Abstract
Time-lapse videos of growing biofilms were analyzed using a background subtraction method, which removed camouflaging effects from the heterogeneous field of view to reveal evidence of streamer formation from optically dense biofilm segments. In addition, quantitative measurements of biofilm velocity and optical density, combined with mathematical modeling, demonstrated that streamer formation occurred from mature, high-viscosity biofilms. We propose a streamer formation mechanism by sudden partial detachment, as opposed to continuous elongation as observed in other microfluidic studies. Additionally, streamer formation occurred in straight microchannels, as opposed to serpentine or pseudo-porous channels, as previously reported.
INTRODUCTION TO STREAMER FORMATION IN MICROCHANNELS
Bacterial biofilms are among Nature's most successful multi-cellular life-forms. A main advantage is their adaptivity, including their ability to modify their mechanical properties and morphology to better suit their environment.1 In addition to interest from pure environmental and biological studies, biofilms have received interest in relation to biofouling and more recently as biocatalysts.2,3 Biofilms benefit from, and respond to, tangential liquid flow to facilitate transport of nutrients and by-products through the biofilm-liquid interface. Microfluidics is among the most promising techniques to closely study biofilm physiochemical properties as it achieves strict control over hydrodynamic, chemical, and thermal conditions and is readily interrogated by regular optical microscopes.4 An exciting nascent area of biofilm research in microchannels concerns biofilm streamer formation. Beneficial aspects of such formations in microscopic algae in pelagic ecosystems, for example, include enhanced nutrient uptake and protection against grazing since they remain suspended in the bulk liquid, above a surface adhered biofilm.5 In contrast to the ease and ubiquity of streamer formation in high Reynolds number flows, streamer formation in laminar flow conditions in microchannels have only been observed at corners and edges of sharp features where complex three-dimensional flow patterns exist.6
In this study, we utilized a background subtraction method to visualize subtle events in time-lapse videos against a highly heterogeneous field of view. With this approach, we were able to see the formation of objects resembling biofilm streamers. In parallel, a particle tracking algorithm was used for video analysis to quantitatively measure the changing downstream velocity and optical density of the biofilms as they matured and changed their mechanical properties. We propose that streamers were formed by sudden partial detachment, which accounts for the observed non-continuous streamer growth and the apparent maturity and high viscosity of the biofilm at the time of formation. As this mechanism is unreported concerning biofilms in microfluidic flow cells, we conclude with a discussion on the strategies for further studies.
GROWTH PHASES AND STREAMER FORMATION
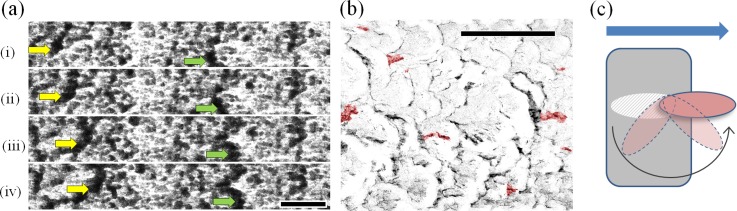
Figure 1(a) shows a zoomed segment of 4 sequential frames from a time-lapse video (one frame per hour) presented in the supplementary material (SMVideo1) in which biofilm segments are seen flowing downstream.7 In this work, we used a Pseudomonas sp. strain of bacteria designated CT07, prepared as described in the supplementary material.7–9 The biofilm could be seen developing over the course of three phases: a lag-phase (0 h < t < 14 h); a flowing phase (14 h ≤ t ≤ 40 h), where biofilm segments moved downstream while coalescing and rapidly increasing optical density (OD); and a mature phase (t > 40 h), where the entire heterogeneous biofilm became almost static and OD stopped increasing. During the third phase, some sudden movements were observed despite being camouflaged by the biofilm. To better see those changes, we used the video frame at t = 46 h for background subtraction for subsequent frames. As seen in supplementary material, the resulting video (SMVideo2) enabled identification of moving biofilm portions relative to frame t = 46 h.7 Particularly striking was the visualization of at least 10 partial detachment events, which resulted in streamer-like formations within the time window 48 h < t < 55 h. This corresponded to 0.15 events·mm−2 h−1. The technique even allowed visualization of subtle motions, such as slight wavering motion expected of streamers in flowing liquid. Figure 1(b) shows a frame at t = 55 h from the background subtracted video showing streamers highlighted in red. It is apparent from close inspection of the video that the streamers were formed by sudden partial detachment from localized biofilm formations with locally large OD (Figure 1(c)). We note that other moving segments could be observed in Fig. 1 (in black), which could also have been streamers, though certainly some are the result of residual viscous flow in the biofilm.
FIG. 1.
