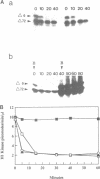
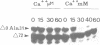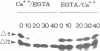Abstract
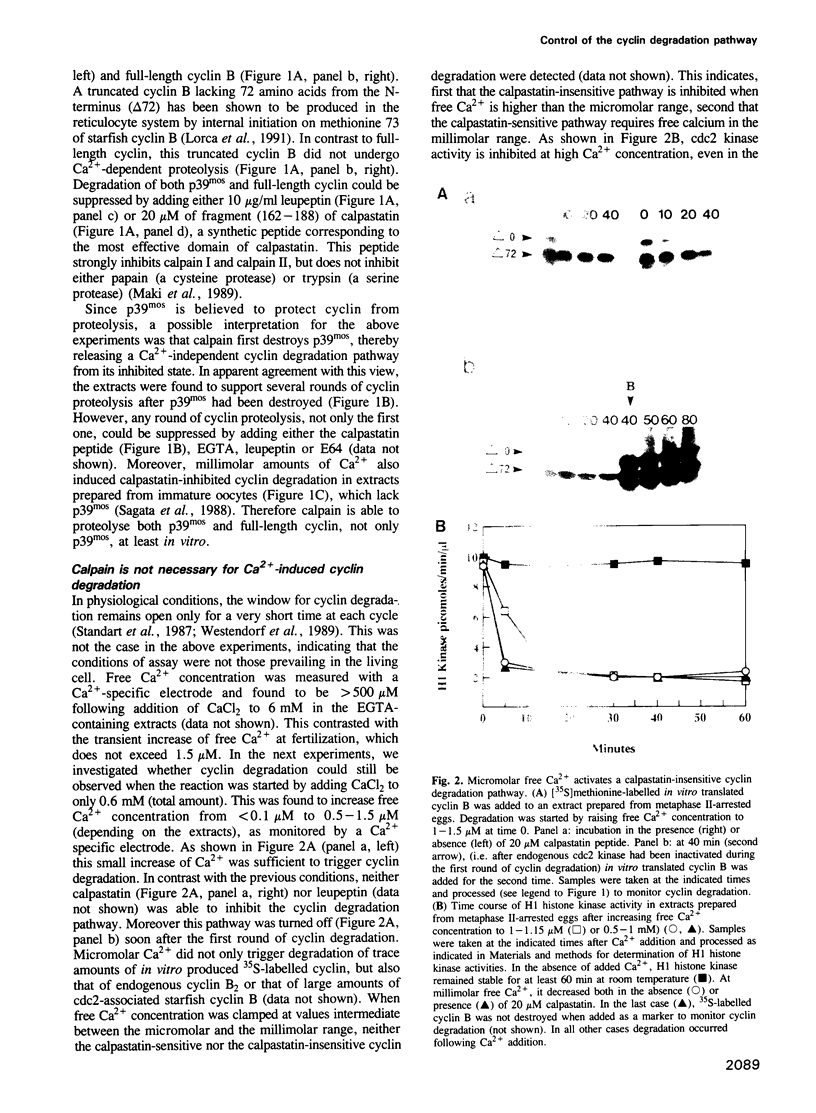
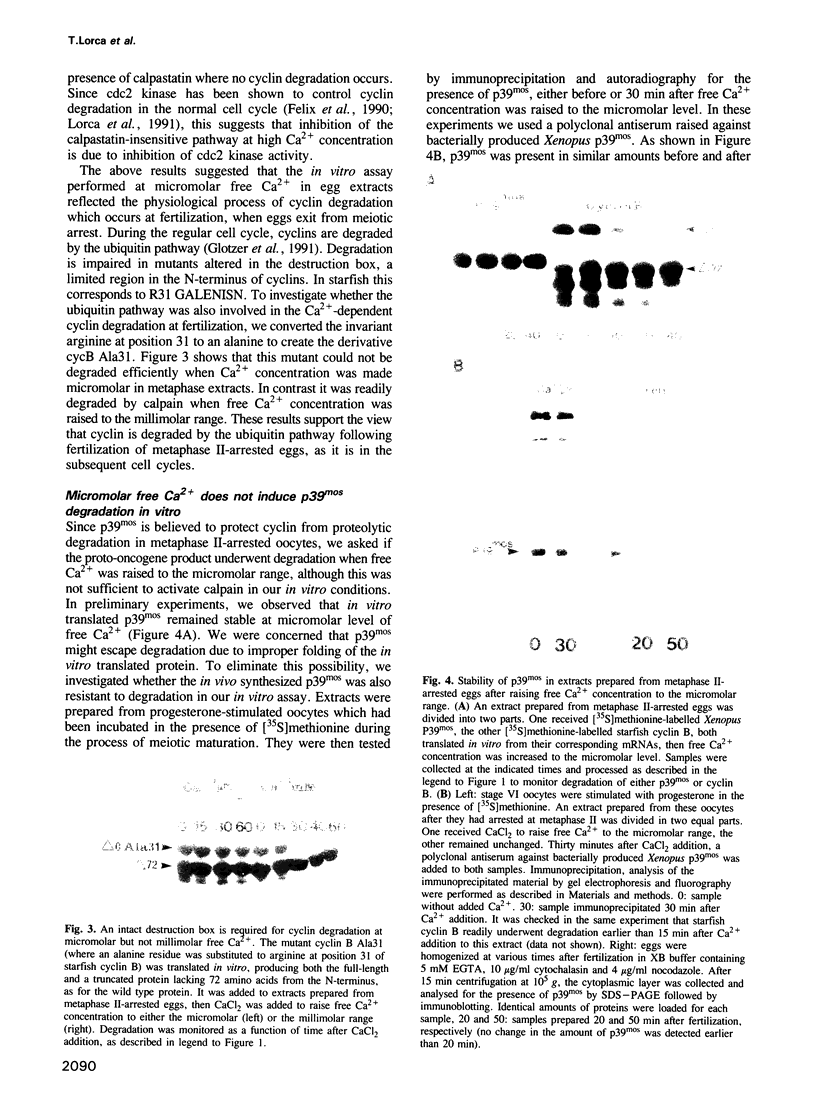
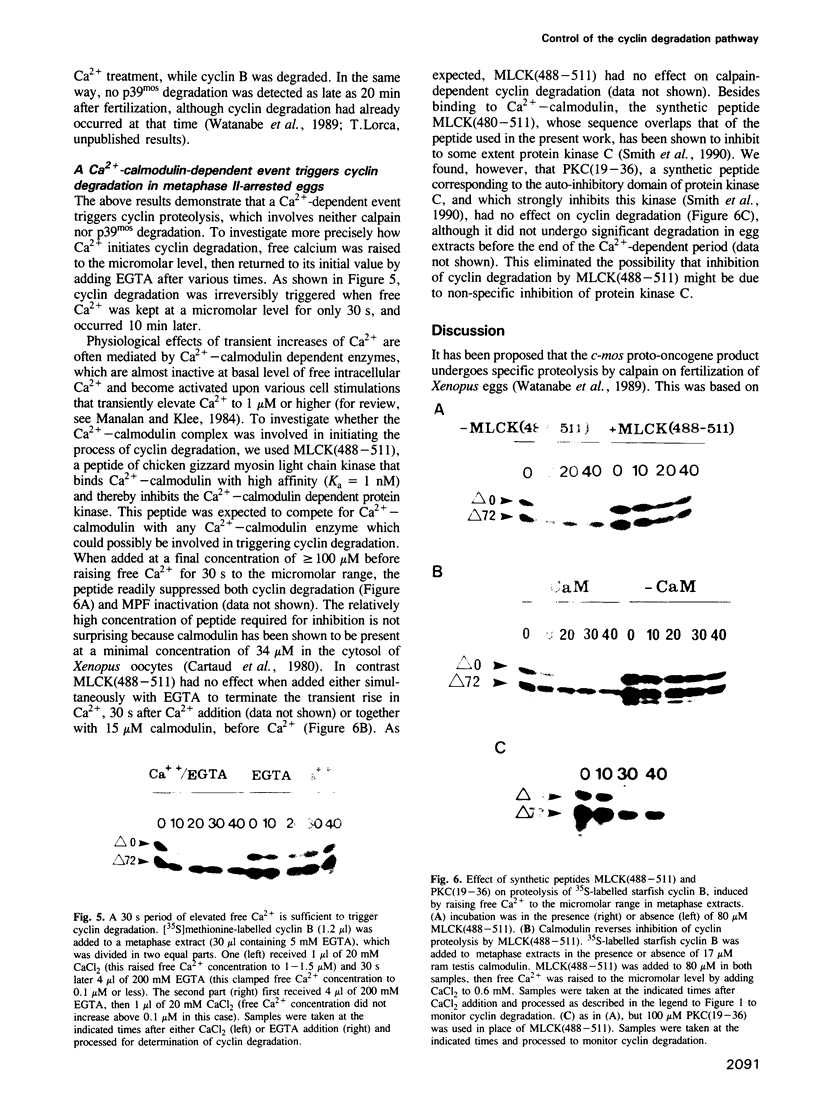
Exit from M phase, which requires cyclin degradation, is prevented from occurring in unfertilized eggs of vertebrates arrested at second meiotic metaphase due to a cytostatic factor recently identified as p39mos, the product of the proto-oncogene c-mos. Calpain can destroy both p39mos and cyclin in vitro in extracts prepared from metaphase-arrested Xenopus eggs, but only when free Ca2+ concentration is raised to the millimolar range. When free Ca2+ concentration is raised for only 30 s to the micromolar range, as occurs in physiological conditions after fertilization, cyclin degradation is induced, byt p39mos is not degraded. Cyclin proteolysis at micromolar free Ca2+, is not inhibited by calpastatin, and therefore does not involve calpain. A cyclin mutant modified in the destruction box is found to be resistant at micromolar, but not millimolar free Ca2+, suggesting that the ubiquitin pathway mediates cyclin degradation at micromolar Ca2+ concentration whereas calpain is involved at the millimolar level. A synthetic peptide which binds Ca(2+)-calmodulin with high affinity suppresses cyclin degradation at micromolar but not millimolar free Ca2+, and this only when it is present in the extract during the first 30 s after raising free Ca2+ concentration. The inhibition of the cyclin degradation pathway by the Ca(2+)-calmodulin binding peptide can be overcome by adding calmodulin. These results strongly suggest that a Ca(2+)-calmodulin process is required as an early event following fertilization to release the cyclin degradation pathway from inhibition in metaphase-arrested eggs. In contrast, p39mos degradation is not required.