Abstract
Background
Traffic-related particulate matter (PM) has been linked to heightened incidence of asthma and allergic diseases. However, molecular mechanisms by which PM exposure promote allergic diseases remain elusive.
Objective
We sought to determine the expression, function and regulation of pathways involved in the promotion by PM of allergic airway inflammation.
Methods
We employed gene expression transcriptional profiling, in vitro culture assays, and vivo murine models of allergic airway inflammation.
Results
We identified genes of the Notch pathway, most notably Jagged 1 (Jag1), as targets of PM induction in human monocytes and murine dendritic cells (DCs). PM, especially ultrafine particles (UFP), upregulated T helper cytokine, IgE production and allergic airway inflammation in mice in a Jag1 and Notch-dependent manner especially in the context of the pro-asthmatic IL-4 receptor allele Il4raR576. PM-induced Jag1 expression was mediated by the aryl hydrocarbon receptor (AhR), which bound to and activated AhR response elements in the Jag1 promoter. Pharmacological antagonism of AhR or its lineage-specific deletion in CD11c+ cells abrogated the augmentation of airway inflammation by PM.
Conclusion
PM activate an AhR-Jag1-Notch cascade to promote allergic airway inflammation in concert with pro-asthmatic alleles.
Keywords: Traffic-related particulate matter, diesel exhaust particles, ultra-fine particles, Asthma, allergic airway inflammation, Jagged1, Notch, aryl hydrocarbon receptor, Airway hyper-responsiveness
INTRODUCTION
Asthma is a major health problem worldwide, whose prevalence has reached unprecedented levels in recent years1. Its rise to the status of a modern epidemic has been driven by the interaction of susceptibility genes in affected individuals with emerging environmental factors unleashed by the industrial revolution and life style changes2. The latter include changes in microbial exposure, integrated under the rubric of the hygiene hypothesis, changes in diet and activity and, significantly, exposure to airborne particles and pollutants in carbon footprint-heavy economies3. A large body of research has linked asthma development with urbanization and exposure to pro-oxidative, traffic-related air pollutants, most notably particulate matter (PM) and ozone, by virtue of their initiation of airway and systemic inflammation and their pro-allergic adjuvant function4–7.
The acute rise in the incidence of atopy and asthma in urban areas has been related in part to fine (FP; ≤2.5 µm in diameter) and ultrafine (UFP; ≤0.18 µm in diameter) particulate matter emitted by vehicular traffic, in particular that associated with diesel engines8–11. These particles have been reported to promote Th2 and Th17 cell responses and upregulate IgE production in the exposed host6, 12, 13. They differentially impact the lung function of asthmatic individuals, suggesting that genetic and epigenetic predispositions to atopy cooperatively promote airway inflammatory responses seen after diesel exhausted particulate (DEP) exposure14.
Ambient PM exposure acts as an adjuvant that primes the immune response to common environmental allergens15. Intranasal instillation of ambient ultrafine particles (UFP) derived from vehicular emissions enables a de novo immune response in the absence of added adjuvants16. Recently, it was demonstrated that simple inhalation of ambient UFP could effectively boost the secondary immune response to an experimental allergen, indicating that vehicular traffic exposure might exacerbate allergic inflammation in already-sensitized subjects12. Neither the precise molecular mechanisms by which PM exposure promotes allergic sensitization nor the identity of the PM subcomponents involved, are clear. However, PM induced oxidative stress can induce Th-skewing of the immune response through impacting the antigen-presenting function of DCs17–21. This could involve antigen uptake, antigen presentation, DCs co-stimulatory activity, and cytokine production.
To elucidate molecular mechanisms by which PM exposure may program antigen presenting cells to promote allergic diseases, a range of genetic, immunological and whole animal approaches were employed. We here provide evidence for a critical role for an AhR-Jag1-Notch pathway in mediating the pro-inflammatory effects of PM in allergic airway inflammation in interaction with pro-atopic Il4ra alleles.
