Abstract
Introduction
Adequate maternal supply and placental delivery of long chain polyunsaturated fatty acids (LCPUFA) is essential for normal fetal development. In humans, maternal obesity alters placental FA uptake, though the impact of diet remains uncertain. The fatty fetal liver observed in offspring of Japanese macaques fed a high fat diet (HFD) was prevented with resveratrol supplementation during pregnancy. We sought to determine the effect of HFD and resveratrol, a supplement with insulin-sensitizing properties, on placental LCPUFA uptake in this model.
Methods
Japanese macaques were fed control chow (15% fat, n=5), HFD (35% fat, n=10) or HFD containing 0.37% resveratrol (n=5) prior to- and throughout pregnancy. At ~130d gestation (term=173d), placentas were collected by caesarean section. Fatty acid uptake studies using 14C-labeled oleic acid, arachidonic acid (AA) and docosahexanoic acid (DHA) were performed in placental explants.
Results
Resveratrol supplementation increased placental uptake of DHA (P<0.05), while HFD alone had no measurable effect. Resveratrol increased AMP-activated protein kinase activity and mRNA expression of the fatty acid transporters FATP-4, CD36 and FABPpm (P<0.05). Placental DHA content was decreased in HFD dams; resveratrol had no effect on tissue fatty acid profiles.
Discussion
Maternal HFD did not significantly affect placental LCPUFA uptake. Furthermore, resveratrol stimulated placental DHA uptake capacity, AMPK activation and transporter expression. Placental handling of DHA is particularly sensitive to the dramatic alterations in the maternal metabolic phenotype and placental AMPK activity associated with resveratrol supplementation.
Keywords: Placenta, fatty acid uptake, high fat diet, non-human primate
Introduction
1 in 5 women who deliver in the US are obese [1]. The offspring of these women have a higher risk of developing obesity and insulin resistance and dying of cardiovascular disease in later life [2–4]. These outcomes may be due to changes in placental function, specifically nutrient transport, that are associated with maternal obesity and a diet high in fat [5, 6]. Indeed, in humans, maternal obesity has been associated with changes in placental fatty acid uptake [7, 8]. The long chain polyunsaturated fatty acids (LCPUFA), specifically the n-3 LCPUFA docosahexanoic acid (DHA), are critical for proper neurological and cardiovascular development of the fetus [reviewed in 9]. Unlike adult mammals, the fetus has limited capacity to synthesize LCPUFA from their essential fatty acid precursors [10–12], and so relies upon placental delivery for their supply. Therefore, conditions that alter placental LCPUFA handling may have important effects on fetal development and long-term wellness. Maternal high fat diet [5, 6] and maternal insulin resistance [13, 14] alter placental fatty acid metabolism and transport and are associated with maternal obesity. The relative contribution of these two variables to obesity-related changes in placental lipid transport and metabolism are not understood.
Japanese macaques fed a diet composed of 35% fat, comparable to a typical western-style diet, before and during pregnancy have offspring born with fatty liver who develop obesity and vascular dysfunction in later life [15, 16]. Characteristics of these pregnancies include maternal insulin resistance, decreased uterine blood flow, decreased plasma LCPUFA levels, high placental triglycerides and placental inflammation [17], mimicking complications observed in pregnancies of obese women [18–20], and possibly altering placental nutrient handling.
Resveratrol is a polyphenol that mimics calorie restriction and has been shown to improve outcomes in animal models of obesity [21–23]; effects in obese humans are more controversial [24–26]. Recently, Roberts et al. reported in the Japanese macaque model, that supplementation of a high fat diet (HFD) with resveratrol during pregnancy improves maternal fasting insulin levels and decreases fat mass in addition to resolving fetal outcomes such as high liver triglycerides [27]. Furthermore, resveratrol infusions were shown to acutely increase uterine blood flow in this model of maternal high fat diet [27].
Resveratrol’s caloric restriction effects may depend upon activation of the AMP-activated protein kinase (AMPK) pathway [28], which, as an energy sensor, in turn stimulates numerous metabolic processes within the cell including fatty acid uptake and metabolism with the goal of increasing cellular energy production [29, 30]. AMPK activity is decreased in placentas of obese women [19]. The consequences of AMPK activation on placental fatty acid handling are unknown.
We sought to determine the effect of HFD with and without resveratrol supplementation on placental fatty acid handling using the Japanese macaque model. We hypothesized that a diet high in fat would suppress placental LCPUFA uptake and resveratrol supplementation would oppose this effect in association with the activation of the cellular energy sensor, AMPK.
Materials and Methods
Experimental Design
All animal procedures were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC) and Oregon Health & Science University. Japanese macaques were fed control chow (15% fat, n=5), HFD (35% fat, n=10) or HFD containing 0.37% resveratrol (n=5) prior to- and throughout pregnancy as previously described [27]. At ~130d gestation (term=173d), placentas were collected by caesarean section. Macaque placentas have two lobes: a primary lobe attached to the umbilical cord and a secondary lobe attached to the primary via interlobar vessels. Whether these lobes have different functional capacity is not well understood and therefore we collected samples (four separate cotyledons) from both lobes. Sets of samples were: 1) flash frozen immediately in liquid nitrogen and stored at −80°C for molecular analyses; 2) formalin fixed for subsequent paraffin embedding for immunohistochemistry analyses; 3) collected fresh for uptake studies as described below.
