeontology 53, 1255–1268. ( 10.1111/j.1475-4983.2010.01007.x) [DOI] [Google Scholar]
- 50.Jablonski D. 2008. Species selection: theory and data. Annu. Rev. Ecol. Evol. Syst. 39, 501–524. ( 10.1146/annurev.ecolsys.39.110707.173510) [DOI] [Google Scholar]
- 51.Van Bakel BWM, Guinot D, Artal P, Fraaije RHB, Jagt JWM. 2012. A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Decapoda, Brachyura, Podotremata). Zootaxa 3215, 1–216. [Google Scholar]
- 52.Guinot D, Tavares M, Castro P. 2013. Significance of the sexual openings and supplementary structures on the phylogeny of brachyuran crabs (Crustacea, Decapoda, Brachyura), with new nomina for higher-ranked podotreme taxa. Zootaxa 3665, 1–414. ( 10.11646/zootaxa.3665.1.1) [DOI] [PubMed] [Google Scholar]
- 53.Karasawa H, Schweitzer CE, Feldmann RM, Luque J. 2014. Phylogeny and classification of Raninoida (Decapoda: Brachyura). J. Crust. Biol. 34, 216–272. ( 10.1163/1937240X-00002216) [DOI] [Google Scholar]
- 54.Rice AL, Chapman CJ. 1971. Observations on the burrows and burrowing behaviour of two mud-dwelling decapod crustaceans, Nephrops norvegicus and Goneplax rhomboides. Mar. Biol. 10, 330–342. ( 10.1007/BF00368093) [DOI] [Google Scholar]
- 55.Cobb JS. 1977. Review of the habitat behaviour of the clawed lobsters (Homarus and Nephrops). Div. Fish. Oceanogr. Circ. 7, 143–157. [Google Scholar]
- 56.Butler MJ, Steneck RS, Herrnkind WF. 2006. Juvenile and adult ecology. In Lobsters: biology, management, aquaculture and fisheries (ed. Phillips B.), pp. 263–309. Ames, IA: Blackwell Publishing. [Google Scholar]
- 57.Abele LG. 1976. Comparative species composition and relative abundance of decapod crustaceans in marine habitats of Panama. Mar. Biol. 38, 263–278. ( 10.1007/BF00388939) [DOI] [Google Scholar]
- 58.Reaka ML. 1986. Biogeographic patterns of body size in stomatopod Crustacea: ecological and evolutionary consequences. In Biogeography of the Crustacea: crustacean issues, vol. 4 (eds Gore RH, Heck KL.), pp. 209–235. Rotterdam, The Netherlands: Balkema Press. [Google Scholar]
- 59.Van Straelen V. 1925. Contribution à l’étude des Crustacés Décapodes de la période jurassique. Mém. Acad. R. Belgique (Sci.) [collected in number 4, series 2 = (2) 4] 7, 1–462, pls. 1–10. [Google Scholar]
- 60.Holthuis LB. 1991. Marine lobsters of the world: an annotated and illustrated catalogue of species of interest to fisheries known to date. FAO Species Catalogue 13 Rome, Italy: FAO Fisheries Synopsis 125. [Google Scholar]
- 61.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 62.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serène R. 1972. On the brachyuran fauna of the Indo-Pacific coral reefs. In Proc. First Int. Symp. on Corals and Coral Reefs, Mandapam Camp, 12–16 January 1969, pp. 419–424. Cochin, India: Marine Biological Association of India. [Google Scholar]
- 64.Legall N, Poupin J. 2015. Internet—CRUSTA: database of Crustacea (Decapoda and Stomatopoda), with special interest for those collected in French overseas territories. See http://crustiesfroverseas.free.fr/ (accessed 23 January 2015). [Google Scholar]
- 65.Steele DH. 1988. Latitudinal variations in body size and species diversity in marine decapod crustaceans of the continental shelf. Int. Rev. gesamten Hydrobiol. Hydrogr. 73, 235–246. ( 10.1002/iroh.19880730209) [DOI] [Google Scholar]
- 66.Sellwood BW, Valdes PJ. 2006. Mesozoic climates: general circulation models and the rock record. Sediment. Geol. 190, 269–287. ( 10.1016/j.sedgeo.2006.05.013) [DOI] [Google Scholar]
- 67.Price GD, Twitchett RJ, Wheeley JR, Buono G. 2013. Isotopic evidence for long term warmth in the Mesozoic. Sci. Rep. 3, 1438 ( 10.1038/srep01438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scotese CR. 2001. Atlas of Earth history, 1: Paleogeography. Arlington, TX: PALEOMAP Project. [Google Scholar]
- 69.Sessa JA, Patzkowsky ME, Bralower TJ. 2009. The impact of lithification on the diversity, size distribution, and recovery dynamics of marine invertebrate assemblages. Geology 37, 115–118. ( 10.1130/G25286A.1) [DOI] [Google Scholar]
- 70.Hendy AJW. 2011. Taphonomic overprints on Phanerozoic trends in biodiversity: lithification and other secular megabiases. In Taphonomy: process and bias through time, Topics in Geobiology, vol. 32 (eds Allison PA, Bottjer DJ.), pp. 19–77. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 71.Dietl GP, Vega FJ. 2008. Specialized shell-breaking crab claws in Cretaceous seas. Biol. Lett. 4, 290–293. ( 10.1098/rsbl.2008.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klompmaker AA, Karasawa H, Portell RW, Fraaije RHB, Ando Y. 2013. An overview of predation evidence found on fossil decapod crustaceans with new examples of drill holes attributed to gastropods and octopods. Palaios 28, 599–613. ( 10.2110/palo.2013.p13-026r) [DOI] [Google Scholar]
- 73.Abele LG. 1974. Species diversity of decapod crustaceans in marine habitats. Ecology 55, 156–161. ( 10.2307/1934629) [DOI] [Google Scholar]
- 74.Bishop GA. 1983. Fossil decapod crustaceans from the Lower Cretaceous, Glen Rose Limestone of central Texas. San Diego Soc. Nat. Hist. 20, 27–55. [Google Scholar]
- 75.Klompmaker AA. 2013. Extreme diversity of decapod crustaceans from the mid-Cretaceous (late Albian) of Spain: implications for Cretaceous decapod paleoecology. Cretaceous Res. 41, 150–185. ( 10.1016/j.cretres.2012.12.003) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Proprietary decapod database on Dryad: http://dx.doi.org/10.5061/dryad.kt57q.