METHODS
Mice
Il4raR576 mice were previously described22. The following mice were obtained from the Jax Lab: BLAB/c (WT), Tlr4−/− (B6.B10ScN-Tlr4lps-del), Ahrfl/fl (Ahrtm3.1Bra), CD11c-cre (B6.Cg-Tg(Itgax-cre)1-1Reiz/J), Nlrp3−/− (B6.129S6-Nlrp3tm1Bhk/J). DO11.10Rag2−/− mice were obtained from Taconic farms. They were crossed with Il4raR576 mice to generate DO11.10Rag2−/− Il4raR576 mice. Nrf2−/− mice were a kind gift of Andre Nel23.
Particles
DEP were a kind gift of Dr. Andre Nel and have been described before24. CB (<500nm) was purchased from Sigma-Aldrich Co. LLC. Ambient FP (≤2.5 µm) and UFP (≤0.18 µm) were collected in an urban area of downtown Los Angeles by means of a high-volume ultrafine particle (HVUP) sampler25, operating at 400L/min and loaded with a zefluor filter (supported PTFE, 3.0µm pore, 8” × 10”, Pall Life Sciences). For investigating the volatile and non-volatile UFP fractions, we collected two UFP samples; N-UFP, one collected during nighttime + morning traffic hours (i.e. rich in semi-volatile organics partitioned to the PM phase) and Af-UFP, another one collected during the afternoon when partitioning is minimal. The respective particles were suspended in an aqueous solution, with the hydrophilic components becoming part of the solution, while the solid non-soluble UFP cores are left in suspension. To transfer the particles into solution, the particle-loaded filters were soaked in ultrapure water, followed by 5 min vortexing and 30 min sonication. The entire mixture was administered intranasally, as indicated below. Endotoxin levels were less than 0.1ng/ml (data not shown).
Derivation of dendritic cells
BMDCs were obtained by culturing bone marrow aspirates for 7 days with GM-CSF at 20 ng/ml, respectively. The cells were either sham treated with PBS or treated with particles at 10 µg/ml in PBS for 18 hr prior to measurement of Notch pathway components by flow cytometry and/or real time PCR.
T cell/BMDCs co-cultures
Splenic CD4+DO11.10+RAG2−/− T cells were purified by magnetic cell sorting negative selection (Miltenyi Biotec). BMDCs were aliquoted at 2×105 cells in 48 well plates, then either sham treated or treated with the respective PM species at 10 µg/ml/culture well overnight. For AhR inhibition experiment, BMDCs were treated with 30nM AhR antagonist, CH223191 and washed after 16 hours. CD4+DO11.10+RAG2−/− T cells were added at 4×105 cells/well in a final volume of 0.5 ml 10% FBS/RPMI. Cultures were treated with the OVA323–339 peptide at 1µM, as indicated. Notch pathway inhibitors included the gamma secretase inhibitor (GSI) (Calbiochem), added at 5 µM, and the anti-murine Jagged 1 antibodies (Biolegend), added at 2.5 µg/ml.
