Abstract
Purpose of review
The aim of this paper was to review the recent literature on potential therapeutic strategies for overcoming resistance to anti-VEGF drugs in ovarian cancer.
Recent findings
Although clinical benefits of anti-VEGF therapy were observed in ovarian cancer treatment trials, this use yielded only modest improvement in progression-free survival, and with the exception of cediranib no effect on overall survival. Adaptive resistance and escape from anti-angiogenesis therapy is likely a multifactorial process, including induction of hypoxia, vascular modulators and the immune response. New drugs targeting the tumor vasculature or other components of the surrounding microenvironment have shown promising results.
Summary
When to start and end antiangiogenesis therapy and the choice of optimal treatment combinations remain controversial. Further evaluation of personalized novel angiogenesis-based therapy is warranted. Defining the critical interaction of these agents and pathways and the appropriate predictive markers will become an increasingly important objective for effective treatment.
Keywords: Angiogenesis, adaptive resistance, ovarian cancer
Introduction
The current standard frontline therapy of ovarian cancer consists of combination surgery and cytotoxic chemotherapy[1]. While inducing lasting clinical remission in some patients, progress has stagnated due to emerging or promoted drug resistance and lack of specificity to mechanisms of disease progression. Angiogenesis plays a critical role in the pathogenesis of epithelial ovarian cancer (OC), promoting tumor growth and metastatic spread[2]. To date, anti-angiogenic therapy has been identified as one of the most promising targeted therapies in OC and worthy of intensive study. The VEGF family is among the most potent proangiogenic factors[3, 4]. Other angiogenic growth factors and chemokines include fibroblast growth factor (FGF), angiopoietins, endothelins, interleukin-8 (IL-8), macrophage chemotactic proteins, and platelet-derived growth factor (PDGF)[2, 5]. Many agents targeting these growth factors have produced clinical benefits in OC[1, 6].
VEGF/VEGFR-targeted therapies
Bevacizumab is a recombinant, humanized, monoclonal antibody that binds to all isoforms of VEGF. Two randomized, phase III trials of bevacizumab in advanced ovarian cancer improved PFS when administered concomitantly with chemotherapy and in maintenance but without extending OS (Table 1). A completed clinical trial (AURELIA) evaluated the efficacy and safety of bevacizumab added to chemotherapy (BEV-CT) versus chemotherapy alone (CT) in patients with EOC with disease progression within 6 months of platinum therapy. All patients received standard chemotherapy with either paclitaxel or topotecan or liposomal doxorubicin. Patients were randomly assigned to receive chemotherapy alone or chemotherapy combined with bevacizumab (15 mg/kg every 3 weeks or 10 mg/kg every 2 weeks) until progressive disease(PD), unacceptable toxicity, or withdrawal of patient consent. BEV-CT treatment resulted in a significant improvement in PFS, compared with CT treatment (6.7 months with bevacizumab-containing therapy vs 3.4 months with chemotherapy alone; hazard ratio: 0.48; 95% CI: 0.38 to 0.60; P<0.001)[7]. Another placebo-controlled phase III trial (OCEANS) tested the efficacy and safety of bevacizumab (BV) with gemcitabine and carboplatin (GC) compared with GC in platinum-sensitive recurrent ovarian, primary peritoneal, or fallopian tube cancer (ROC) for 6 to 10 cycles; GC plus BV followed by BV until progression resulted in a statistically significant improvement in PFS compared with GC plus placebo in platinum-sensitive (median PFS was 8.4 and 12.4 months for the GC with placebo and BV with GC arms, HR: 0.484; 95% CI: 0.388 to 0.605; P<.0001)[8] (Table 1). Bevacizumab has thus regulatory approval in many countries (not USA) for this setting[7, 15–18].
Table1.
