Abstract
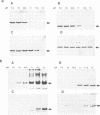
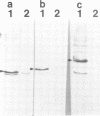
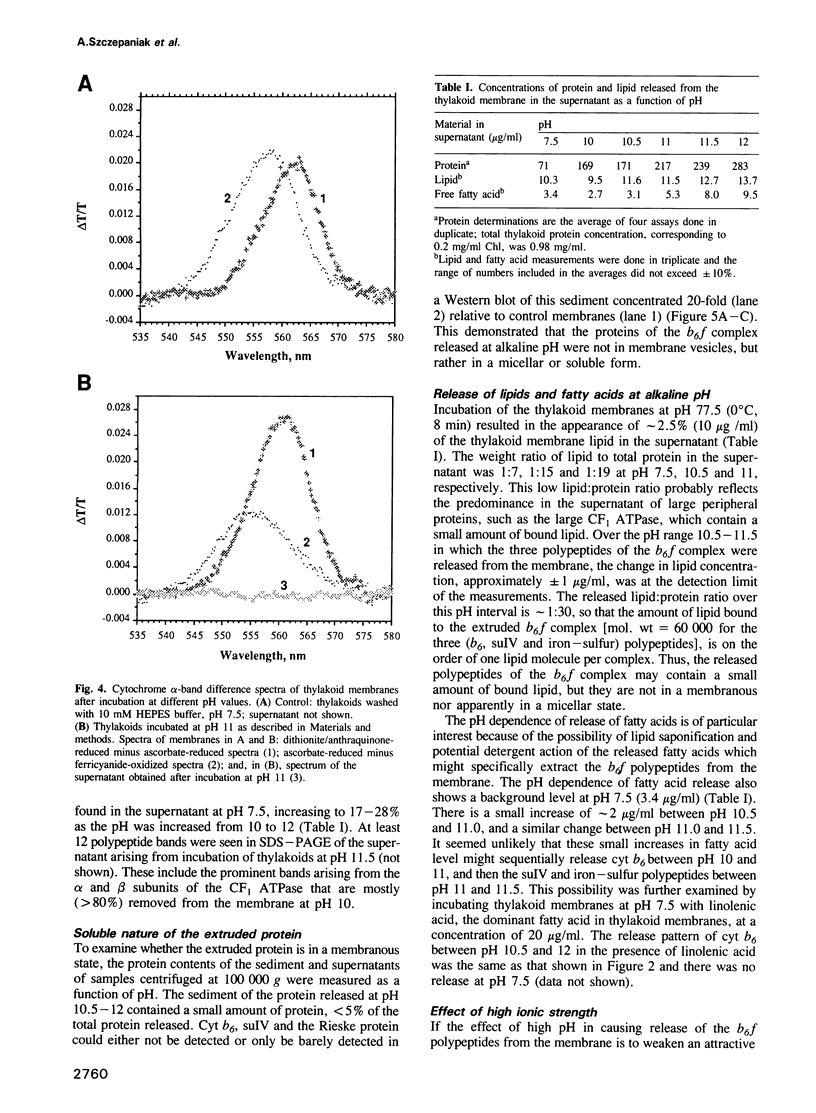
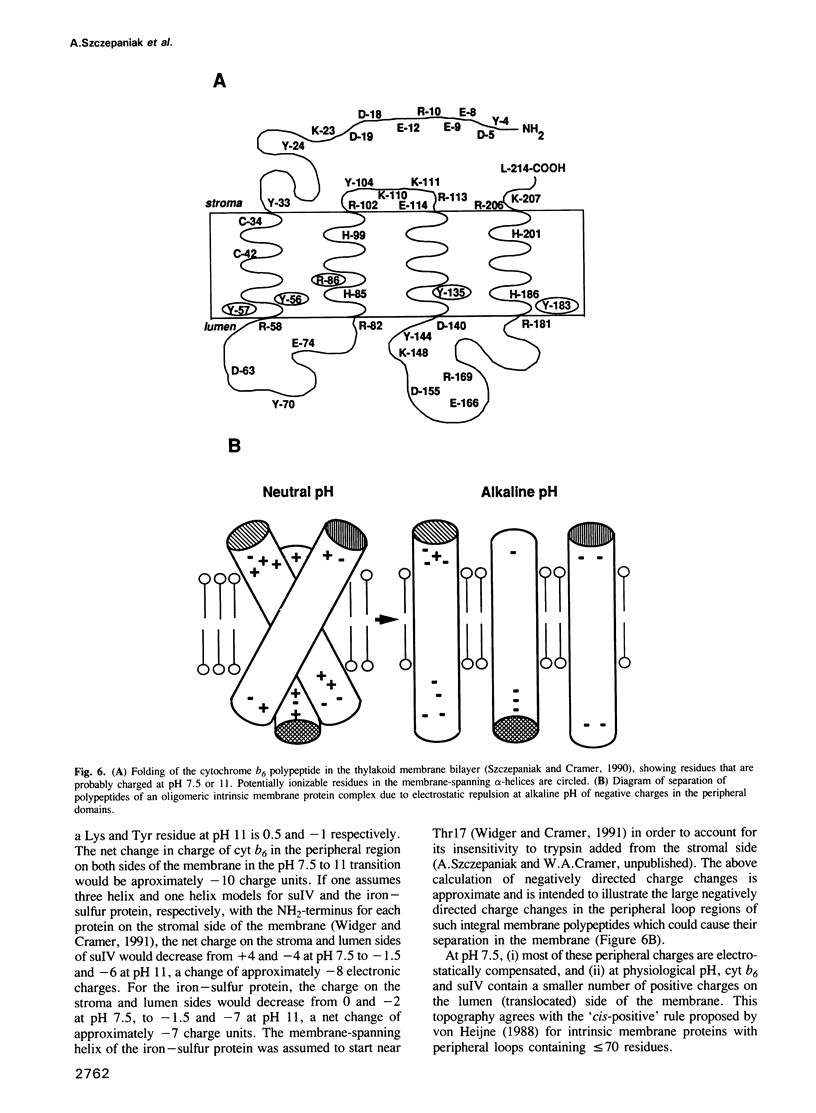
Three of the membrane-spanning polypeptides of the chloroplast cytochrome (cyt) b6f complex were sequentially released from the thylakoid membrane, in the order cyt b6, suIV and Rieske iron-sulfur protein, as the pH was increased from 10 to 12, a protocol usually employed to remove peripheral proteins from membranes. The fourth polypeptide of the cyt b6f complex, cyt f, which spans the membrane once, was apparently not released. The pH values for half-release at low ionic strength were approximately 10.7, 11.1 and 11.3 respectively. The separation of the polypeptides of the complex and the sequential release is readily seen at pH 11, where the loss from the membrane of cyt b6, suIV and Fe iron-sulfur center is approximately 90%, 50% and 20%, respectively. the release of cyt b6 from the membrane was reflected by the absence of its characteristic reduced minus oxidized absorbance signal. The pH values at which the release occurred increased as the ionic strength was raised, implying that the release of the b6f polypeptides arises from extrusion due to repulsive electrostatic interactions probably caused by deprotonation of tyrosine and lysine residues. The lipid content of the released polypeptides was very low, consistent with the observation of a non-membranous state. It is proposed that the pH-dependent extrusion requires two electrostatic effects at alkaline pH higher than approximately 10.5: (i) increased electrostatic repulsion between neighbouring polypeptides of the complex, arising from increased net negative charge in the peripheral segments of these polypeptides, which can cause separation of the polypeptides from the complex; and (ii) ionization of residues such as tyrosine in the membrane-spanning alpha-helices, and neutralization of residues such as lysine which can bind to the negative membrane surface.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black M. T., Widger W. R., Cramer W. A. Large-scale purification of active cytochrome b6/f complex from spinach chloroplasts. Arch Biochem Biophys. 1987 Feb 1;252(2):655–661. doi: 10.1016/0003-9861(87)90071-3. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Furbacher P. N., Girvin M. E., Cramer W. A. On the question of interheme electron transfer in the chloroplast cytochrome b6 in situ. Biochemistry. 1989 Nov 14;28(23):8990–8998. doi: 10.1021/bi00449a006. [DOI] [PubMed] [Google Scholar]