Allergic sensitization and challenge
Mice were sensitized to OVA by intraperitoneal (i.p.) injection of 100 µg OVA in 100 µl PBS, then boosted two weeks later with a second i.p. injection of OVA in PBS. Control mice were sham sensitized and boosted with PBS alone. Starting on day 29, both OVA and sham sensitized mice were challenged with aerosolized OVA at 1%, delivered through a Schuco 2000 nebulizer (Allied Health Care Products, St. Louis, MO), for 30 minutes daily for 3 days. Two hours before each OVA aerosol exposure, subgroups of mice were given intransally (i.n.) either PBS or PM (UFP, FP or CB) at 10 µg/100µl PBS/instillation. Where indicated, GSI (0.3mg/kg in DMSO, i.n) or CH223191 (0.5 mg/kg in corn oil, i.p) was introduced 4 hr prior to OVA challenge, with untreated mice receiving the DMSO vehicle, also intranasally. For anti-Jag1 antibody blocking, 150 µg anti-Jag1 or isotype control antibodies in 100 µl PBS buffer were administered daily for three consecutive days during OVA aerosol challenge. Mice were euthanized on day 32 post sensitization and analyzed. For dust mite-induced allergic airway inflammation, mice received 5µg of lyophilized D. Pteronyssinus extract in 100 µl PBS intranasally for 3 days at the start of the protocol then challenged with the same dose of D. Pteronyssinus extract on days 15–17 with or without PM. Where indicated, mice were treated with isotype control or anti-Jag1 antibodies, or with DMSO (sham) or AhR antagonist CH223191 as indicated, on the same days (15–17) of the D. Pteronyssinus challenge. Mice were euthanized on day 18 and analyzed for measures of airway inflammation.
Lung histopathology staining
Paraffin-embedded lung sections were stained with hemtoxylin and eosin (H&E) as described26. Lung inflammation was scored separately for cellular infiltration around blood vessels and airways: 0, no infiltrates; 1, few inflammatory cells; 2, a ring of inflammatory cells 1 cell layer deep; 3, a ring of inflammatory cells 2–4 cells deep; 4, a ring of inflammatory cells of >4 cells deep27. A composite score was determined by the adding the inflammatory scores for both vessels and airways. The number and distribution of goblet cells was assessed by Periodic Acid Schiff (PAS) staining of mucin granules. Individual airways (bronchi/bronchioles) were scored for goblet cell hyperplasia according to the following scale: 0, no PAS-positive cells; 1, <5% PAS-positive cells; 2, 5 to 10% PAS-positive cells; 3, 10 to 25% PAS-positive cells; and 4, > 25% PAS-positive cells28.
Statistical analysis
Student’s two tailed t-test, one and two way ANOVA and repeat measures two way ANOVA with Bonferroni posttest analysis of groups were used to compare test groups, as appropriate. A p value <0.05 was considered statistically significant.
Study approval
All animal studies were reviewed and approved by the Boston Children’s Hospital office of Animal Care Resources.
Other Methods
Information on additional chemical reagents employed, gene expression profiling, real time PCR analysis, flow cytometry and intracellular staining reagents, antibodies and methods, IgE and cytokine ELISAs, measurement of airway hyper-responsiveness, chromatin immunoprecipitation (ChIP) assays and luciferase assays is provided in the Methods section in this article's Online Repository at www.jacionline.org.