Summary of anti-angiogenesis drugs tested in phase 3 clinical trials for ovarian cancer treatment
| Study | Drug | Eligibility | Arms | Sample size |
Median PFS(M) |
Hazard ratio |
p-value | Median OS(M) |
p-value | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| AURELIA | Bevacizumab | Platinum resistant recurrent ovarian cancer |
CT alone CT with bevacizumab |
361 | 3.4 6.7 |
0.48 | < 0.001 |
13.3 16.6 |
0.174 | [7] |
| OCEANS | Bevacizumab | Platinum sensitive recurrent EOC, FTC, or PPC |
GC with placebo GC with bevacizumab |
484 | 8.4 12.4 |
0.484 | < 0.001 |
OS data immature |
[8] | |
| AGO-OVAR12 | Nintedanib | FIGO IIB-IV ovarian cancer | CP alone CP with nintedanib |
1366 | 16.6 17.3 |
0.84 |
0.0239 |
OS data immature |
[9] | |
| TRINOVA-1 | Trebananib | Recurrent EOC, PPC, or FTC | Paclitaxel with placebo Paclitaxel with trebananib |
919 | 5.4 7.2 |
0.66 | 0.0001 | 17.3 19.0 |
0.19 | [10] |
| ICON6 | Cediranib | platinum-sensitive/relapsed ovarian cancer |
CP alone CP with cediranib, followed by placebo or cediranib |
456 | 9.4 12.6 |
0.68 | 0.002 | 17.6 20.3 |
0.0419 | [11] |
| GOG 218 | Bevacizumab | Stage III/IV EOC | CP with placebo CP with bevacizumab from cycles 2–6 (bevacizumab initiation) CP with bevacizumab (bevacizumab throughout) |
1873 | 10.3 11.2 14.1 |
0.908 0.717 |
<0.16 0.001 |
39.3 38.7 39.7 |
0.76 0.45 |
[12] |
| ICON7 | Bevacizumab | EOC,FTC, or PPC | CP alone CP plus bevacizumab followed by bevacizumab for a maximum of 12 months |
1528 | 17.5 19.9 |
0.93 |
0.025 | 58.6 58 |
0.85 | [13] |
| AGO-OVAR16 | Pazopanib | Stage II to IV ovarian, fallopian tube |
pazopanib alone Placebo alone |
940 | 17.9 12.3 |
0.77 | 0.0021 | OS data immature |
[14] |
M= Month, Caboplatin+Paclitaxel=CP, Carboplatin+Topotecan=CT, Carboplatin+Gemcitabine=CG, PFS = progression free survival; OS = overall survival, EOC=epithelial ovarian cancers, PPC= primary peritoneal cancers, FTC=fallopian tube cancers
Several combinations of bevacizumab with other antitumor agents have been tested. In a phase II study, the effect of combination of docetaxel, oxaliplatin, and bevacizumab as first-line treatment of advanced EOC was investigated. The 12-month PFS rate was 65.7%, median PFS was 16.3 months. Median OS was 47.3 months, indicating that this novel treatment regimen may provide a promising therapeutic approach[19]. Carboplatin and bevacizumab applied in a neoadjuvant setting resulted in optimal cytoreductive surgery (ICS) in all patients, in which 78% had no gross residual tumor[20]. Besides, bevacizumab showed activity in the treatment of recurrent sex cord-stromal tumors of the ovary with acceptable toxicity[21].
The most common adverse events(AEs) were neutropenia, leukopenia, hypertension, fatigue, nausea, proteinuria and even fatal gastrointestinal perforation[22, 23]. A history of treatment for inflammatory bowel disease (IBD), or bowel resection at primary surgery was found to increase the odds of gastrointestinal AEs[24].
Small molecular inhibitors
Sorafenib is a nonselective multi-kinase-inhibitor that has broad activity against tyrosine kinase receptors, including VEGFR and PDGFR, as well as angiogenic factors[25, 26]. Modest activity, but with noted adverse effects were observed when sorafenib was administered alone or combined with carboplatin and paclitaxel in several phase 2 trials[27]. One study that combined sorafenib with bevacizumab showed partial remission (PR) in 46% patients and stable disease (SD) in 37% patients[26]. However, a randomized phase II trial of sorafenib as a maintenance strategy failed to meet its primary endpoint of PFS[28].
Nintedanib (BIBF 1120) is another tyrosine kinase inhibitor that targets VEGFR, PDGFR, and fibroblast growth factor receptor. In a phase 2 trial of patients with relapsed OC, the PFS rate was 16.3% in the nintedanib arm versus 5% in the placebo arm (HR = 0.65; 95% CI: 0.42–1.02; p = 0.06). A significantly higher grade 3/4 hepatotoxicity was detected in nintedanib-treated patients than in the placebo arm (51.2% vs. 7.5%; p < 0.001). In a phase 3 AGO-OVAR 12 study, favorable results were reported (Table 1)[9]. Another planned phase 2 trial will investigate nintedanib in bevacizumab-resistant, recurrent, or persistent OC. The most common AEs with tyrosine kinase inhibitor drugs included diarrhea, thrombocytopenia, hepatic toxicity, anemia or even neuropathic syndrome[29].
Cediranib is a potent inhibitor of all receptors of the VEGF family, and has activity against PDGF-b and c-Kit with generally well tolerated AE[30]. A two-stage, multicenter phase 2 clinical trial was initiated to evaluate the activity of cediranib in patients with recurrent ovarian, peritoneal or fallopian tube cancer. The median time to progression (TTP) and median survival time for all patients was 4.1 months (95% CI: 3.4–7.6) and 11.9 months (95% CI: 9.9-not reached), respectively which shows significant activity in recurrent ovarian cancer[31]. Cediranib combined with Olaparib, a poly (ADP-ribose) polymerase inhibitor resulted in improved PFS (17.7months, 95% CI: 14.7–not reached, VS 9.0 months, 95% CI: 5.7–16.5) compared with olaparib monotherapy in women with recurrent platinum-sensitive ovarian cancer[32]. In the ICON6 trial (3-arm, 3-stage, double-blind, placebo-controlled randomized trial in first relapse of platinum-sensitive ovarian cancer), patients were randomized (2: 3: 3) to receive six cycles of carboplatin (AUC5/6) plus paclitaxel (175 mg/m2) with either placebo (reference), cediranib 20 mg per day, followed by placebo (concurrent), or cediranib 20 mg per day, followed by cediranib (concurrent plus maintenance). They reported longer restricted mean PFS and OS in the cediranib arm[33] (Table 1).