Fatty acid uptake
Placental fatty acid uptake studies using 14C-labeled oleic acid (OA) arachidonic acid (AA) and docosahexanoic acid (DHA) were performed in placental explants as previously described [7]. Briefly, villous tissue was collected from both placental lobes and washed in warm PBS. Small fragments (<2mm3) were dissected and one from each cotyledon was placed in each Netwell insert in warm uptake buffer (HBSS with 10mM HEPES, pH 7.4) for 30 minute equilibration at 37°C. Buffer was removed and 200 μM albumin-bound fatty acids (ratio of 1:1 fatty acid:BSA) in uptake buffer were added. Fatty acids were prepared using radio-labeled 1-14C-oleic acid, 1-14C-arachidonic acid or 1-14C–DHA (Moravek Biochemicals, Brea, CA) and corresponding non-labeled fatty acids (oleic acid [OA; Acros Organics, NJ, USA], arachidonic acid [AA; Sigma, St. Louis, MO] and DHA [Cayman Chem, Ann Arbor, MI]). Reactions were stopped at 0, 15, 30, 45 and 60 minutes and explants were washed 3x in cold stop solution (uptake buffer with 0.1% BSA and 200μM phloretin (MP Biomedicals, Solon, OH)) and finally in cold PBS. Explants were dissolved in BioSol (National Diagnostics, Atlanta, GA) overnight at 37°C and an aliquot was counted on a Beckman LS3801 Liquid Scintillation Counter (Beckman Coulter, Brea, CA). Total protein concentration was determined by BCA assay following the manufacturer’s instructions (Pierce, Rockford, IL, USA). Uptake was calculated as nmol fatty acid/mg/minute.
Real-Time PCR analysis
Total RNA was isolated from ~50 mg of placental villous tissue collected from the primary placental lobe using TriReagent (Sigma) following manufacturer’s directions. Real-Time PCR analysis was performed using the Stratagene Mx3005P Thermocycler as described in detail previously [7]. Gene-specific primers (Table 1) were designed for AMPK-α, fatty acid transporters (CD36, FABPpm, FATP-1,2,4,6) and fatty acid binding proteins (FABP)-3,4 and 5 using primer design software (Clone Manager Professional Suite, v.8). GAPDH was used as a housekeeping gene. PCR amplicons were detected by fluorescent detection of SYBR Green (Applied Biosystems, USA Power SYBR® Green Master Mix, 4368708). Cycling conditions were the same for all primer pairs: 95°C 10 min (for enzyme activation) followed by 40 cycles of 95°C 20s, 55°C for 30s, 72°C 30s. NTCs were not detectable ≤ 40 cycles. Comparative quantification corrected for the efficiency of the respective standard curve was used to generate values for each replicate based on the Cq using MxPro software. The same sample was used as the “calibrator” in all assays. Values were expressed as a ratio of the gene of interest to GAPDH in each sample.
Table 1.
Primer Sequences
| Gene | Gene ID | Sense (5′→3′) | Antisense (5′→3′) | Product Size (bp) | Efficiency (%) |
|---|---|---|---|---|---|
| FABPpm | NM_002080.2 | TTGCTGCTGCCATTCTGAAC | AGGCTTTAGCCCTGTGAAAC | 188 | 85 |
| CD36 | NM_001001548 | AAACCTCCTTGGCCTGATAG | GAATTGGCCACCCAGAAACC | 173 | 120 |
| FABP-3 | NM_004102.3 | GACACTGGATGGAGGGAAAC | GTAGCCGATTGGCAGAGTAG | 191 | 99 |
| FABP-4 | NM_001442.2 | TACTGGGCCAGGAATTTGAC | GAAGTGACGCCTTTCATGAC | 178 | 120 |
| FABP-5 | NM_001444.2 | TTTCTTGTACCCTGGGAGAG | TCCGAGTACAGGTGACATTG | 195 | 115 |
| FATP-1 | NM_198580.1 | TTCCAGGAGTGGAGGGTAAG | TTGAAGGTGCCTGTGGTGTC | 163 | 101 |
| FATP-2 | NM_001159629.1 | TTCTATGCTGCCACTGAAGG | GGAACTCTGACGCAATATCC | 173 | 112 |
| FATP-4 | NM_005094.3 | GCTGCCCTGGTGTACTATGG | GGAGGCTGAAGAACTTCTTCC | 153 | 95 |
| FATP-6 | NM_014031.3 | TTTGGGACCGTACTGGAGAC | GCCATTCCTGCTCTTCCTTC | 151 | 96 |
| GADPH | NM_002046.4 | AGGTGGTCTCCTCTGACTTC | TACTCCTTGGAGGCCATGTG | 169 | 95 |
| AMPKα | NM_206907.3 | AGGCATATGGTGGTCCATAG | CTTCTGGTGCAGCATAGTTG | 157 | 104 |
Immunoblotting analysis
Phosphorylated and total AMPKα, FABPpm, CD36 and FATP4 protein levels were determined in placental protein extracts by western blot analysis. Plasma membrane and cytosolic protein was isolated from ~50 mg of placental villous tissue from the primary lobe following manufacturer’s directions (Abcam, Plasma Membrane Protein Extraction Kit, ab65400). Protein concentration was quantified by BCA assay. Equal amounts of total protein/sample (20 μg) were separated by SDS-PAGE on a 7.5% gel (TGX™ Precast Gels, BioRad) and transferred to 0.2 μm nitrocellulose membrane (BioRad). Membranes were blocked with 5% milk TBS-T (TBS+0.01% Tween) buffer for 1 h. Membranes were incubated with primary antibodies rabbit anti-pAMPKα (Thr172) (1:1000, Cell Signaling #9957), rabbit anti-AMPKα (1:1000, Cell Signaling), mouse anti-FABPpm (1:1000 Abcam #ab93928), mouse anti-FATP4 (1:1000 Abnova #H00010999-M01) and rabbit anti-CD36 (1:2000 ThermoFisher #PA1-16813) overnight at 4°C. Membranes were washed in TBS-T before exposure to the appropriate secondary antibody (1:3000, Santa Cruz) for 1 h at room temperature. Antibody binding was detected using a chemiluminescence system (Amersham ECL™ Prime, GE Healthcare); protein expression was quantified from a digitized image of the blot and expressed as a ratio of phosphorylated to total protein or protein of interest to ponceau S staining.