RESULTS
PM activates Jag1-Notch transcriptional circuitry
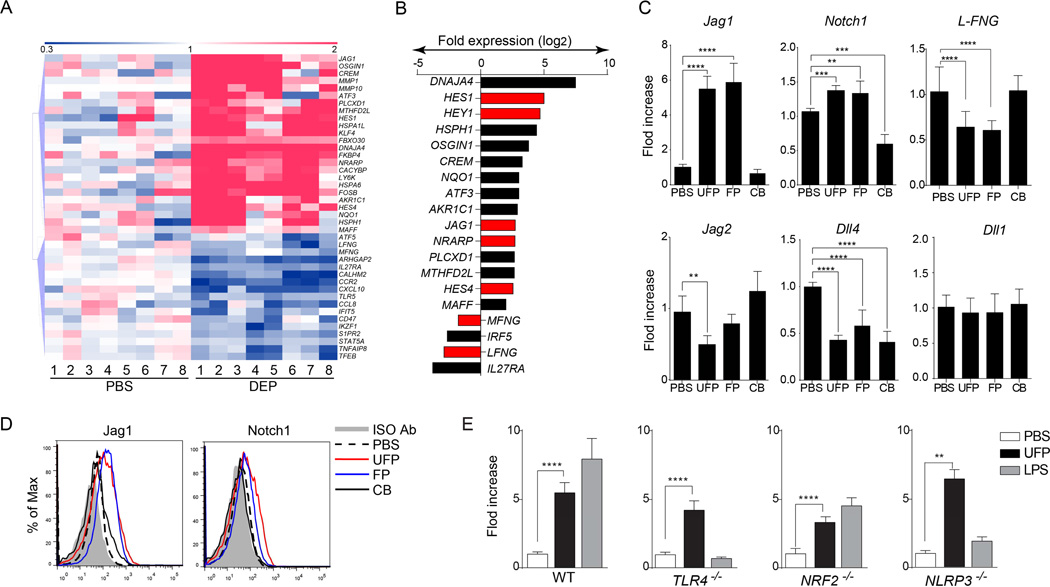
PM has been noted to reprogram antigen-presenting cells in favor of Th2 responses. To understand mechanisms by which PM mediates this function, we analyzed the gene expression profiles of human monocytes, isolated from healthy volunteers, in response to short term in vitro exposure to DEP (4 h, 10 µg/ml) by using Affymetrix human genome U133 plus 2.0 arrays. Expression levels of 757 probes were found to be significantly changed between exposed and unexposed cultures at 2 fold cutoff (Table E1 in this article's Online Repository). Affected genes included ones involved in oxidative stress (GCLM, AKR1C2), heat shock response (DNAJB4, HSP1AB), transcription factors (CREM, FOSB) and other cellular pathways (Fig. 1A–B)29, 30. Importantly, DEP exposure affected the expression of several genetic components of the Notch pathway, which plays a key role in instructing Th cell differentiation31. It upregulated the expression of the Notch ligand gene JAG1 and the Notch downstream genetic targets HES1, HES4, HEY1 and NRARP. DEP also suppressed the expression of LFNG (Lunatic Fringe) and MFNG (manic Fringe), encoding two enzymes that modify Notch receptor species in favor of interaction with JAG1 and JAG2 (pro-Th2) but not Delta type ligands (pro-Th1) skewing32, 33.
Fig 1.
PM induce components of the Notch pathway in human peripheral blood monocytes and mouse BMDCs. (A) Hierarchical clustering analysis of gene transcripts of human monocytes that were either sham treated with PBS or treated with DEP (10 µg/ml; 4h). (B) Gene expression fold change in human monocytes after treatment with DEP. Genes related to the Notch pathway are indicated in red. (C) mRNA expression of the indicated Notch pathway component in BMDCs treated with PBS, UFP, FP or CB (10 µg/ml each). (D) Flow cytometric analysis of Jag1 and Notch1 in murine BMDCs treated with PBS, FP, UFP or CB. (E) Fold change of Jag1 transcripts in BMDCs derived from either WT, Tlr4−/−, Nrf2−/− or Nlrp3−/− mice and treated with PBS, UFP (10 µg/ml) or LPS (1 µg/ml). For panels (A) and (B), results are representative of 1 experiment with N=8. For panels (C–E), results are representative of 3 independent experiments. N=10/15 mice per group. *p<0.05; **p<0.01; ***p<0.001. 1-way ANOVA with post-test analysis and Student’s unpaired two tailed t test.
DEP is composed primarily of fine and ultrafine particles (FP and UFP) with effective aerodynamic diameters of <2.5 µm and <0.18 µm, respectively34, 35. We examined the induction of components of the Notch pathway in bone marrow-derived murine dendritic cells (BMDCs) using size-fractionated FP and UFP as well as chemically inert carbon black particles (CB, effective aerodynamic diameter 0.5 µm). Expression of mRNA encoding Jag1, and to a lesser extent Notch1, was upregulated in BMDCs treated with FP and UFP but not CB, as detected by real-time PCR analysis (Fig. 1C). In contrast, other Notch type receptors and ligands were either unaffected such as Notch2, Notch 4, and delta like ligand (DLL1 and DLL3) or down-regulated such as DLL4 and Jag2 (Fig. 1C and unpublished data). Flow cytometric analysis confirmed the upregulation of Jag1 and Notch1 expression in BMD treated with UFP and FP but not CB (Fig. 1D).