Pazopanib (GW786034) is a second-generation pan-VEGFR, PDGFR-α and –β, and c-Kit inhibitor[34]. In a phase 2 study, patients with EOC were treated with pazopanib; 11 patients (31%) had a CA-125 response to pazopanib, and the overall response rate was 18%[35]. However, another phase 2 study was discontinued due to the lack of activity of pazopanib in patients with recurrent EOC, PPC, or FTC[36]. In a recent randomized phase 3 trial (AGO-OVAR16), pazopanib showed a role in the treatment of selected women with EOC, especially use in women with bevacizumab-resistant disease (Table 1). Questions remain about optimal timing, the ideal patient population, and the efficacy of combination therapy with cytotoxic agents and other biologics.
Although clinical benefits of anti-VEGF therapy were observed in OC, anti-VEGF therapy, however, has yielded only modest improvement in PFS or OS. The greatest challenge today is that a substantial number of cancer patients eventually develop disease resistant to anti-VEGF therapy.
Resistance mechanisms in anti-angiogenic therapy
Evidence suggests that mechanisms of resistance to anti-VEGF therapy might be mediated by tumor cells and by members of the microenvironment[37–39]. Hypoxia is a major molecular controller of angiogenic switch[37, 40, 41]; hypoxia inducible factor 1-alpha (HIF-1α), and interleukin-8 (IL-8) expression have been shown to support angiogenesis and resistance to apoptosis[40–42]. It is known that VEGF pathway inhibition can cause hypoxia and promote recruitment of vascular progenitors (eg, endothelial and pericyte progenitors) and vascular modulators (tumor-associated macrophages, immature monocytes, VEGFR-1 hemangiocytes, and CD11b-myeloid cells). This environment favors selection of tumor clones expressing proangiogenic factors such as Bv8, which has been shown to be partially responsible for angiogenesis promotion and escape from VEGF blockade. In addition, hypoxia was demonstrated to drive translocation of the MDM2 to the cytoplasm, with binding and stabilization of VEGF mRNA[43].
Growing evidence indicates that inflammation controls angiogenesis. Tumor-infiltrating myeloid cells including neutrophils, eosinophils, and activated dendritic cells (DCs) play a crucial role in OC progression)[40]. Myeloid leukocytes were recently shown to drive both inhibition of tumor growth (first) and aggressive malignant expansion (later). CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs) confer resistance to initially sensitive tumors. In one study, myeloid cell–driven angiogenesis was selectively ablated in a mouse model of breast cancer, resulting in reduced VEGF, reduced vascular density, increased blood vessels maturation and normalization.
Infiltrating tumor associated macrophages (TAMs) and enrichment of a macrophage-related gene signature are associated with cancer progression and escape from anti-angiogenic therapy in some types of human cancer[41, 44]. TAM of the M2 phenotype promote tumor vascularization by producing proangiogenic factors and growth factors, including transforming growth factor (TGF-β) and VEGF, and attracting leukocytes to further enhance angiogenesis[44, 45]. Depletion of these macrophages reduces angiogenesis and tumor progression.
Pericytes are smooth muscle-like cells found in close contact with endothelial cells in small blood vessels and capillaries, whereas functionally associate with regulating vessel stabilization, providing endothelial survival factors, such as VEGF. Immature vessels with poor pericyte investment are vulnerable to anti-VEGF treatment, while richer pericyte coverage may protect vessels from VEGF-targeted therapy[46]. VEGF blockade increases the signaling of angiopoietin-1, resulting in improved endothelial cell function and pericyte recruitment, which in turn is implicated in the rescue and escape from VEGF blockade[46].
