Abstract
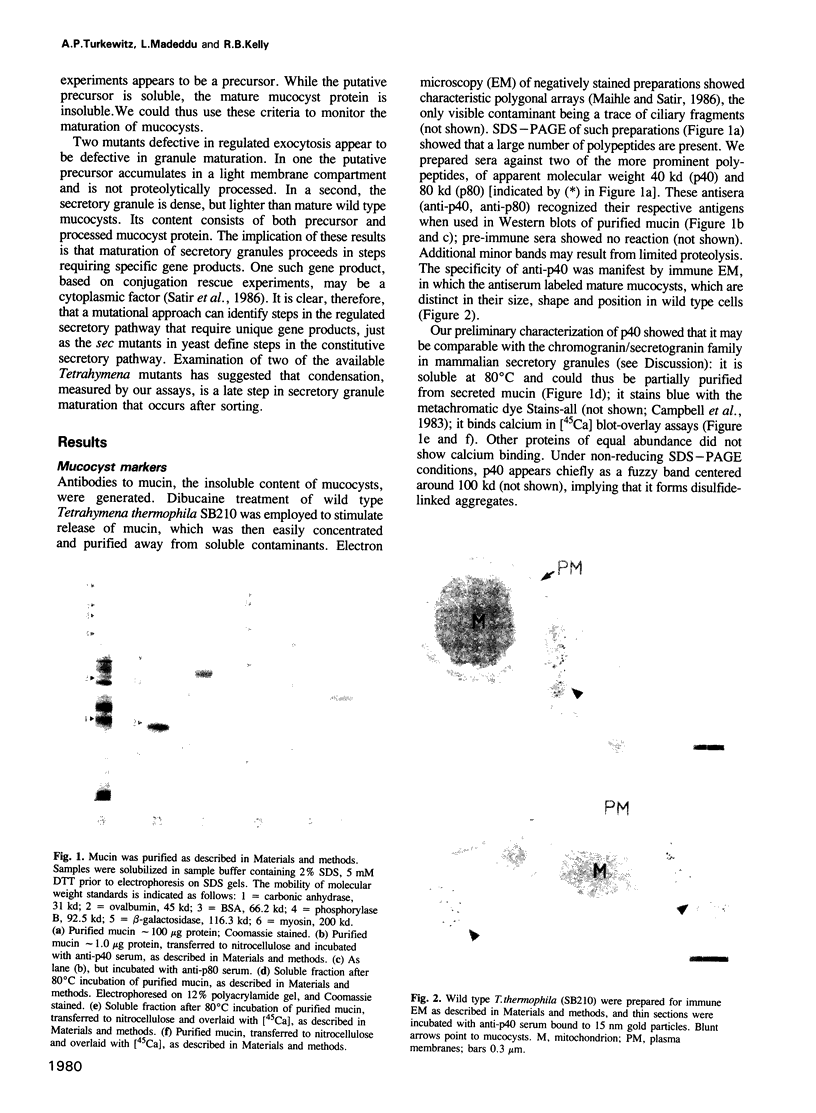
Tetrahymena thermophila, a ciliated protozoan, has a well-developed pathway of regulated secretion from dense core granules called mucocysts. Since exocytosis-defective mutants are available, steps in the biogenesis of dense core granules and their fusion with the plasma membrane may be resolved genetically. To describe the steps in biochemical terms, we have generated antisera against mucocyst content proteins. One antiserum is directed against a calcium binding protein, p40, that is released on stimulation of exocytosis. p40 is shown to associate with an insoluble matrix in mature mucocysts. In addition, the antiserum recognizes a larger protein, p60, that is soluble, is not found in mature mucocysts and is not released on stimulation. Pulse-chase experiments support a precursor-product relationship between p60 and p40. Using these proteins as markers, two mutant Tetrahymena strains defective in exocytosis have been shown to accumulate the putative precursor p60 in organelles that can be distinguished from one another and from wild type mucocysts on the basis of density. The kinetics of appearance of insoluble p40 and the mutant phenotypes suggest a model of mucocyst maturation in which sorting precedes matrix condensation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., de Loubresse N. G., Beisson J. Proteolytic cleavage and maturation of the crystalline secretion products of Paramecium. J Mol Biol. 1984 Dec 25;180(4):1065–1081. doi: 10.1016/0022-2836(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Bannon G. A., Perkins-Dameron R., Allen-Nash A. Structure and expression of two temperature-specific surface proteins in the ciliated protozoan Tetrahymena thermophila. Mol Cell Biol. 1986 Sep;6(9):3240–3245. doi: 10.1128/mcb.6.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L. M., Robinson J. S., Klionsky D. J., Emr S. D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988 Oct;107(4):1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson J., Lefort-Tran M., Pouphile M., Rossignol M., Satir B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J Cell Biol. 1976 Apr;69(1):126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Craik C. S., Matsuuchi L., Kelly R. B. In vitro mutagenesis of trypsinogen: role of the amino terminus in intracellular protein targeting to secretory granules. J Cell Biol. 1987 Aug;105(2):659–668. doi: 10.1083/jcb.105.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Chung K. N., Walter P., Aponte G. W., Moore H. P. Molecular sorting in the secretory pathway. Science. 1989 Jan 13;243(4888):192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Cohen J., Beisson J. Genetic analysis of the relationships between the cell surface and the nuclei in Paramecium tetraurella. Genetics. 1980 Aug;95(4):797–818. doi: 10.1093/genetics/95.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Wilhelm J. M. Post-translational cleavage of mucocyst precursors in Tetrahymena. J Biol Chem. 1981 Oct 25;256(20):10475–10484. [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S. Purification and partial characterization of the H immobilization antigens of Tetrahymena thermophila. J Protozool. 1986 May;33(2):204–208. doi: 10.1111/j.1550-7408.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Hagn C., Schober M. Chromogranins A, B, and C: widespread constituents of secretory vesicles. Ann N Y Acad Sci. 1987;493:120–134. doi: 10.1111/j.1749-6632.1987.tb27189.x. [DOI] [PubMed] [Google Scholar]
- Frankel J. An analysis of the recovery of tetrahymena from effects of cycloheximide. J Cell Physiol. 1970 Aug;76(1):55–63. doi: 10.1002/jcp.1040760109. [DOI] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Hausmann K. Extrusive organelles in protists. Int Rev Cytol. 1978;52:197–276. doi: 10.1016/s0074-7696(08)60757-3. [DOI] [PubMed] [Google Scholar]