To determine the mechanisms involved in Jag1 induction by PM, we examined the role of the Toll like receptor 4 (TLR4) pathway, previously implicated in Jag1 regulation36. Unlike LPS, which induced Jag1 expression in WT but not TLR4-deficient BMDCs, UFP induced Jag1 expression in both cell types (Fig. 1E). Similarly, DEP induction of Notch pathway components proceeded unaffected in BMDCs lacking in the adaptor MyD88, common to most TLRs (data not shown). We further examined the role of Nrf2-dependent oxidative stress pathway and the inflammasome pathway in the induction by PM of Jag1 expression in BMDCs. Both the Nrf2 and Nlrp3 pathways are known to be activated by DEP exposure, and Nrf2 activates Notch1 expression37–39. Our results revealed that induction by UFP of Jag1 transcription proceeded unaffected in Nrf2 and NLRP3-deficient BMDCs. In contrast, LPS induced Jag1 expression was abrogated in the setting of NLRP3 deficiency (Fig. 1E). Together, these data indicated that UFP-mediated induction of Jag1 transcription proceeds independent of TLR4/MyD88, Nrf2 or NLRP3 pathways.
PM upregulate Th cytokine expressions by a Jag1-depdendent mechanism
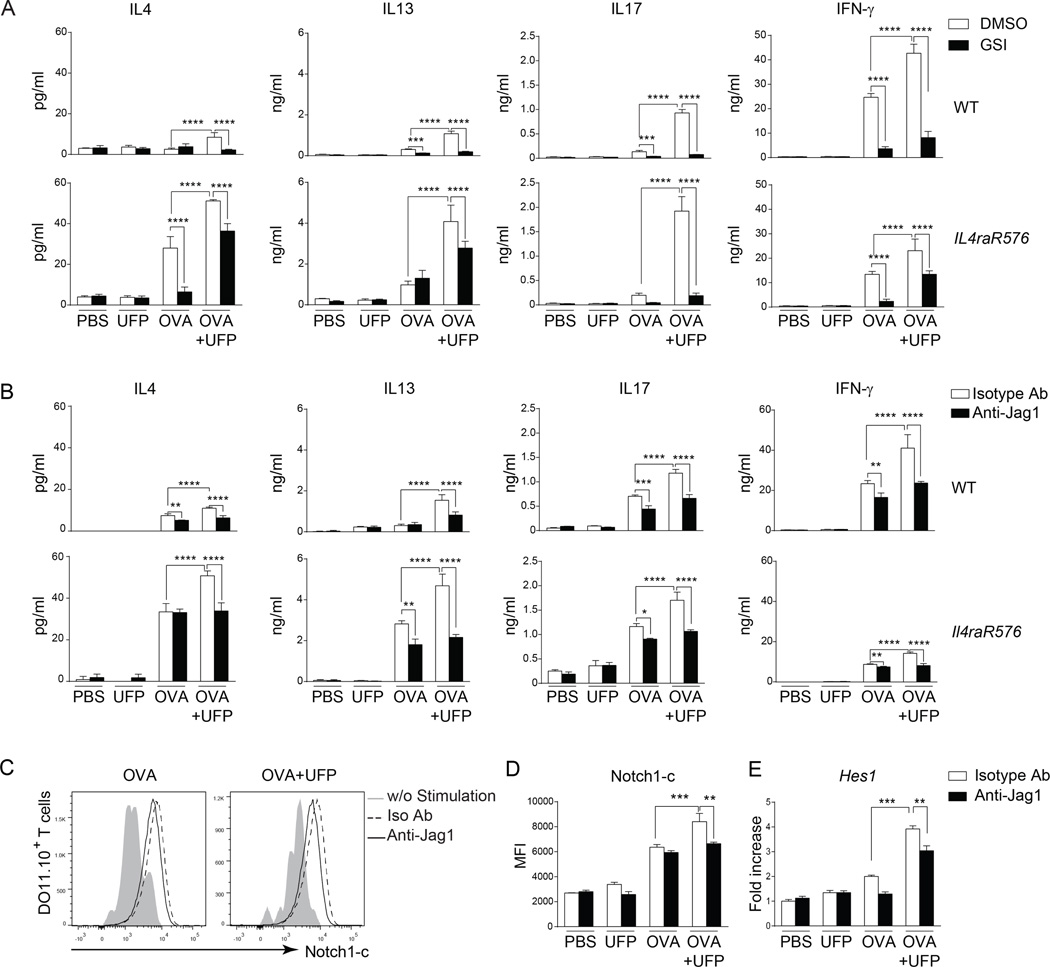
To examine if Notch pathway activation by PM contributes to Th cell skewing, we employed an in vitro Th cell differentiation system involving naïve DO11.10+CD4+ T cells, derived from DO11.10+Rag2−/− mice. Naïve DO11.10+CD4+ T cells were incubated with BMDCs that had either been sham pulsed with PBS or pulsed with the OVA peptide OVA323–339. Culture supernatants were analyzed by ELISA for levels of the Th2 cytokines IL-4 and IL-13, the Th1 cytokine IFN-γ and the Th17 cytokine IL-17. Treatment of cell co-cultures with OVA323–339 peptide induced increased production of all four Th cytokines, which was further upregulated by the addition of UFP (10 µg/ml). Treatment with a gamma secretase inhibitor (GSI), an inhibitor of Notch signaling, suppressed the increase in Th cytokines in response to UFP (Fig. 2A). In parallel set of studies, treatment of cell co-cultures with an anti-Jag1 but not an isotype control antibody blocked the super-induction by UFP of OVA peptide-induced cytokine production. In contrast, anti-Jag1 antibody treatment had a modest effect on cytokine production induced by the OVA peptide in the absence of UFP (Fig. 2B).