Immunohistochemistry
Paraffin sections, 5μm thick were prepared using standard methodology. To block non-specific binding of the peroxidase to the blood cells, the tissue was incubated in 0.3% H2O2 in PBS for 15 min followed by several more PBS rinses. Sections were then blocked for non-specific staining in PBS containing 1% BSA (Jackson ImmunoResearch) 10% Normal Goat serum (NGS), 0.3% Triton-X 100 for 30 min. Sections with primary antibodies (1:800 anti-GOT2 (FABPpm), Abcam #ab93928; 1:500 anti-CD36, ThermoFisher #PA1-16813; 1:200 anti-ACSVL4 (FATP4), SantaCruz #sc25670) diluted in PBS containing 1% BSA, 0.3% TX-100 were incubated 12–14 hours at 4°C in a humidified chamber. Vector Laboratories elite ABC kit was used according to directions except for 90 min incubations. DAB was used for visualization. The counterstain was Hematoxylin QS (Vector Laboratories). Slides were imaged using E600 (Nikon) with Spot RT camera and software version 4.7 (Diagnostic Instruments).
Placental tissue fatty acid analysis
Frozen placental tissue was homogenized in PBS and kept cold on ice. Fatty acid profiles were analyzed by a modification of the methods described by Lagerstedt, et al. [31]. Deuterated free fatty acids d3C10:0, d3C14:0, d3C16:0, d3C18:0, d3C20:0 and d4C22:0, were added to samples prior to extraction as internal standards. Fatty acid pentafluorobenzyl (PFB)-esters were analyzed by GC-MS on a Trace DSQ (Thermoelectron) operating in the negative ion chemical ionization mode with methane as the reagent gas. Fatty acid-PFB esters were separated on a DB-5ms capillary column (30 m x 0.25 mm x 0.25 μm) with helium as the carrier gas at a flow rate of 1 ml/min. Individual fatty acids were monitored with selected ion monitoring and a dwell time of 50 ms for each ion species. Calibration curves were generated to quantify unknowns using Xcalibur software (Thermo Scientific).
Statistical analysis
One-way analysis of variance followed by Tukey’s post-test was used to examine differences between groups (GraphPad Prism 6.02). Q-PCR data was log transformed prior to statistical analysis to normalize the data. Data are presented as mean ± SEM unless noted otherwise. P value <0.05 was considered statistically significant.
Results
Maternal and fetal characteristics are described in Table 2; metabolic data from this cohort were previously reported [27]. The majority of the animals on a HFD gain weight and have a reduced insulin response as measured by an intravenous glucose tolerance test before pregnancy and 1 week before delivery and are considered “diet sensitive”, the remainder are “diet resistant” [15]. For all measurements, results were first separated by diet sensitivity, but in every case, diet sensitivity did not have a detectable impact on the reported measurements and thus animals were grouped by diet alone. Maternal and fetal weights were not different between groups (Table 2). Placental weight was 18% lower in the mothers supplemented with resveratrol as compared to control or HFD alone (P<0.01).
Table 2.
Maternal, placental and fetal characteristics
| CTR | HFD | HFD-RES | |
|---|---|---|---|
| n | 5 | 10 | 5 |
| Pre-pregnancy Weight (kg) | 9.7 ± 2.0 | 12.8 ± 2.9 | 12.9 ± 1.5 |
| 3rd Trimester Weight (kg) | 11.3 ± 2.9 | 14.5 ± 3.3 | 12.8 ± 2.3 |
| Birth Weight (g) | 316 ± 24 | 342 ± 32 | 326 ± 40 |
| Total Placental Weight (g) | 106 ± 2 | 108 ± 13 | 88 ± 8** |
| Birth weight: Placental Weight | 3.0 ± 0.2 | 3.2 ± 0.4 | 3.7 ± 0.3** |
| Female/Male | 3/2 | 5/5 | 1/4 |
Placental fatty acid uptake
Fatty acid uptake values were calculated for both the primary and secondary placental lobes separately. We did not observe detectable differences between the lobes in terms of rate of uptake for any fatty acid studied, nor association of uptake with diet. Thus, we report values from the primary lobe only.
Resveratrol supplementation of the high fat diet was associated with an 87% increase in placental uptake of the LCPUFA DHA (Fig 1C) over control diet levels. LCPUFA uptake was not significantly altered by a high fat diet alone. Placental oleic acid and arachidonic acid uptake was not different between groups (Fig 1A & B).
Figure 1.
