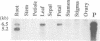Abstract
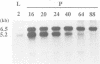
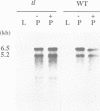
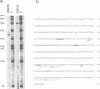
The Tnt1 transposable element of tobacco belongs to the retrotransposon family and shares the structural features of viral retroelements including two long terminal repeats (LTRs) which are known to contain promoter regions. We show that two Tnt1 RNAs of 5.2 and 6.5 kb can be found. The 5.2 kb RNA matches with the size of the Tnt1 elements so far isolated (5.3 kb), whilst the evidence suggests that the 6.5 kb RNA could be a chimaeric RNA initiated in a gene in which Tnt1 has inserted. The Tnt1 5.2 kb RNA starts in the LTR, and the LTR can promote the expression of a translational LTR-beta-glucuronidase (GUS) fusion at a high level in transient expression assays. The Tnt1 5.2 kb RNA and the LTR-GUS fusion of transgenic tobacco plants are specifically expressed in leaf-derived protoplasts whereas they are not expressed in leaf tissue. The 5.2 kb RNA is also transcribed at low levels in roots. This RNA is induced after 2 h of maceration in the protoplast isolation medium, and its level declines rapidly after protoplast isolation. The induction requires only the presence of cell wall hydrolases, and is independent of wounding and plasmolysis. The induction of Tnt1 expression is not mediated by typical oligosaccharide elicitors released from the cell wall known to mediate defense gene responses. Tnt1 transcription features provide a first example of tissue culture-induced mutagenesis in plants and a molecular basis for some of the somaclonal variation events.
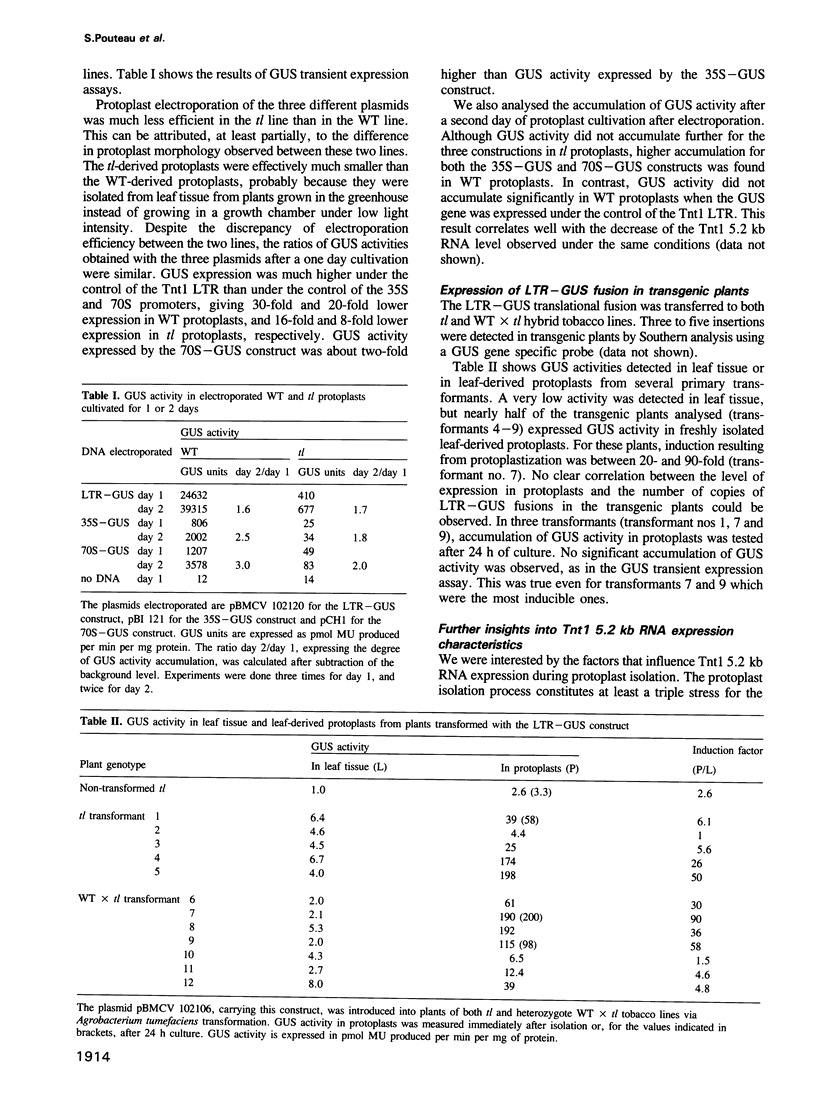
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Primer extension analysis of RNA. Methods Enzymol. 1989;180:347–369. doi: 10.1016/0076-6879(89)80111-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradshaw V. A., McEntee K. DNA damage activates transcription and transposition of yeast Ty retrotransposons. Mol Gen Genet. 1989 Sep;218(3):465–474. doi: 10.1007/BF00332411. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Moore C. Determination of 3' end processing in retroelements. Trends Genet. 1990 Sep;6(9):276–277. doi: 10.1016/0168-9525(90)90215-r. [DOI] [PubMed] [Google Scholar]
- Curcio M. J., Hedge A. M., Boeke J. D., Garfinkel D. J. Ty RNA levels determine the spectrum of retrotransposition events that activate gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1990 Jan;220(2):213–221. doi: 10.1007/BF00260484. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ruby S. W., Toole J. J., Roberts B. E., Rubin G. M. Translation and developmental regulation of RNA encoded by the eukaryotic transposable element copia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7107–7111. doi: 10.1073/pnas.77.12.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Day H. M., Turton J. F., Shen W. J., Cullimore J. V., Oliver J. E. Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. Plant Cell. 1989 Apr;1(4):391–401. doi: 10.1105/tpc.1.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Lucas W. J., Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989 Mar;1(3):301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandbastien M. A., Spielmann A., Caboche M. Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature. 1989 Jan 26;337(6205):376–380. doi: 10.1038/337376a0. [DOI] [PubMed] [Google Scholar]
- Grosset J., Marty I., Chartier Y., Meyer Y. mRNAs newly synthesized by tobacco mesophyll protoplasts are wound-inducible. Plant Mol Biol. 1990 Sep;15(3):485–496. doi: 10.1007/BF00019165. [DOI] [PubMed] [Google Scholar]