Fig 2.
UFP promote Th cytokine production in a Jag1-dependent manner. (A and B) Th cytokine production by naïve WT and Il4raR576 CD4+DO11.10+Rag2−/− T cells co-cultured with OVA-pulsed BMDCs in the presence of UFP (10 µg/ml), sham, GSI (5 µM), anti-Jag1 mAb or an isotype control (2.5 µg/ml). (C and D) Flow cytometric analysis (C) and mean fluorescent intensity (D) of intracellular Notch1 (Notch1-c) expression in Il4raR576 CD4+DO11.10+Rag2−/−T cells co-cultured with the OVA323–339-pulsed BMDCs in the presence of UFP and anti-Jag1 mAb. (E) Hes1 mRNA expression by T cell co-cultured as described in panel (B). Results are representative of 3 independent experiments. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by two way ANOVA with Bonferroni post test analysis.
We also investigated the capacity of UFP treatment to promote Th2 responses in the context of a Th2-skewed genetic background. To that end, we employed naïve DO11.10+CD4+ T cells homozygous for Il4raR576, an allergy and asthma-associated human IL-4 receptor alpha chain (IL-4Ra) polymorphism which is especially common in the populations of African ancestry, including African Americans, and which promotes Th2 responses and allergic airway inflammation22, 40. Naïve DO11.10+CD4+ T cells, derived from DO11.10Rag2−/−Il4raR576 mice, expressed substantially more Th2 cytokines, and less IFN-γ when incubated with OVA323–339-pulsed BMDCs than their counterparts of DO11.10Rag2−/− mice (Fig. 2A–B). UFP upregulated the production of Th2 cytokines as well as IL-17 and IFN-γ and by antigen-stimulated DO11.10+CD4+Il4raR576 T cells in a manner that was completely blocked by GSI and Jag1 antibody treatment relative to isotype control (Fig. 2A–B). Analysis of the expression of the active Notch1 intracellular domain (Notch1c), which mediates intracellular Notch 1 signaling revealed increased levels in OVA+UFP treated cells along with increased transcripts of the Notch target gene Hes1 (Fig. 2C–E). Increased Notch1c expression and Hes1 transcripts induced by UFP were inhibited by treatment with the anti-Jag1 antibody (Fig. 2C–E). Overall, these results indicate that the Jag1/Notch pathway amplifies the production of Th cytokines by naïve T cells, with a skewing towards Th2 cytokines in the context of a pro-Th2 human polymorphism.