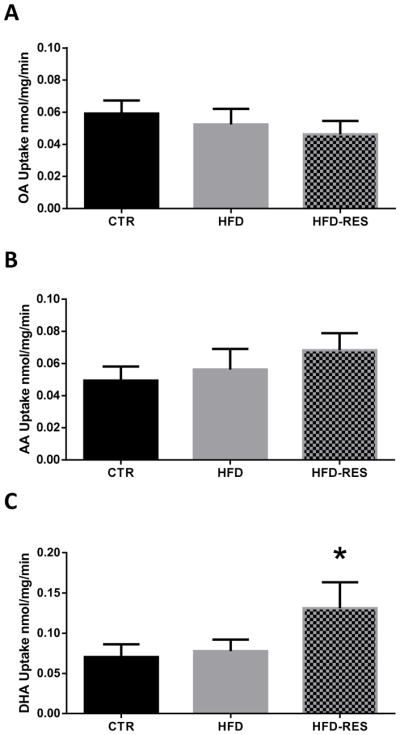
Unsaturated fatty acid uptake in placentas of control (CTR), high fat fed (HFD) and resveratrol supplemented (HFD-RES) non-human primates. Resveratrol supplementation stimulated placental uptake of the n-3 LCPUFA DHA (C) over animals on a control diet and high fat diet without resveratrol supplementation. Oleic acid uptake (A) and arachidonic acid uptake (B) were not affected by a high fat diet or resveratrol supplementation. Diet sensitivity was not associated with detectable differences in placental LCPUFA uptake (data not shown). Data are mean ± SEM **P<0.05 vs HFD alone and vs CTR by ANOVA and Tukey posthoc test.
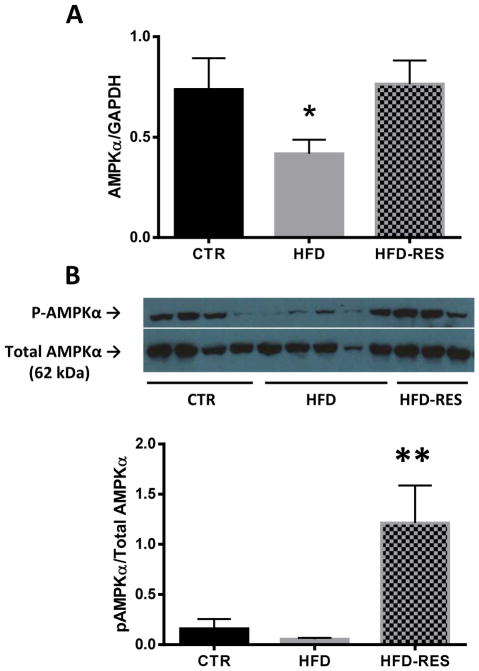
AMPK activation
To investigate possible mechanistic pathways underlying resveratrol’s effect on placental LCPUFA uptake, we first measured the placental expression and phosphorylation of the catalytic α subunit of AMPK (Fig 2). Placental AMPKα mRNA expression was significantly lower in animals fed a high fat diet (P=0.02) as compared to control diet animals (Fig 2A). AMPKα expression in HFD-RES was not different from control animals, suggesting that resveratrol normalized AMPKα expression. Though differences in AMPKα phosphorylation were not detected between placentas of control and HFD animals, resveratrol supplemented animals had a 22-fold increase in phosphorylated AMPKα as compared to HFD-alone (Fig 2B), suggesting that resveratrol supplementation activates placental AMPK.
Figure 2.
Placental AMP-activated protein kinase mRNA and protein levels in control (CTR), high fat fed (HFD) and resveratrol supplemented (HFD RES) non-human primates. AMPKα mRNA expression was decreased in placentas of HFD animals, but resveratrol supplementation normalized these levels (A). Resveratrol supplementation stimulated the phosphorylation of AMPKα protein in the placenta (B). Representative blot of phosphorylated and total AMPKα in the placenta shown. Diet sensitivity was not associated with detectable differences in placental AMPK expression or phosphorylation (data not shown). Data are mean ± SEM *P<0.05 vs CTR, **P<0.05 vs CTR, HFD by ANOVA and Tukey posthoc test.
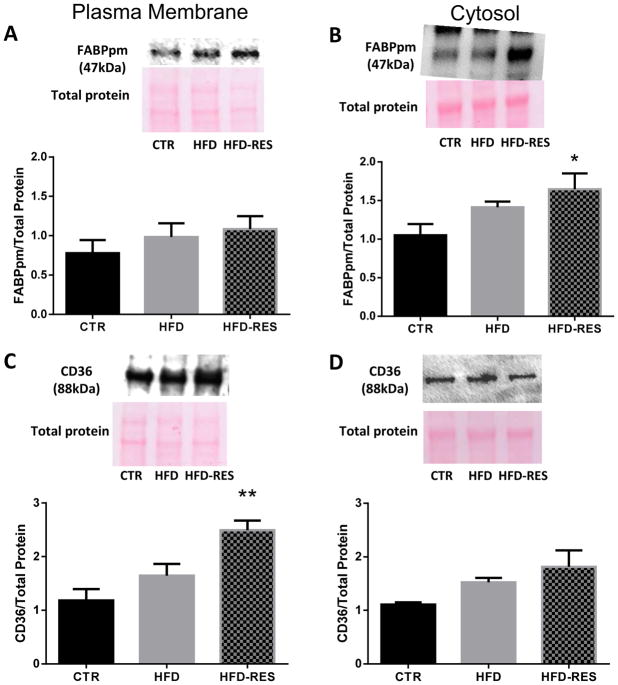
Placental fatty acid transporter expression and localization
The expression of several placental fatty acid transporters was measured to assess their possible role in resveratrol-induced changes in fatty acid uptake (Fig 3). HFD alone was associated with lower mRNA expression of placental plasma membrane fatty acid binding protein (FABPpm, Fig 3A) and fatty acid transporter (FATP)-4 (Fig 3B). Resveratrol supplementation of the HFD normalized (FATP4) or increased mRNA expression (FABPpm) above control diet levels. Placental fatty acid translocase (CD36) mRNA expression was not affected by HFD alone, but was 138% higher than control diet and 162% higher than HFD animals in the resveratrol supplemented group (Fig 3C). As the fatty acid transporters are most active when localized to the plasma membrane, we analyzed the protein expression of these transporters in the plasma membrane and cytosol fractions of the placenta. FABPpm levels were not different between diet groups in the plasma membrane fraction (Fig 4A), but were higher in the placental cytosol of resveratrol supplemented animals (Fig 4B). In contrast, CD36 levels were higher in the plasma membrane of resveratrol-supplemented animals (Fig 4C), but not the cytosol (Fig 4D). Placental FATP4 protein levels were not different between groups (data not shown). We were unable to detect a statistically significant effect of diet or resveratrol supplementation on the placental mRNA expression of FATP1, FATP2, FATP6, FABP3, FABP4 or FABP5 (data not shown).