Targeting other pathways beyond the VEGF/VEGFR pathway
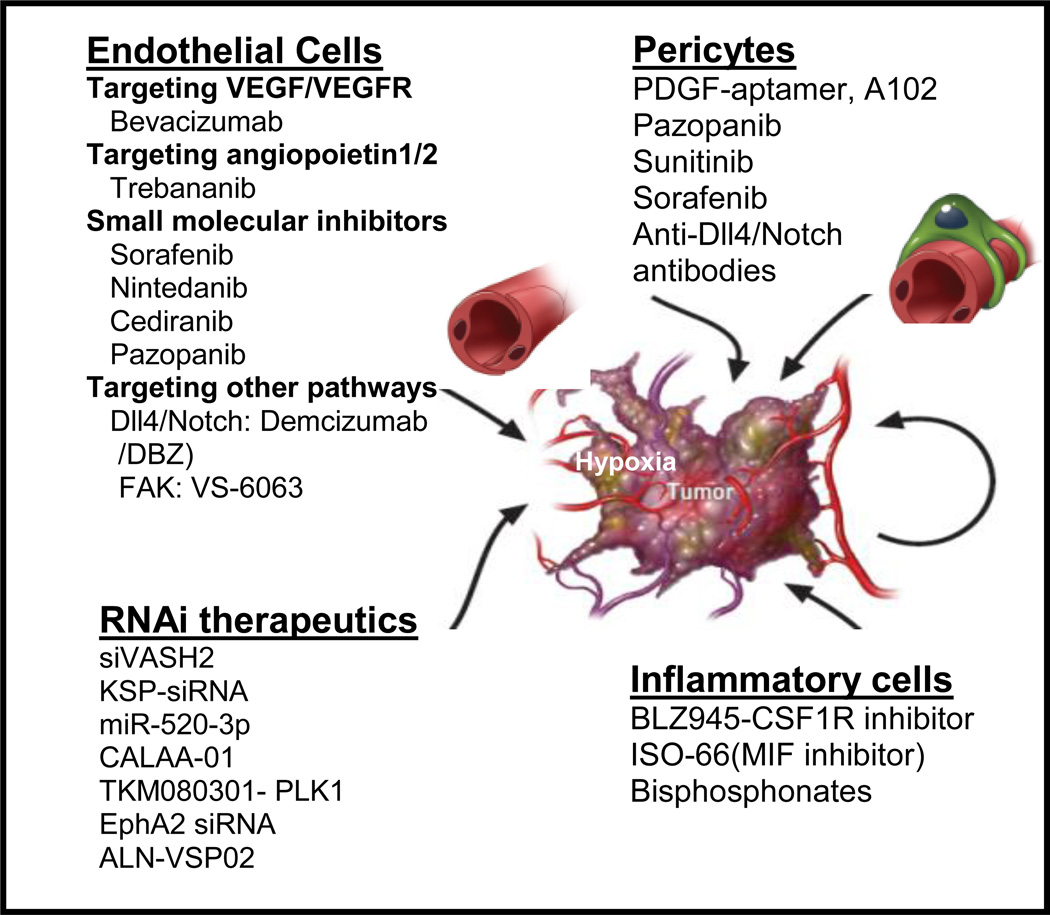
Several proangiogenic molecules and mechanisms underlying angiogenesis beyond VEGF/VEGFR pathway such as Dll4/notch, focal adhesion kinase (FAK) and microRNAs have been recognized(Figure 1). The Notch signaling pathway is comprised of five trans-membrane Notch ligands (Jagged 1, Jagged 2, and Delta-like ligands [Dll] 1, 3, and 4) and four Notch receptors (Notch 1–4)[47]. We have demonstrated that Notch pathway alterations, especially in Notch3 (amplification or upregulation of expression), are prevalent in HGS-OvCa and are associated with shorter OS[48]. Dll4 is an endothelium-specific ligand expressed at sites of vascular development and angiogenesis. Blockage of Dll4 inhibits tumor growth by inducing non-productive angiogenesis, manifested by increased vascular density and decreased perfusion in tumors[47, 49]. We investigated the clinical and biological significance of Dll4 in ovarian cancer and found that Dll4 was overexpressed in 72% of tumors examined where it was also an independent predictor of poor survival. Patients with tumors responding to anti-VEGF therapy had lower levels of Dll4 than patients with stable or progressive disease. Dll4-silencing in ovarian tumor cells and associated endothelial cells inhibited tumor growth and angiogenesis[50]. Clinical enthusiasm in targeting the Dll4/Notch signaling pathway is high, particularly with the availability of γ-secretase inhibitors (GSIs) and anti-Dll4 antibodies (demcizumab and REGN 421, available at http://clinical trials.gov).
FIGURE 1.
Overcoming anti-VEGF resistance by targeting other pathways beyond VEGF and by targeting inflammatory cells in the tumor microenvironment (modified from JCO 2012: 30 (34); 4026–4034) [77]. Abbreviations: TLR: toll-like receptor, DBZ: dibenzazepine, γ secretase inhibitor; PLK1: polo-like kinase 1, KSP: kinesin spindle protein.
Focal adhesion kinase (FAK) is a 125-kDa non-receptor kinase. VS-6063 is a FAK inhibitor, which blocks phosphorylation at the Tyr397 site. Combination of VS-6063 and paclitaxel markedly decreased proliferation and increased apoptosis[51]. FAK silencing in combination with docetaxel resulted in decreased microvessel density, increased apoptosis, and reduced tumor growth.
MicroRNAs (miRNAs) are small non-coding RNAs (21–23 nt) that regulate gene expression through translational repression and mRNA degradation. Recent studies have shown that some miRNAs are involved in the regulation of vascular development and angiogenesis. MiR-10b and miR-196b have been identified to promote angiogenesis by directly regulating bone marrow-derived endothelial progenitor cells, whereas miR-126 induces angiogenesis by increasing VEGF expression. Conversely, miR-221 and miR-222 inhibit angiogenesis by targeting c-Kit receptors in endothelial cells. MiR-718 directly represses VEGF expression and inhibits ovarian cancer proliferation both in vitro and in vivo[52]. The miR-200 family inhibits angiogenesis through direct and indirect mechanisms by targeting IL-8 and CXCL1, whereas over expression of miR-199a and miR-125b inhibited angiogenesis associated with the decrease of HIF-1a and VEGF expression in EOC cells[53].
Novel angiogenesis-targeted therapy
Growing evidence suggested that adaptive resistance and escape from anti-angiogenesis therapy is likely a multifactorial process, including induction of hypoxia, vascular modulators and the immune response. New drugs targeting the tumor vasculature or other components of the surrounding microenvironment have shown promising results.