UFP upregulate allergic airway inflammation in interaction with a pro-allergic human IL4RA polymorphism
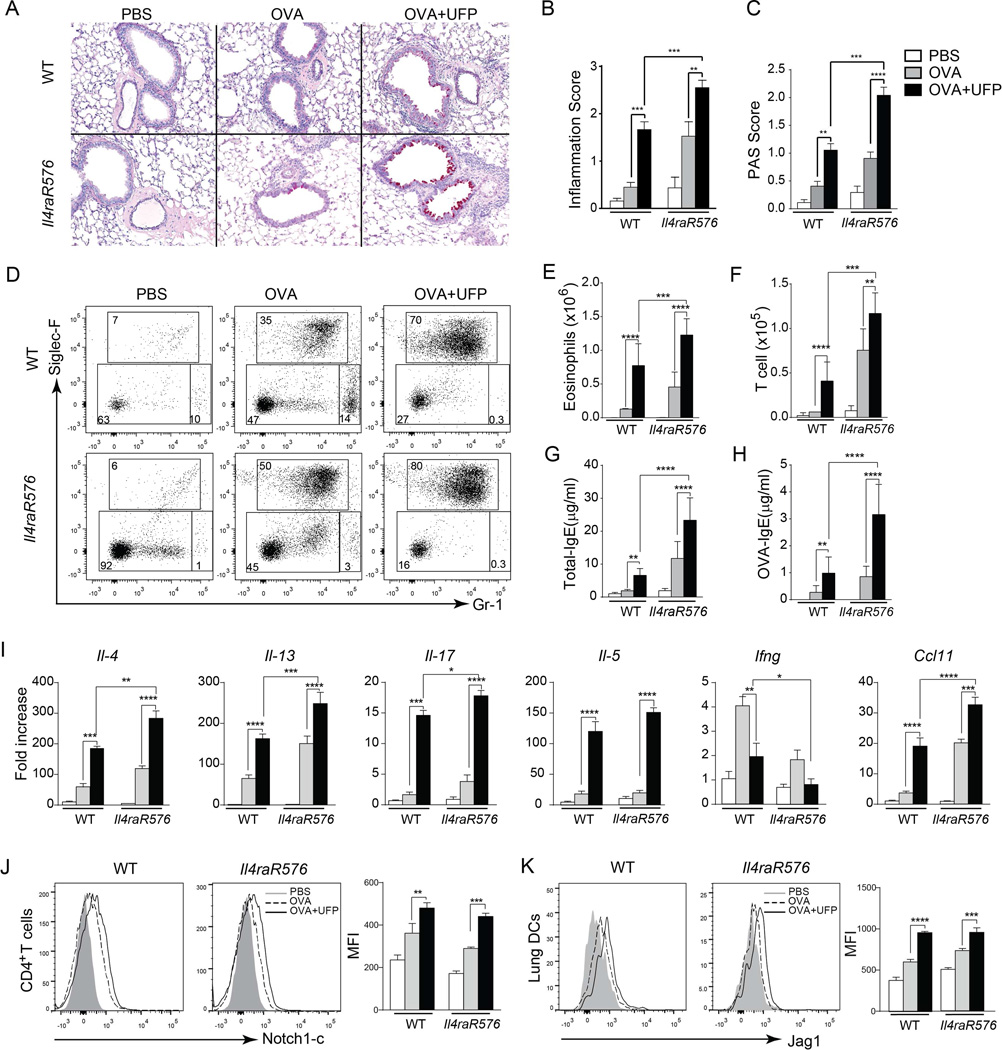
To determine the impact of PM treatment in vivo, we examined the influence of exposure to PM on the allergic response of WT BALB/c mice as compared to mice harboring the Il4raR576 polymorphism22. Accordingly, WT and Il4raR576 mice were sensitized by intra-peritoneal injection of OVA and then challenged by inhalation of 1% aerosolized OVA once daily over 3 successive days. Control mice were sham sensitized with PBS and challenged with aerosolized OVA. Subgroups of mice were treated intra-nasally with UFP (10 µg/instillation) or PBS 2 hr before the OVA aerosol challenge41. Sensitization and challenge of WT mice with OVA resulted in a modest eosinophilic airway inflammatory response that was amplified by the inclusion of PM exposure during the OVA challenge phase. In contrast, OVA sensitization and challenge of Il4raR576 mice resulted in robust airway inflammation that was also amplified by PM exposure (Fig. 3A–I). Airway eosinophilia and T cell infiltration, total and OVA-specific IgE responses, and Th2 and Th17 responses in OVA-sensitized and challenged mice were all augmented by UFP exposure. Furthermore, there was synergistic interaction between UFP and the Il4raR576 allele in eliciting many of these responses (Fig. 3A–I). UFP treatment induced increased Notch1c expression of similar magnitude in lung T cells of OVA sensitized and challenged WT and Il4raR576 mice (Fig. 3J). UFP also upregulated to a similar degree the expression of Jag1 in DCs of treated mice of both genotypes (Fig. 3K). These results establish a gene-environment interaction between UFP and the Il4raR576 allele in inducing allergic airway inflammation in vivo.
Fig 3.