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autric F., Ferraz C., Kilhoffer M. C., Cavadore J. C., Demaille J. G. Large-scale purification and characterization of calmodulin from ram testis: its metal-ion-dependent conformers. Biochim Biophys Acta. 1980 Aug 1;631(1):139–147. doi: 10.1016/0304-4165(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Bement W. M., Capco D. G. Protein kinase C acts downstream of calcium at entry into the first mitotic interphase of Xenopus laevis. Cell Regul. 1990 Feb;1(3):315–326. doi: 10.1091/mbc.1.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J Cell Biol. 1985 Apr;100(4):1325–1329. doi: 10.1083/jcb.100.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoly J. P., Picard A., Peaucellier G., Labbé J. C., Dorée M. Changes in the activity of the maturation-promoting factor during meiotic maturation and following activation of amphibian and starfish oocytes: their correlations with protein phosphorylation. Dev Biol. 1986 Sep;117(1):1–12. doi: 10.1016/0012-1606(86)90342-8. [DOI] [PubMed] [Google Scholar]
- Cartaud A., Ozon R., Walsh M. P., Haiech J., Demaille J. G. Xenopus laevis oocyte calmodulin in the process of meiotic maturation. J Biol Chem. 1980 Oct 10;255(19):9404–9408. [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Cross N. L. Initiation of the activation potential by an increase in intracellular calcium in eggs of the frog, Rana pipiens. Dev Biol. 1981 Jul 30;85(2):380–384. doi: 10.1016/0012-1606(81)90269-4. [DOI] [PubMed] [Google Scholar]