(a) Time-series optical micrograph in transmission mode from the microchannel center at (i) 29 h, (ii) 30 h, (iii) 31 h, and (iv) 32 h after inoculation. Dark portions show high optical density biofilm features. Yellow and green arrows highlight the displacement of visible segments in time. Scale bar is 700 μm. (b) Biofilm after 55 h with background subtraction using the t = 46 h frame. Differences between images at 55 h and 46 h are shown, with sudden partial detachment events coloured in red and continuous biofilm motion coloured in black. Scale bar is 1 mm. Flow is from left to right in (a) and (b). (c) A simplified schematic for the proposed streamer formation by partial detachment. A shear force applied to a biofilm segment (grey) by a liquid flow (blue arrow) causes a small biofilm portion to become partially detached, leaving behind a void (cross-hatched). The partially detached biofilm segment (red) rotates to align itself in the direction of the liquid flow, becoming a streamer.
QUANTIFYING BIOFILM DYNAMICS AND GROWTH RATE
The current thought is that streamers form when the biofilm is in a liquid-like (viscous) state.10 In order to correlate the viscosity and the growth rate of the biofilm segments during the streamer formation, first we used a software tracking algorithm which quantified velocity and the OD of the moving biofilm segments (referred to herein as “tracks”). Due to the low-magnification used in these studies, tracks could be followed over relatively large distances (millimetres). We measured the average downstream track velocity track) from the displacement of individual tracks (dtrack,i) between video frames using Eq. (1). Figure 2(a) shows that tracks accelerated until reaching a maximum of 105.5 μm h−1 after 33 h, followed by a sharp deceleration. Therefore, we believed that biofilm viscosity must have strongly increased in order to account for the reduction in track with time, despite the growing shear stress against tracks resulting from local growth-induced flow constrictions. To draw a link between velocity and viscosity, we developed a simplified mathematical model based on a lubrication/thin-film approximation model that calculated various h and m pairs (biofilm height normalized to the channel height and viscosity ratio between nutrient solution, , and biofilm, , respectively) given knowledge of track and the average nutrient solution velocity 0 = 0.25 m h−1, Eq. (2),
| (1) |
| (2) |
where n was the total number of tracks, Δt was 1 h in (1), and track was the track velocity made dimensionless by 0 in (2). As an example, Figure 2(b) shows the h,m combinations that would lead to the maximum velocity, as measured at t = 33 h. Interestingly, the two-phase viscous flow model also predicts that viscosity was increasing in the biofilm, even before 33 h. We are concerned, however, with the time interval in which streamer formation occurred (46 h < t < 56 h). Assuming the sudden reduction in track was due to h reaching some limiting value due to erosion or stabilizing itself at the far microchannel wall, we could determine the necessary value of that would result in the measured track for t > 33 h (Fig. 2(c)). If, on the other hand, h continued to increase, then viscosity would have had to increase even faster.
FIG. 2.
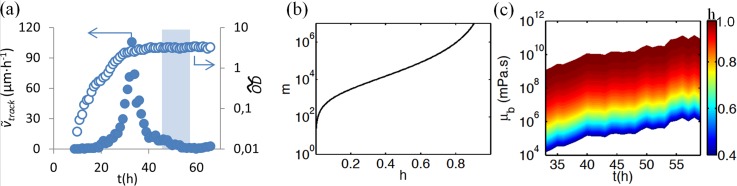
(a) Time-dependent track and (solid and hollow circles, respectively) based on 40 tracks. The blue box shows the time interval for observed streamers formation. (b) The contour of track = 0.000422 versus m and h. (c) Biofilm viscosity changes during transformation to maturity for 0.4 ≤ h ≤ 0.98, where the colour represents the normalized biofilm height.
Average optical density () was calculated from tracking data in order to have a measurement that was directly proportional to total biomass.11 The was higher than that of the average biofilm across the entire channel, indicating that the tracks likely stood taller than surrounding biofilms, explaining why these biofilm segments were the only ones to exhibit significant movement. Figure 2(a) shows a nearly exponential growth phase for t < 28 h. However, in the time interval t > 40 h, the growth rate was negligible for all tracked biofilms, marking the onset of maturity.
DISCUSSION
The streamer formation observed here was different than those in other microfluidic studies in that it was sudden and occurred in straight microchannels. Also, we note that streamers are formed from high OD biofilm segments, which were likely taller than average biofilm height. This means that, in addition to the increased overall fluid velocity accompanying the continuous reduction in free volume related to biofilm growth, the tall segments were exposed to particularly high stresses due to their protrusion into regions of faster flow near to the center of the channel. Sudden partial detachment events at these sites formed flag-like streamers, which extend from a fixed point of attachment. Fingering of tall segments into biofilm streamers has been observed in non-microfluidic studies12 and in simulations,5,13,14 though none discussed a mechanism for sudden formation. From our observations and simulations, we conclude that sudden streamer formation occurred after the biofilm segments became highly viscous. With regard to the elasticity, which we have not studied here, it has been shown previously that biofilms' stiffness increases when exposed to higher stresses.15 This supports our proposed formation mechanism by sudden partial detachment, which is a priori consistent with stress applied to mechanically stiff structures. Additionally, we have observed this phenomenon when supplying the biofilm with modified LB media, varying slightly in their tryptone and NaCl concentrations. However, we have never observed such phenomena with citrate-containing AB media solutions, likely due to the stabilization of the biofilm matrix due to the presence of divalent cation (Mg2+ and Ca2+) bridges.16
OUTLOOK AND PERSPECTIVES
The type of streamer we observed in a simple straight microchannel has likely not been observed before because their formation is modest and easy to miss. While it is tempting to tout their potential importance, more work is required. For example, it is recommended to look more deeply into the cause of the apparent instability that initiates the partial detachment events, which goes beyond our simple two-phase viscous flow model and velocity tracking experiments. Experimental techniques such as digital image correlation will likely be helpful.17 In the short-term, height measurements (for example, by confocal microscopy) can be added as the approach shown here for absolute viscosity measurements and to better study the streamer morphology. As well, long-term experiments are required to determine streamer fate after formation. Specifically, it will be important to know if they remain independent from surface-bound biofilms and, if so, how their growth kinetics change. As well, monitoring biofilms later in their growth stages can reveal if streamer formation by this mechanism continues indefinitely.