- Harnisch U., Weiss H., Sebald W. The primary structure of the iron-sulfur subunit of ubiquinol-cytochrome c reductase from Neurospora, determined by cDNA and gene sequencing. Eur J Biochem. 1985 May 15;149(1):95–99. doi: 10.1111/j.1432-1033.1985.tb08898.x. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Heape A. M., Juguelin H., Boiron F., Cassagne C. Improved one-dimensional thin-layer chromatographic technique for polar lipids. J Chromatogr. 1985 Apr 5;322(2):391–395. doi: 10.1016/s0021-9673(01)97702-7. [DOI] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990 May 1;29(17):4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- Schägger H., Borchart U., Machleidt W., Link T. A., Von Jagow G. Isolation and amino acid sequence of the 'Rieske' iron sulfur protein of beef heart ubiquinol:cytochrome c reductase. FEBS Lett. 1987 Jul 13;219(1):161–168. doi: 10.1016/0014-5793(87)81210-3. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Szczepaniak A., Black M. T., Cramer W. A. Topography of the chloroplast cytochrome b6: orientation of the cytochrome and accessibility of the lumen-side interhelix loops. Z Naturforsch C. 1989 May-Jun;44(5-6):453–461. doi: 10.1515/znc-1989-5-619. [DOI] [PubMed] [Google Scholar]
- Szczepaniak A., Cramer W. A. Thylakoid membrane protein topography. Location of the termini of the chloroplast cytochrome b6 on the stromal side of the membrane. J Biol Chem. 1990 Oct 15;265(29):17720–17726. [PubMed] [Google Scholar]
- Tae G. S., Black M. T., Cramer W. A., Vallon O., Bogorad L. Thylakoid membrane protein topography: transmembrane orientation of the chloroplast cytochrome b-559 psbE gene product. Biochemistry. 1988 Dec 27;27(26):9075–9080. doi: 10.1021/bi00426a002. [DOI] [PubMed] [Google Scholar]
- Usui S., Yu L., Yu C. A. Cloning and sequencing of a cDNA encoding the Rieske iron-sulfur protein of bovine heart mitochondrial ubiquinol-cytochrome c reductase. Biochem Biophys Res Commun. 1990 Mar 16;167(2):575–579. doi: 10.1016/0006-291x(90)92063-6. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Hermodson M., Meyer D., Gullifor M. Purification and partial amino acid sequence of the chloroplast cytochrome b-559. J Biol Chem. 1984 Mar 25;259(6):3870–3876. [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]