Figure 3.
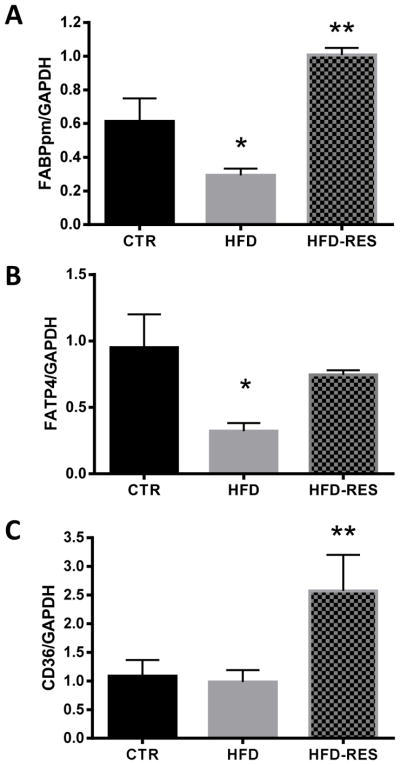
Placental fatty acid transporter mRNA expression in control (CTR), high fat fed (HFD) and resveratrol supplemented (HFD RES) non-human primates. FABPpm (A) and FATP4 (B) mRNA expression was decreased in placentas of HFD animals, but resveratrol supplementation normalized these levels, and in the case of FABPpm, upregulated them above control. CD36 mRNA expression was increased with resveratrol supplementation (C). Diet sensitivity was not associated with detectable differences in placental fatty acid transporter expression (data not shown). Data are mean ± SEM *P<0.05 vs CTR; **P<0.05 vs CTR, HFD by ANOVA and Tukey’s posthoc test.
Figure 4.
Placental fatty acid transporter protein levels in control (CTR), high fat fed (HFD) and resveratrol supplemented (HFD RES) non-human primates. Plasma membrane FABPpm levels (A) were not different between groups, but were higher in the cytosol with resveratrol supplementation (B); CD36 levels in the plasma membrane (C), but not cytosol (D) were increased with resveratrol supplementation. Representative immunoblots and ponceau S stained membranes are shown. Diet sensitivity was not associated with detectable differences in placental fatty acid transporter expression (data not shown). Data are mean ± SEM *P<0.05 vs CTR; **P<0.05 vs CTR, HFD by ANOVA and Tukey’s posthoc test.
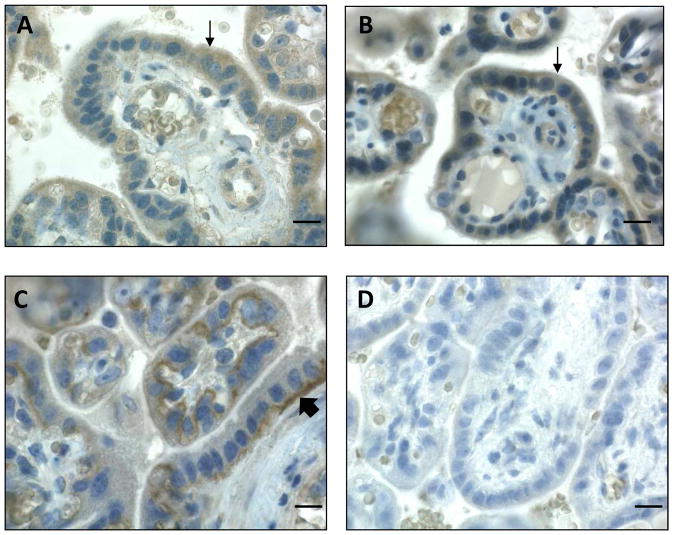
The localization of these fatty acid transporters within the macaque placenta was determined using immunohistochemistry. All transporters studied were localized primarily to the syncytiotrophoblast layer of the placenta. FABPpm (Fig 5A) and FATP4 (Fig 5B) showed diffuse staining within this cellular layer, whereas CD36 strongly localized to the basal membrane of the syncytium (Fig 5C). PBS without any primary antibody served as the negative control (Fig 5D).
Figure 5.
Fatty acid transporter protein localization in the non-human primate placenta. Representative bright field microscope images of FABPpm (A), FATP4 (B) and CD36 (C) in CTR diet non-human primate placenta are shown. FABPpm and FATP4 are detected throughout the syncytiotrophoblast layer (thin arrows, A&B), whereas CD36 is localized most strongly in the basal membrane of the syncytium (thick arrow, C). PBS control is shown (D). Scale bars = 10 μm
Placental fatty acid profiles
The fatty acid composition of placental tissue was analyzed to determine the effect of changes in uptake on tissue storage and lipid profiles (Table 3). Resveratrol supplementation did not measurably alter the placental fatty acid composition as compared to HFD alone. HFD was associated with a decrease in the concentration of placental DHA levels by 40% (P<0.05); though the decrease in placental DHA in HFD-RES dams was similar (37%), it did not reach statistical significance (P=0.12). The proportion of monounsaturated fatty acids (palmitoleic, C16:1 and oleic, C18:1) was significantly higher in the placentas of HFD dams (P=0.007 by ANOVA), with or without resveratrol supplementation. As oleic acid was the most abundant MUFA measured, we calculated the delta-9-desaturase index (C18:1/C18:0) for all animals. This index tended to be higher in HFD placentas, regardless of resveratrol supplementation, though it did not reach statistical significance (P=0.06 by ANOVA). The placental N-6/N-3 ratio was altered by diet (P=0.03 by ANOVA), however multiple comparison testing failed to detect significant differences between groups (CTR vs HFD, P=0.057).