- Dorée M., Cavadore J. C., Picard A. Facts and hypotheses of calcium regulation of MPF activity during meiotic maturation of starfish oocytes. J Reprod Fertil Suppl. 1990;42:135–140. [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Sources of calcium in egg activation: a review and hypothesis. Dev Biol. 1983 Oct;99(2):265–276. doi: 10.1016/0012-1606(83)90276-2. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Newport J., Hubble R., Kirschner M. Interconversion of metaphase and interphase microtubule arrays, as studied by the injection of centrosomes and nuclei into Xenopus eggs. J Cell Biol. 1984 May;98(5):1730–1745. doi: 10.1083/jcb.98.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., Guerriero V., Jr, Bagchi I. C., Means A. R. The calmodulin binding domain of chicken smooth muscle myosin light chain kinase contains a pseudosubstrate sequence. J Biol Chem. 1987 Feb 25;262(6):2542–2548. [PubMed] [Google Scholar]
- Kubota H. Y., Yoshimoto Y., Yoneda M., Hiramoto Y. Free calcium wave upon activation in Xenopus eggs. Dev Biol. 1987 Jan;119(1):129–136. doi: 10.1016/0012-1606(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Picard A., Karsenti E., Dorée M. An M-phase-specific protein kinase of Xenopus oocytes: partial purification and possible mechanism of its periodic activation. Dev Biol. 1988 May;127(1):157–169. doi: 10.1016/0012-1606(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Lorca T., Fesquet D., Zindy F., Le Bouffant F., Cerruti M., Brechot C., Devauchelle G., Dorée M. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol Cell Biol. 1991 Feb;11(2):1171–1175. doi: 10.1128/mcb.11.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M., Bagci H., Hamaguchi K., Ueda M., Murachi T., Hatanaka M. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J Biol Chem. 1989 Nov 15;264(32):18866–18869. [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Calmodulin. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:227–278. [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S. The calpains. Trends Neurosci. 1989 Nov;12(11):438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Meyerhof P. G., Masui Y. Ca and Mg control of cytostatic factors from Rana pipiens oocytes which cause metaphase and cleavage arrest. Dev Biol. 1977 Dec;61(2):214–229. doi: 10.1016/0012-1606(77)90293-7. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Ogita K., Koide H., Kikkawa U., Kishimoto A., Nishizuka Y. The heterogeneity of protein kinase C in signal transduction cascade. Adv Second Messenger Phosphoprotein Res. 1990;24:218–223. [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Shibuya E. K., Masui Y. Stabilization and enhancement of primary cytostatic factor (CSF) by ATP and NaF in amphibian egg cytosols. Dev Biol. 1988 Sep;129(1):253–264. doi: 10.1016/0012-1606(88)90179-0. [DOI] [PubMed] [Google Scholar]
- Smith M. K., Colbran R. J., Soderling T. R. Specificities of autoinhibitory domain peptides for four protein kinases. Implications for intact cell studies of protein kinase function. J Biol Chem. 1990 Feb 5;265(4):1837–1840. [PubMed] [Google Scholar]
- Standart N., Minshull J., Pines J., Hunt T. Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev Biol. 1987 Nov;124(1):248–258. doi: 10.1016/0012-1606(87)90476-3. [DOI] [PubMed] [Google Scholar]
- Verde F., Labbé J. C., Dorée M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990 Jan 18;343(6255):233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Hosoya H., Matsumura F. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 1991 Jan 10;349(6305):169–172. doi: 10.1038/349169a0. [DOI] [PubMed] [Google Scholar]