ACKNOWLEDGMENTS
The authors thank the following funding sources: Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation, and Fonds de recherche du Québec—Nature et technologies for funding support.
References
- 1. Shaw T., Winston M., Rupp C. J., Klapper I., and Stoodley P., Phys. Rev. Lett. 93, 098102 (2004). 10.1103/PhysRevLett.93.098102 [DOI] [PubMed] [Google Scholar]
- 2. Bachmann R. T. and Edyvean R. G. J., Biofilms 2, 197–227 (2005). 10.1017/S1479050506001979 [DOI] [Google Scholar]
- 3. Logan B. E., Hamelers B., Rozendal R., Schröder U., Keller J., Freguia S., Aelterman P., Verstraete W., and Rabaey K., Environ. Sci. Technol. 40, 5181–5192 (2006). 10.1021/es0605016 [DOI] [PubMed] [Google Scholar]
- 4. Karimi A., Karig D., Kumar A., and Ardekani A. M., Lab Chip 15, 23 (2015). 10.1039/C4LC01095G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celler K., Hödl I., Simone A., Battin T. J., and Picioreanu C., Sci. Rep. 4, 3649 (2014). 10.1038/srep03649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drescher K., Shen Y., Bassler B. L., and Stone H. A., Proc. Natl. Acad. Sci. U.S.A. 110, 4345–4350 (2013). 10.1073/pnas.1300321110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See supplementary material at http://dx.doi.org/10.1063/1.4928296E-BIOMGB-9-019504 for a representative video in which a biofilm's segments are seen flowing downstream; for a background subtracted video, which reveals streamer formation by sudden partial detachment; for a description of all experimental procedures; and a description of the mathematical model used here.
- 8. Wolfaardt G. M., Hendry M. J., Birkham T., Bressel A., Gardner M. N., Sousa A. J., Korber D. R., and Pilaski M., Biotechnol. Bioeng. 100, 141–149 (2008). 10.1002/bit.21736 [DOI] [PubMed] [Google Scholar]
- 9. Bester E., Wolfaardt G., Joubert L., Garny K., and Saftic S., Appl. Environ. Microbiol. 71, 7792–7798 (2005). 10.1128/AEM.71.12.7792-7798.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das S. and Kumar A., Sci. Rep. 4, 7126 (2014). 10.1038/srep07126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakke R., Kommedal R., and Kalvenes S., J. Microbiol. Methods 44, 13–26 (2001). 10.1016/S0167-7012(00)00236-0 [DOI] [PubMed] [Google Scholar]
- 12. Stewart P. S., Biofouling 28, 187–198 (2012). 10.1080/08927014.2012.662641 [DOI] [PubMed] [Google Scholar]
- 13. Böl M., Ehret A. E., Bolea Albero A., Hellriegel J., and Krull R., Crit. Rev. Biotechnol. 33, 145–171 (2013). 10.3109/07388551.2012.679250 [DOI] [PubMed] [Google Scholar]
- 14. Alpkvist E. and Klapper I., Water Sci. Technol. 55, 265–273 (2007). 10.2166/wst.2007.267 [DOI] [PubMed] [Google Scholar]
- 15. Wilking J. N., Angelini T. E., Seminara A., Brenner M. P., and Weitz D. A., MRS Bull. 36, 385 (2011). 10.1557/mrs.2011.71 [DOI] [Google Scholar]
- 16. Banin E., Brady K. M., and Greenberg E. P., Appl. Environ. Microbiol. 72, 2064–2069 (2006). 10.1128/AEM.72.3.2064-2069.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathias J. D. and Stoodley P., Biofouling 25, 695–703 (2009). 10.1080/08927010903104984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4928296E-BIOMGB-9-019504 for a representative video in which a biofilm's segments are seen flowing downstream; for a background subtracted video, which reveals streamer formation by sudden partial detachment; for a description of all experimental procedures; and a description of the mathematical model used here.