- Hünseler P., Scheidgen-Kleyboldt G., Tiedtke A. Isolation and characterization of a mutant of Tetrahymena thermophila blocked in secretion of lysosomal enzymes. J Cell Sci. 1987 Aug;88(Pt 1):47–55. doi: 10.1242/jcs.88.1.47. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefort-Tran M., Aufderheide K., Pouphile M., Rossignol M., Beisson J. Control of exocytotic processes: cytological and physiological studies of trichocyst mutants in Paramecium tetraurelia. J Cell Biol. 1981 Feb;88(2):301–311. doi: 10.1083/jcb.88.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihle N. J., Satir B. H. Protein secretion in Tetrahymena thermophila. Characterization of the major proteinaceous secretory proteins. J Biol Chem. 1986 Jun 5;261(16):7566–7570. [PubMed] [Google Scholar]
- Maihle N. J., Satir B. H. Protein secretion in Tetrahymena thermophila: characterization of the secretory mutant strain SB281. J Cell Sci. 1985 Oct;78:49–65. doi: 10.1242/jcs.78.1.49. [DOI] [PubMed] [Google Scholar]
- Moore H. H., Kelly R. B. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986 May 22;321(6068):443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Nozawa Y., Thompson G. A., Jr Studies of membrane formation in Tetrahymena pyriformis. II. Isolation and lipid analysis of cell fractions. J Cell Biol. 1971 Jun;49(3):712–721. doi: 10.1083/jcb.49.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Orias E., Flacks M., Satir B. H. Isolation and ultrastructural characterization of secretory mutants of Tetrahymena thermophila. J Cell Sci. 1983 Nov;64:49–67. doi: 10.1242/jcs.64.1.49. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Heuser J. E., Nelson D. L. Dissociation and reassociation of trichocyst proteins: biochemical and ultrastructural studies. J Cell Sci. 1987 Feb;87(Pt 1):3–25. doi: 10.1242/jcs.87.1.3. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Nelson D. L., Ling E., Angeletti R. H. Chromogranin A-like proteins in the secretory granules of a protozoan, Paramecium tetraurelia. J Biol Chem. 1987 Dec 25;262(36):17264–17267. [PubMed] [Google Scholar]
- Roberts C. T., Jr, Orias E. On the mechanism of adaptation to protein synthesis inhibitors by Tetrahymena. Facilitation, cross adaptation, and resensitization. J Cell Biol. 1974 Sep;62(3):707–716. doi: 10.1083/jcb.62.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt J., Schekman R. A hitchhiker's guide to analysis of the secretory pathway in yeast. Methods Cell Biol. 1989;32:3–36. doi: 10.1016/s0091-679x(08)61165-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Yamashiro C. T., Kane P. M., Stevens T. H. Protein targeting to the yeast vacuole. Trends Biochem Sci. 1989 Aug;14(8):347–350. doi: 10.1016/0968-0004(89)90170-9. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Reichman M., Orias E. Conjugation rescue of an exocytosis-competent membrane microdomain in Tetrahymena thermophila mutants. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8221–8225. doi: 10.1073/pnas.83.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Stoller T. J., Shields D. Retrovirus-mediated expression of preprosomatostatin: posttranslational processing, intracellular storage, and secretion in GH3 pituitary cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2087–2095. doi: 10.1083/jcb.107.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A., Fuller S. D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987 Sep;105(3):1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Bravin M., Zorzato F., Margreth A. Isolation of terminal cisternae of frog skeletal muscle. Calcium storage and release properties. J Biol Chem. 1988 Jul 15;263(20):9901–9907. [PubMed] [Google Scholar]