Angiopoietin inhibitors
Angiopoietin-1 is critical for vessel maturation, adhesion, migration, and survival. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling[54, 55]. Trebananib (AMG 386) is a peptide-Fc fusion protein (or peptibody) that targets angiogenesis by inhibiting the binding of both angiopoietin 1 and 2 to the Tie2 receptor[56, 57]. Combined inhibition of Ang1 and Ang2 may provide superior therapeutic efficacy to that mediated by single targeting alone[58]. In several Phase I trials, objective responses were demonstrated in patients with advanced OC treated with trebananib alone or in combination with chemotherapy[59]. A phase II randomized trial has evaluated weekly paclitaxel plus trebananib in 161 patients with recurrent EOC. A significantly improved median PFS with trebananib was reported than placebo (HR 0.76; 95% CI, 0.52–1.12; P = 0.165), with evidence of a significant dose–response effect (P = 0.037)[57, 59]. Three phase III clinical trials (TRINOVA-1 [NCT01204749], TRINOVA-2 [NCT01281254], and TRINOVA-3 [NCT01493505]) evaluated trebananib in EOC, PPC and FTC, and favorable results were observed in TRINOVA-1 (7.2 months vs 5.4 months, p<0.0001)[58, 60, 10](Table 1). The AEs attributable to trebananib have been mild and reversible, including hypokalemia and/or edema[10, 60]. LC06 a novel angiopoietin-2 selective human antibody with potent anti-tumoral and anti-angiogenic efficacy and a superior AE profile compared to pan-angiopoietin-1/-2 Inhibitors. LC06 neutralizes specifically the binding of Ang-2 to its receptor Tie2, the inhibition appears to be largely restricted to tumor vasculature without obvious effects on normal vasculature[61].
RNAi approaches
Early-phase trials have reported clinical responses in cancer patients after RNAi therapies targeting VEGF and kinesin spindle protein (KSP). The results showed target down regulation and antitumor activity in regression of liver metastases. We have demonstrated that EZH2 silencing in the tumor vasculature results in anti-angiogenic and anti-tumor effects[62]. Vasohibin-2 siRNA (siVASH2) targeted to a xenograft model of ovarian cancer significantly inhibited tumor growth by abrogating tumor angiogenesis[63]. We have also demonstrated the feasibility of combined miRNA-siRNA therapy in preclinical models. Synergistic anti-tumor efficacy was noted with miR-520d-3p combined with EphA2-siRNA using DOPC nanoliposomes [64]. Studies to address the safety and efficacy of several siRNA, CALAA-01[65], TKM 080301[66] and siRNA-EphA2-DOPC (NCT01591356)[67], in solid tumors and their liver metastasis are ongoing.
Targeting macrophages
TAM depletion by clodrolip or a CSF1R inhibitor increased the anti-angiogenic and anti-tumor effects of VEGF/VEGFR2 antibodies in subcutaneous tumor models. Depletion of macrophages by zoledronic acid (ZOMETA) in combination with sorafenib significantly inhibited tumor progression, tumor angiogenesis with mice treated with sorafenib alone[68].These data support the rationale for combining antiangiogenic drugs with macrophage targeting strategies to increase the efficacy of the former, particularly in tumors that are refractory or develop resistance to anti-VEGF therapy.
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine that has been reported to promote tumor progression and enhance angiogenesis[69]. MIF levels in serum of patients with ovarian cancer correlates with poor prognosis[70]. Current therapeutic strategies for targeting MIF mainly focus on developing small inhibitors(ISO-66) toward its tautomerase activities or biologic activities[71].
Conclusions and future direction
To improve the therapeutic benefit and counteract compensatory escape mechanisms, it is likely that simultaneous targeting of multiple angiogenic pathways will be required[72]. Additional studies are necessary to determine optimal combinations that could involve either vertical (e.g., bevacizumab with other angiogenesis inhibitors like sorafenib[31], vandetanib[73], sunitinib[74]), horizontal (e.g., inhibitors of PI3K pathway, MEK, immune, angiopoeitin), or direct (e.g., bevacizumab with thrombospondin-1[75] or vascular disrupting agents such as combretastatin A1 phosphate (OXi4503))[76].
KEY POINTS.
Anti-VEGF therapy has yielded only modest improvements in progression-free and overall survival.
Adaptive resistance and escape from anti-angiogenesis therapy is multifactorial.
Controversy exists regarding when to start and end anti-angiogenesis therapy as well as the choice of the optimal combination.
Predictive markers for response to anti-angiogenesis therapy are needed.
Acknowledgements
This work was supported in part by the NIH (grants P50 CA083639, CA109298, P50 CA098258, UH2 TR000943, CA128797, CA177909, CA016672 and U54 CA151668), the Ovarian Cancer Research Fund (a Program Project Development Grant), the United States Department of Defense (grants OC120547 and OC093146), the Chapman Foundation, the Meyer and Ida Gordon Foundation #2, the Betty Anne Asche Murray Distinguished Professorship, MD Anderson Cancer Center Support Grant CA016672, the Gilder Foundation, and the RGK Foundation. We thank Tamara Locke, Department of Scientific Publications, for editing the manuscript.
Footnotes
Conflicts of interest
The author has no conflicts of interest to declare.