- Guerche P., Bellini C., Le Moullec J. M., Caboche M. Use of a transient expression assay for the optimization of direct gene transfer into tobacco mesophyll protoplasts by electroporation. Biochimie. 1987 Jun-Jul;69(6-7):621–628. doi: 10.1016/0300-9084(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Harberd N P, Flavell R B, Thompson R D. Identification of a transposon-like insertion in a Glu-1 allele of wheat. Mol Gen Genet. 1987 Sep;209(2):326–332. doi: 10.1007/BF00329661. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M. A., Mottinger J., Freeling M. A low copy number, copia-like transposon in maize. EMBO J. 1985 May;4(5):1093–1101. doi: 10.1002/j.1460-2075.1985.tb03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junakovic N., Di Franco C., Best-Belpomme M., Echalier G. On the transposition of copia-like nomadic elements in cultured Drosophila cells. Chromosoma. 1988 Nov;97(3):212–218. doi: 10.1007/BF00292963. [DOI] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984 Nov 16;226(4676):792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Developmental expression of Drosophila melanogaster retrovirus-like transposable elements. EMBO J. 1987 Feb;6(2):419–424. doi: 10.1002/j.1460-2075.1987.tb04771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham I., Sobel S., Comer J., Jaenisch R., Barklis E. Retrovirus activation in embryonal carcinoma cells by cellular promoters. Genes Dev. 1989 Dec;3(12B):2062–2071. doi: 10.1101/gad.3.12b.2062. [DOI] [PubMed] [Google Scholar]
- Peschke V. M., Phillips R. L., Gengenbach B. G. Discovery of transposable element activity among progeny of tissue culture--derived maize plants. Science. 1987 Nov 6;238(4828):804–807. doi: 10.1126/science.238.4828.804. [DOI] [PubMed] [Google Scholar]
- Planckaert F., Walbot V. Molecular and genetic characterization of Mu transposable elements in Zea mays: behavior in callus culture and regenerated plants. Genetics. 1989 Nov;123(3):567–578. doi: 10.1093/genetics/123.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S., Cherel I., Vaucheret H., Caboche M. Nitrate Reductase mRNA Regulation in Nicotiana plumbaginifolia Nitrate Reductase-Deficient Mutants. Plant Cell. 1989 Nov;1(11):1111–1120. doi: 10.1105/tpc.1.11.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. E., Lockett T. J., Young M. W. Analysis of transcripts from two families of nomadic DNA. J Mol Biol. 1982 May 5;157(1):49–68. doi: 10.1016/0022-2836(82)90512-5. [DOI] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. R., Kalitsis P., Joseph J. L., Sentry J. W. Plant retrotransposon from Lilium henryi is related to Ty3 of yeast and the gypsy group of Drosophila. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5015–5019. doi: 10.1073/pnas.86.13.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand D. J., McDonald J. F. Copia is transcriptionally responsive to environmental stress. Nucleic Acids Res. 1985 Jun 25;13(12):4401–4410. doi: 10.1093/nar/13.12.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas D. F., Ausubel F. M. A copia-like transposable element family in Arabidopsis thaliana. Nature. 1988 Nov 17;336(6196):242–244. doi: 10.1038/336242a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Yun Y. D., Davis R. L. Copia RNA levels are elevated in dunce mutants and modulated by cAMP. Nucleic Acids Res. 1989 Oct 25;17(20):8313–8326. doi: 10.1093/nar/17.20.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziarczyk P., Fourcade-Peronnet F., Simonart S., Maisonhaute C., Best-Belpomme M. Functional analysis of the long terminal repeats of Drosophila 1731 retrotransposon: promoter function and steroid regulation. Nucleic Acids Res. 1989 Nov 11;17(21):8631–8644. doi: 10.1093/nar/17.21.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]