Table 3.
Placental fatty acid profiles in control diet, high fat diet and resveratrol-supplemented dams.
| CTR | HFD | HFD-RES | |
|---|---|---|---|
| n | 6 | 6 | 5 |
| Palmitate, C16:0 | 7.18 ± 0.48 | 6.61 ± 1.20 | 6.21 ± 1.07 |
| Stearate, C18:0 | 4.89 ± 0.63 | 4.34 ± 0.77 | 4.49 ± 0.77 |
| Total SFA | 12.07 ± 1.03 | 10.95 ± 1.90 | 10.70 ± 1.74 |
| Palmitoleate, C16:1 | 0.36 ± 0.13 | 0.27 ± 0.08 | 0.24 ± 0.04 |
| Oleate, C18:1 | 1.69 ± 0.36 | 2.20 ± 0.38 | 2.27 ± 0.50 |
| Total MUFA | 2.05 ± 0.47 | 2.47 ± 0.42 | 2.51 ± 0.52 |
| Linoleate, C18:2 | 3.91 ± 0.61 | 2.69 ± 0.52 | 2.82 ± 0.55 |
| Arachidonate, C20:4 | 8.79 ± 1.62 | 9.13 ± 2.05 | 8.99 ± 2.00 |
| Total n-6 | 12.69 ± 2.05 | 11.83 ± 2.14 | 11.81 ± 2.40 |
| α-Linolenate, C18:3 | 0.10 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| Eicosapentanoate, C20:5 | 0.83 ± 0.05 | 0.82 ± 0.06 | 0.83 ± 0.07 |
| Docosahexanoate, C22:6 | 1.66 ± 0.70 | 1.00 ± 0.11* | 1.04 ± 0.19 |
| Total n-3 | 2.59 ± 0.76 | 1.91 ± 0.15 | 1.96 ± 0.25 |
| n-6/n-3 | 5.06 ± 0.66 | 6.17 ± 0.84 | 5.98 ± 0.63 |
| Total FA | 29.40 ± 2.87 | 27.16 ± 4.37 | 26.99 ± 4.69 |
| %SFA | 41.36 ± 5.11 | 40.34 ± 2.00 | 39.74 ± 1.35 |
| %MUFA | 6.95 ± 1.25 | 9.11 ± 0.52* | 9.35 ± 1.24* |
| %n-6 | 43.02 ± 3.62 | 43.44 ± 2.49 | 43.57 ± 1.42 |
| %n-3 | 8.67 ± 1.68 | 7.11 ± 0.73 | 7.34 ± 0.62 |
| D9D Index | 0.35 ± 0.12 | 0.51 ± 0.05 | 0.51 ± 0.07 |
Values are mean ± SD and expressed as μmol/g tissue, unless otherwise indicated. SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; D9D, delta-9-desaturase index.
P<0.05 vs CTR.
Discussion
The main findings of this study were that maternal HFD did not alter the ability of the placenta to take up fatty acids; however, supplementation of HFD with resveratrol prior to and throughout pregnancy significantly increased placental DHA uptake capacity. This increase in DHA uptake capacity was associated with activation of AMPK and higher expression of multiple fatty acid transporters in the early third trimester macaque placenta.
In our model, plasma n-3 LCPUFA levels are 77% lower in mothers on HFD (vs. control diet) and 71% lower in their offspring [32]. HFD offspring of this model display anxiety-like behavior [33] and increased vascular reactivity [16, 34], consistent with exposure to low levels of n-3 LCPUFA during fetal development [35, 36]. Though we found that resveratrol supplementation improves the ability of the HFD placenta to take up DHA, fetal n-3 LCPUFA levels in circulation remain low [27]. As fetal plasma fatty acid profiles are influenced by fetal metabolism as well as placental transport, it is unclear whether changes in placental uptake capacity altered transport of LCPUFA to the fetus. Resveratrol supplementation improves uterine and umbilical blood flow in the HFD animals [27], suggesting that when paired with enhanced capacity for placental uptake, LCPUFA transfer may be increased in vivo. Offspring of resveratrol-supplemented HFD mothers showed dramatic improvements in liver triglyceride deposition at birth [27]. The n-3 LCPUFA, namely DHA and EPA reduce lipid esterification in several tissues, including the liver [37, 38] and increased exposure to these fatty acids may explain the findings in offspring of resveratrol-supplemented mothers. Alternatively, the reduced placental size associated with resveratrol supplementation suggests that despite an increase in DHA uptake capacity per mg placental tissue, a decrease in amount of tissue may result in no change in overall placental DHA uptake. However, these studies were not designed to conclusively determine maternal-fetal fatty acid transfer.
Tracer studies found that in vivo placental DHA transport to the fetus was lower in obese women with gestational diabetes and this was associated with lower placental DHA uptake [14], supporting an important connection between placental uptake and transport in vivo. The effect of maternal obesity on placental transport of DHA to the fetus in non-diabetic women is unknown, though we have previously reported that DHA uptake in placental explants from obese non-diabetic women at term is not different from lean women [7]. These findings are consistent with our observation that placental DHA uptake was not altered by HFD alone in the macaque model. The uptake values measured in the macaque placentas (~0.07 nmol DHA/mg/min) were comparable to human placentas (~0.1 nmol DHA/mg/min) [7], suggesting that placental DHA uptake capacity is similar between human and non-human primates.