References
- 1.Gonzalez-Martin A, Sanchez-Lorenzo L, Bratos R, et al. First-line and maintenance therapy for ovarian cancer: current status and future directions. Drugs. 2014;74:879–889. doi: 10.1007/s40265-014-0221-9. [DOI] [PubMed] [Google Scholar]
- 2.Gavalas NG, Liontos M, Trachana SP, et al. Angiogenesis-related pathways in the pathogenesis of ovarian cancer. Int J Mol Sci. 2013;14:15885–15909. doi: 10.3390/ijms140815885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Liu X, Wang H, Wang S. Correlation of the expression of vascular endothelial growth factor and its receptors with microvessel density in ovarian cancer. Oncol Lett. 2013;6:175–180. doi: 10.3892/ol.2013.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimberger P, Chebouti I, Kasimir-Bauer S, et al. Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol Oncol. 2014;133:467–472. doi: 10.1016/j.ygyno.2014.03.574. [DOI] [PubMed] [Google Scholar]
- 5.Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma--mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol. 2014;132:496–505. doi: 10.1016/j.ygyno.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 6. Shaw D, Clamp A, Jayson GC. Angiogenesis as a target for the treatment of ovarian cancer. Curr Opin Oncol. 2013;25:558–565. doi: 10.1097/CCO.0b013e328363e0da. • These trials [the International Collaborative Ovarian Neoplasm Group trial (ICON7), the Gynecologic Oncology Group trial (GOG218), OCEANS and AURELIA] have shown that tumour vasculature is avalid target.
- 7.Stockler MR, Hilpert F, Friedlander M, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32:1309–1316. doi: 10.1200/JCO.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harter P, Kimmig R, de Gregorio N, et al. AGO-OVAR 12: A Randomized Placebo- controlled GCIG/ENGOT-Intergroup Phase III trial of standard frontline chemotherapy plus /− Nintedanib for advanced ovarian cancer. Oncol. Res. Treat. 2014;37:74–75. [Google Scholar]
- 10. Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:799–808. doi: 10.1016/S1470-2045(14)70244-X. • Inhibition of angiopoietins 1 and 2 with trebananib provided a clinically meaningful prolongation in progression-free survival.
- 11.Raja FA, Perren TJ, Embleton A, et al. RANDOMISED DOUBLE-BLIND PHASE III TRIAL OF CEDIRANIB (AZD 2171) IN RELAPSED PLATINUM SENSITIVE OVARIAN CANCER: RESULTS OF THE ICON6 TRIAL. Int. J. Gynecol. Cancer. 2013;23:1. [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.Pujade-Lauraine E, Oza AM, Perren TJ, et al. ICON7: FINAL OVERALL SURVIVAL RESULTS IN THE GCIG PHASE III RANDOMISED TRIAL OF BEVACIZUMAB IN NEWLY DIAGNOSED OVARIAN CANCER.nt. J. Gynecol. Cancer. 2013;23:1. [Google Scholar]
- 14.Davidson BA, Secord AA. Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer. Int J Womens Health. 2014;6:289–300. doi: 10.2147/IJWH.S49781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monk BJ, Pujade-Lauraine E, Burger RA. Integrating bevacizumab into the management of epithelial ovarian cancer: the controversy of front-line versus recurrent disease. Ann Oncol. 2013;24(Suppl 10):x53–x58. doi: 10.1093/annonc/mdt472. • An overview about optimal setting for bevacizumab treatment?'
- 16.Aghajanian C, Goff B, Nycum LR, et al. Independent radiologic review: bevacizumab in combination with gemcitabine and carboplatin in recurrent ovarian cancer. Gynecol Oncol. 2014;133:105–110. doi: 10.1016/j.ygyno.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open- label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 18.Liu JF, Cannistra SA. Emerging role for bevacizumab in combination with chemotherapy for patients with platinum-resistant ovarian cancer. J Clin Oncol. 2014;32:1287–1289. doi: 10.1200/JCO.2013.54.7299. [DOI] [PubMed] [Google Scholar]
- 19.Herzog TJ, Monk BJ, Rose PG, et al. A phase II trial of oxaliplatin, docetaxel, and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol Oncol. 2014;132:517–525. doi: 10.1016/j.ygyno.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salani R, O'Malley DM, Copeland LJ, et al. Feasibility of interval cytoreduction following neoadjuvant chemotherapy with carboplatin, weekly paclitaxel, and bevacizumab for advanced ovarian cancer--a phase 1 study. Int J Gynecol Cancer. 2014;24:682–686. doi: 10.1097/IGC.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 21. Brown J, Brady WE, Schink J, et al. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120:344–351. doi: 10.1002/cncr.28421. • Bevacizumab has activity in the treatment of recurrent sex cord-stromal tumors of the ovary, and its toxicity is acceptable. The median progression-free survival was 9.3 months, and the median overall survival was not reached in during reporting period.
- 22.Sawaya R, Radwan W, Hammoud S. Benign reversible encephalopathy syndrome after bevacizumab therapy for metastatic ovarian cancer. Med Oncol. 2014;31:831. doi: 10.1007/s12032-013-0831-1. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Zheng Y, Zhu J, et al. An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One. 2014;9:e89960. doi: 10.1371/journal.pone.0089960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger RA, Brady MF, Bookman MA, et al. Risk factors for GI adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2014;32:1210–1217. doi: 10.1200/JCO.2013.53.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leone Roberti Maggiore U, Valenzano Menada M, Venturini PL, Ferrero S. Sorafenib for ovarian cancer. Expert Opin Investig Drugs. 2013;22:1049–1062. doi: 10.1517/13543784.2013.802769. • Promising results have been achieved combining sorafenib with bevacizumab, although overlapping and cumulative toxicities should be taken into consideration.
- 26.Smolle E, Taucher V, Petru E, Haybaeck J. Targeted treatment of ovarian cancer--the multiple -kinase - inhibitor sorafenib as a potential option. Anticancer Res. 2014;34:1519–1530. [PubMed] [Google Scholar]
- 27.Ramasubbaiah R, Perkins SM, Schilder J, et al. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol Oncol. 2011;123:499–504. doi: 10.1016/j.ygyno.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Herzog TJ, Scambia G, Kim BG, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol. 2013;130:25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Patejdl R, Markmann S, Benecke R, Wittstock M. Severe acute motor neuropathy after treatment with triple tyrosine kinase inhibitor BIBF 1120 (Nintedanib) Clin Neurol Neurosurg. 2013;115:1851–1852. doi: 10.1016/j.clineuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Sahade M, Caparelli F, Hoff PM. Cediranib: a VEGF receptor tyrosine kinase inhibitor. Future Oncol. 2012;8:775–781. doi: 10.2217/fon.12.73. [DOI] [PubMed] [Google Scholar]
- 31.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raja FA, Griffin CL, Qian W, et al. Initial toxicity assessment of ICON6: a randomised trial of cediranib plus chemotherapy in platinum-sensitive relapsed ovarian cancer. Br J Cancer. 2011;105:884–889. doi: 10.1038/bjc.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson BA, Secord AA. Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer. Int J Womens Health. 2014;6:289–300. doi: 10.2147/IJWH.S49781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedlander M, Hancock KC, Rischin D, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119:32–37. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez Martin A, Redondo A, Jurado M, et al. GEICO (Spanish Group for Investigation on Ovarian Cancer) treatment guidelines in ovarian cancer 2012. Clin Transl Oncol. 2013;15:509–525. doi: 10.1007/s12094-012-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomao F, Papa A, Rossi L, et al. Beyond bevacizumab: investigating new angiogenesis inhibitors in ovarian cancer. Expert Opin Investig Drugs. 2014;23:37–53. doi: 10.1517/13543784.2013.839657. [DOI] [PubMed] [Google Scholar]
- 38.Daniele G, Di Maio M, Piccirillo MC, et al. New biological treatments for gynecological tumors: focus on angiogenesis. Expert Opin Biol Ther. 2014;14:337–346. doi: 10.1517/14712598.2014.873401. [DOI] [PubMed] [Google Scholar]
- 39.Winiarski BK, Wolanska KI, Rai S, et al. Epithelial ovarian cancer-induced angiogenic phenotype of human omental microvascular endothelial cells may occur independently of VEGF signaling. Transl Oncol. 2013;6:703–714. doi: 10.1593/tlo.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarlett UK, Conejo-Garcia JR. Modulating the tumor immune microenvironment as an ovarian cancer treatment strategy. Expert Rev Obstet Gynecol. 2012;7:413–419. doi: 10.1586/eog.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawrychowski K, Szewczyk G, Skopinska-Rozewska E, et al. The angiogenic activity of ascites in the course of ovarian cancer as a marker of disease progression. Dis Markers. 2014;2014:683757. doi: 10.1155/2014/683757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou S, Gu L, He J, et al. MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol Cell Biol. 2011;31:4928–4937. doi: 10.1128/MCB.06085-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahat MA, Hemmerlein B, Iragavarapu-Charyulu V. The regulation of angiogenesis by tissue cell-macrophage interactions. Front Physiol. 2014;5:262. doi: 10.3389/fphys.2014.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Zhao X, Wang K, et al. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci. 2013;104:516–523. doi: 10.1111/cas.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasumi Y, Klosowska-Wardega A, Furuhashi M, et al. Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int J Cancer. 2007;121:2606–2614. doi: 10.1002/ijc.22999. [DOI] [PubMed] [Google Scholar]
- 47.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu W, Liu T, Ivan C, et al. Notch3 pathway alterations in ovarian cancer. Cancer Res. 2014;74:3282–3293. doi: 10.1158/0008-5472.CAN-13-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng YH, Li FD, Tian C, et al. Notch gamma-secretase inhibitor dibenzazepine attenuates angiotensin II-induced abdominal aortic aneurysm in ApoE knockout mice by multiple mechanisms. PLoS One. 2013;8:e83310. doi: 10.1371/journal.pone.0083310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu W, Lu C, Dong HH, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–6039. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang Y, Hu W, Ivan C, et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013;105:1485–1495. doi: 10.1093/jnci/djt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leng R, Zha L, Tang L. MiR-718 represses VEGF and inhibits ovarian cancer cell progression. FEBS Lett. 2014;588:2078–2086. doi: 10.1016/j.febslet.2014.04.040. • MiR-718 directly targets and represses VEGF expression. MiR-718 restoration inhibits ovarian cancer proliferation both in vitro and in vivo.