Normal changes in maternal metabolism during pregnancy includes a reduction in insulin sensitivity which drives increases in maternal fat mass and circulating triglycerides – important fuel for fetal growth and the main source of fatty acids for placental transfer [39–41]. Roberts et al. reported that resveratrol supplementation of HFD dams decreased maternal body weight (both fat and lean mass), improved fasting insulin during pregnancy and reduced circulating triglyceride concentrations, though leptin levels increased normally [27]. Dams supplemented with resveratrol did not gain significant weight during pregnancy, unlike their control and HFD counterparts. Reductions in weight and triglycerides before pregnancy are beneficial for long-term health. However these changes during pregnancy may impede fetal growth and development [42]. Indeed, Roberts reported that fetal pancreatic development was altered in resveratrol-supplemented pregnancies which may have negative effects on metabolism later in life [27].
The metabolic effects of resveratrol have been shown to depend upon activation of AMPK [28]. Indeed, we demonstrated in our model that chronic resveratrol supplementation of HFD prior to and during pregnancy was associated with 22-fold higher levels of phosphorylated AMPK in the placenta as compared to HFD alone. Activation of AMPK leads to translocation of CD36 and FABPpm to the membrane and is associated with an increase in fatty acid uptake in vivo and in vitro [29, 30]. Consistent with this, in our model, the placental mRNA expression of these transporters was increased and CD36 protein levels were higher in placental plasma membrane with resveratrol treatment. These findings highlight an important role for placental AMPK activation in the upregulation of fatty acid transporter expression and enhanced ability to take up DHA in placentas of resveratrol-supplemented dams.
Our immunolocalization data showed that all three transporters (CD36, FABPpm, FATP4) localized primarily to the syncytiotrophoblast layer, suggesting that the observed changes in transporter expression affected levels mainly in the cellular layer responsible for fatty acid uptake from the maternal circulation. Interestingly, though FABPpm and FATP4 protein appeared to be distributed throughout the syncytiotrophoblast, CD36 proteins were localized overwhelmingly to the basal membrane of the trophoblast in the Japanese macaque placenta, a finding that has not previously been reported. We speculate that the observed increase in plasma membrane CD36 levels in resveratrol-supplemented placentas may result in increased shuttling of fatty acids from the syncytiotrophoblast to the fetal circulation. This in turn would drive a favorable concentration gradient for DHA uptake from the maternal circulation across the maternal villous membrane. Though Roberts et al. did not detect a difference in fetal fatty acid levels with resveratrol supplementation, fetal metabolism will also influence these levels and if resveratrol (which was found to cross the placenta) modified fatty acid uptake in other fetal organs, circulating levels may remain low. The delta-9-desaturase index (measure of steroyl CoA desaturase 1 activity) was increased in HFD placentas as compared to controls, though this was not statistically significant. However, it displays a trend consistent with findings in the liver of other models of HFD, that steroyl CoA desaturase activity is increased which coincides with an increase in esterification of lipids and tissue storage [43, 44]. Interestingly, resveratrol supplementation did not alter this effect of HFD in the placenta, in opposition to findings in other tissues [45], suggesting that resveratrol may have unique effects on placental lipid metabolism.
Due to the nature of our model, we are limited by the number of animals in our study, which left us underpowered to thoroughly assess differences between HFD resistant and sensitive dams or between male and female fetuses. Thus, though we did not detect any trends, we cannot conclusively rule out an effect of either diet sensitivity or fetal sex on these relationships. Detection of non-specific binding of labeled fatty acids to the explants may have contributed to the uptake values. However, it is unlikely that this non-specific component explains the differences in DHA uptake between dietary groups. Our plasma membrane extraction protocol was not specific for syncytiotrophoblast membranes, thus we cannot say for certain that changes in transporter expression in the membrane fraction is specific to this cellular layer, though our immunohistochemistry data suggest that the overwhelming localization of these transporters is in the trophoblast. It remains to be established which specific plasma membranes are enriched in this fraction and whether enrichments differ between dietary groups. We did not directly measure fatty acid delivery to the fetus, and therefore can only speculate that an increase in placental DHA uptake and increase in blood flow to the placenta [27] may alter the transfer of fatty acids from mother to baby.
In summary, we found that maternal HFD led to a decrease in placental fatty acid transporter and AMPK mRNA expression, but did not significantly affect placental LCPUFA uptake. Resveratrol supplementation - associated with decreased maternal fat mass and fasting insulin and triglyceride levels, without changes in dietary fat intake - stimulated placental DHA uptake capacity, AMPK activation and transporter expression. These findings suggest that though placental fatty acid uptake capacity appears to be unaffected by a HFD, handling of the n-3 LCPUFA DHA is particularly sensitive to the dramatic alterations in the maternal metabolic phenotype and placental AMPK activity associated with resveratrol supplementation.
Highlights.
Resveratrol is an insulin sensitizer and may act via AMPK activation.
Macaques were fed a high fat diet with and without resveratrol during pregnancy.
Placental fatty acid transporter and AMPK expression was lower in HFD dams.
Resveratrol increased placental transporter expression and AMPK activity.
Resveratrol increased placental polyunsaturated fatty acid uptake capacity.