- 53.Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Felcht M, Luck R, Schering A, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coxon A, Bready J, Min H, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-3 neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu JF, Rasmussen E, Karlan BY, et al. Exposure-response relationship of AMG 386 in combination with weekly paclitaxel in recurrent ovarian cancer and its implication for dose selection. Cancer Chemother Pharmacol. 2012;69:1135–1144. doi: 10.1007/s00280-011-1787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Villanueva MT. Ovarian cancer: TRINOVA-1, beyond VEGF inhibition. Nat Rev Clin Oncol. 2014;11:442. doi: 10.1038/nrclinonc.2014.118. • Trebananib inhibits angiogenesis through the angiopoietin axis—involved in vascular growth, remodelling, and stabilization—by preventing the interaction of the ligands angiopoietin 1 and 2 (ANG1 and ANG2) with the Tie2 receptor. The agent has shown antiangiogenic activity in preclinical models of ovarian cancer, and prolonged progression-free survival (PFS) in a randomized phase II trial in patients with recurrent epithelial ovarian cancer.
- 59.Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo- controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 60.Monk BJ, Minion L, Lambrechts S, et al. Incidence and management of edema associated with trebananib (AMG 386) Gynecol Oncol. 2013;130:636–641. doi: 10.1016/j.ygyno.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Thomas M, Kienast Y, Scheuer W, et al. A Novel Angiopoietin-2 Selective Fully Human Antibody with Potent Anti-Tumoral and Anti-Angiogenic Efficacy and Superior Side Effect Profile Compared to Pan-Angiopoietin-1/-2 Inhibitors. PLoS One. 2013;8:11. doi: 10.1371/journal.pone.0054923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu C, Han HD, Mangala LS, et al. Regulation of tumor angiogenesis by EZH2. Cancer cell. 2010;18:185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koyanagi T, Suzuki Y, Saga Y, et al. In vivo delivery of siRNA targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer Sci. 2013;104:1705–1710. doi: 10.1111/cas.12297. • The siVASH2-treated tumor contained more blood vessels covered with pericytes, indicating that knockdown of VASH2 contributes to normalization of tumor blood vessels.
- 64. Nishimura M, Jung EJ, Shah MY, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013;3:1302–1315. doi: 10.1158/2159-8290.CD-13-0159. • Combined targeting of the Eph pathway using EphA2-targeting siRNA and miR-520d-3p exhibits remarkable therapeutic synergy and enhanced tumor suppression in vitro and in vivo compared with either monotherapy alone.
- 65.Marra E, Palombo F, Ciliberto G, Aurisicchio L. Kinesin spindle protein SiRNA slows tumor progression. J Cell Physiol. 2013;228:58–64. doi: 10.1002/jcp.24103. [DOI] [PubMed] [Google Scholar]
- 66.Ramanathan RK, Hamburg SI, Halfdanarson TR, et al. A Phase I/II Dose Escalation Study of TKM-080301, a RNAi Therapeutic Directed Against PLK1, in Patients with Advanced Solid Tumors, with an Expansion Cohort of Patients with NET or ACC. Pancreas. 2014;43:502–502. [Google Scholar]
- 67.Landen CN, Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 69.Hudson JD, Shoaibi MA, Maestro R, et al. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krockenberger M, Kranke P, Hausler S, et al. Macrophage migration-inhibitory factor levels in serum of patients with ovarian cancer correlates with poor prognosis. Anticancer Res. 2012;32:5233–5238. [PubMed] [Google Scholar]
- 71. Ioannou K, Cheng KF, Crichlow GV, et al. ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int J Oncol. 2014;45:1457–1468. doi: 10.3892/ijo.2014.2551. • Subsequent ex vivo analysis of mouse splenocytes revealed that the observed decrease in tumor growth rates was likely mediated by the selective in vivo expansion of antitumor-reactive effector cells induced by ISO-66.
- 72.Moreno Garcia V, Basu B, Molife LR, Kaye SB. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin Cancer Res. 2012;18:3750–3761. doi: 10.1158/1078-0432.CCR-11-1275. [DOI] [PubMed] [Google Scholar]
- 73.Gutierrez M, Murgo AJ, Allen D, et al. Phase I study of vandetanib (V) and bevacizumab (B) combination therapy evaluating the VEGF and EGF signal transduction pathways in adults with solid tumors and NHL. J Clin Oncol. 2009;27 [Google Scholar]
- 74.Rini BI, Garcia JA, Cooney MM, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–6283. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uronis HE, Cushman SM, Bendell JC, et al. A phase I study of ABT-510 plus bevacizumab in advanced solid tumors. Cancer Med. 2013;2:316–324. doi: 10.1002/cam4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siemann DW, Shi W. Dual targeting of tumor vasculature: combining Avastin and vascular disrupting agents (CA4P or OXi4503) Anticancer Res. 2008;28:2027–2031. [PMC free article] [PubMed] [Google Scholar]
- 77.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30:4026–4034. doi: 10.1200/JCO.2012.41.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]