Acknowledgments
This research is supported by the National Institutes of Child Health & Development (R00HD062841 to PFOG) and NIDDK (R24DK090964 to KG; P51OD011092 to AF and KG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chu SY, Kim SY, Bish CL. Prepregnancy Obesity Prevalence in the United States, 2004–2005. MaternChild Health J. 2008 doi: 10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsen T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315(7112):837–40. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. IntJObes(Lond) 2009;33(7):743–52. doi: 10.1038/ijo.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23(1):271–8. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1224–R31. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–9. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14, 1-,1. doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 9.Hornstra G, Al MD, van Houwelingen AC, Foreman-van Drongelen MM. Essential fatty acids in pregnancy and early human development. Eur J Obstet Gynecol Reprod Biol. 1995;61(1):57–62. doi: 10.1016/0028-2243(95)02153-j. [DOI] [PubMed] [Google Scholar]
- 10.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. The American journal of clinical nutrition. 1994;60(2):189–94. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- 11.Salem N, Jr, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA. 1996;93(1):49–54. doi: 10.1073/pnas.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greiner RC, Winter J, Nathanielsz PW, Brenna JT. Brain docosahexaenoate accretion in fetal baboons: bioequivalence of dietary alpha-linolenic and docosahexaenoic acids. Pediatr Res. 1997;42(6):826–34. doi: 10.1203/00006450-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-De Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. American journal of obstetrics and gynecology. 2009;201(2):209.e1–e10. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagan A, Prieto-Sanchez MT, Blanco-Carnero JE, Gil-Sanchez A, Parrilla JJ, Demmelmair H, Koletzko B, Larque E. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. American journal of physiology Endocrinology and metabolism. 2013;305(7):E826–33. doi: 10.1152/ajpendo.00291.2013. [DOI] [PubMed] [Google Scholar]
- 15.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–35. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, Thornburg KL, Grove KL. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. International journal of obesity (2005) 2013;37(2):254–62. doi: 10.1038/ijo.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–64. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, Barbour LA, Bessesen DH. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care. 2011;34(10):2198–204. doi: 10.2337/dc11-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–7. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35(6):411–6. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, Longo DL, Belman JP, Malagon MM, Navas P, Sanghvi M, Moaddel R, Tilmont EM, Herbert RL, Morrell CH, Egan JM, Baur JA, Ferrucci L, Bogan JS, Bernier M, de Cabo R. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell metabolism. 2013;18(4):533–45. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Zorita S, Fernandez-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition (Burbank, Los Angeles County, Calif) 2013;29(11–12):1374–80. doi: 10.1016/j.nut.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Sun J, Li L, Zheng J, Shi Y, Le G. Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food & function. 2014;5(7):1452–63. doi: 10.1039/c3fo60714c. [DOI] [PubMed] [Google Scholar]
- 24.Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(12):2895–901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- 25.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell metabolism. 2011;14(5):612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62(4):1186–95. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE, Grove KL. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28(6):2466–77. doi: 10.1096/fj.13-245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP508 activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Oort MM, van Doorn JM, Hasnaoui ME, Glatz JF, Bonen A, van der Horst DJ, Rodenburg KW, JJPL Effects of AMPK activators on the sub-cellular distribution of fatty acid transporters CD36 and FABPpm. Arch Physiol Biochem. 2009;115(3):137–46. doi: 10.1080/13813450902975090. [DOI] [PubMed] [Google Scholar]
- 30.Chabowski A, Momken I, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. Prolonged AMPK activation increases the expression of fatty acid transporters in cardiac myocytes and perfused hearts. Mol Cell Biochem. 2006;288(1–2):201–12. doi: 10.1007/s11010-006-9140-8. [DOI] [PubMed] [Google Scholar]
- 31.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73(1):38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 32.Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, Braun TP, Grove KL, Friedman JE, Marks DL. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One. 2011;6(2):e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(10):3826–30. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadderdon SM, Belcik JT, Bader L, Kirigiti MA, Peters DM, Kievit P, Grove KL, Lindner JR. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation. 2014;129(4):471–8. doi: 10.1161/CIRCULATIONAHA.113.003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armitage JA, Pearce AD, Sinclair AJ, Vingrys AJ, Weisinger RS, Weisinger HS. Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids. 2003;38(4):459–64. doi: 10.1007/s11745-003-1084-y. [DOI] [PubMed] [Google Scholar]
- 36.Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med. 2001;7(3):258–9. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- 37.Berge RK, Madsen L, Vaagenes H, Tronstad KJ, Gottlicher M, Rustan AC. In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by decreased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem J. 1999;343(Pt 1):191–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Huang LL, Wan JB, Wang B, He CW, Ma H, Li TW, Kang JX. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot Essent Fatty Acids. 2013;88(5):347–53. doi: 10.1016/j.plefa.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Catalano PM, Drago NM, Amini SB. Maternal carbohydrate metabolism and its relationship to fetal growth and body composition. Am J Obstet Gynecol. 1995;172(5):1464–70. doi: 10.1016/0002-9378(95)90479-4. [DOI] [PubMed] [Google Scholar]
- 40.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–48. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 41.Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res. 2006;65 (Suppl 3):59–64. doi: 10.1159/000091507. [DOI] [PubMed] [Google Scholar]
- 42.Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, Saade G, Sorokin Y, Peaceman AM, Tolosa JE. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enser M. Desaturation of stearic acid by liver and adipose tissue from obese-hyperglycaemic mice (ob/ob) The Biochemical journal. 1975;148(3):551–5. doi: 10.1042/bj1480551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enser M, Roberts JL. The regulation of hepatic stearoyl-coenzyme A desaturase in obese-hyperglycaemic (ob/ob) mice by food intake and the fatty acid composition of the diet. The Biochemical journal. 1982;206(3):561–70. doi: 10.1042/bj2060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food & function. 2014;5(6):1241–9. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]