UFP synergizes with Il4raR576 to promote OVA-induced allergic airway inflammation. (A) Representative PAS staining of lung sections isolated from WT and Il4raR576 mice immunized/challenged with PBS/OVA (PBS), OVA/OVA (OVA) or OVA/ OVA+UFP (OVA+UFP) (200× magnification). (B and C) Inflammation score (B) and PAS scores (C) from the mouse groups shown in (A). (D) Flow cytometric analysis of eosinophils (Siglec-F+Gr-1intermediate) and neutrophils (Siglec-F−Gr-1high) in the BAL fluid of them mouse groups described in panel (A). Numbers indicate the average frequencies of the respective population in each rectangle. (E and F). Eosinophils (E) and T cells (F) absolute numbers in the BAL fluid of the mouse groups described in panel (A). (G and H) Total (G) and OVA-specific (H) IgE levels in the serum of the mouse groups described in panel (A). (I) Il4, Il13, Il17, Il5, Ifng, Ccl11 mRNA expression in WT and Il4raR576 mice lung tissues after PBS, OVA or OVA-UFP treatment. (J and K) Flow cytometric analysis of Notch1-c (J) and Jag1 (K) expression by the CD4+ T cells isolated from the lungs of the mouse groups described in panel (A). Results are representative of 3 independent experiments. N=5 mice/group. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by two way ANOVA with Bonferroni post test analysis.
We also examined the capacity of UFP to complement the effects of a naturally occurring allergen, house dust mite (D. pteronyssinus) in association with the Il4ra4576 allele. Results were concordant with those observed with OVA sensitization and challenge. Whereas WT mice showed augmented inflammation with a combination of UFP and a low dose of D. pteronyssinus (5 µg) in inducing airway inflammation, this interaction was markedly amplified in the context of the Il4ra4576 allele (Fig. E1 in this article's Online Repository). These results confirm that UFP promote airway inflammation induced by a human allergen in the context of a pro asthmatic human allele.
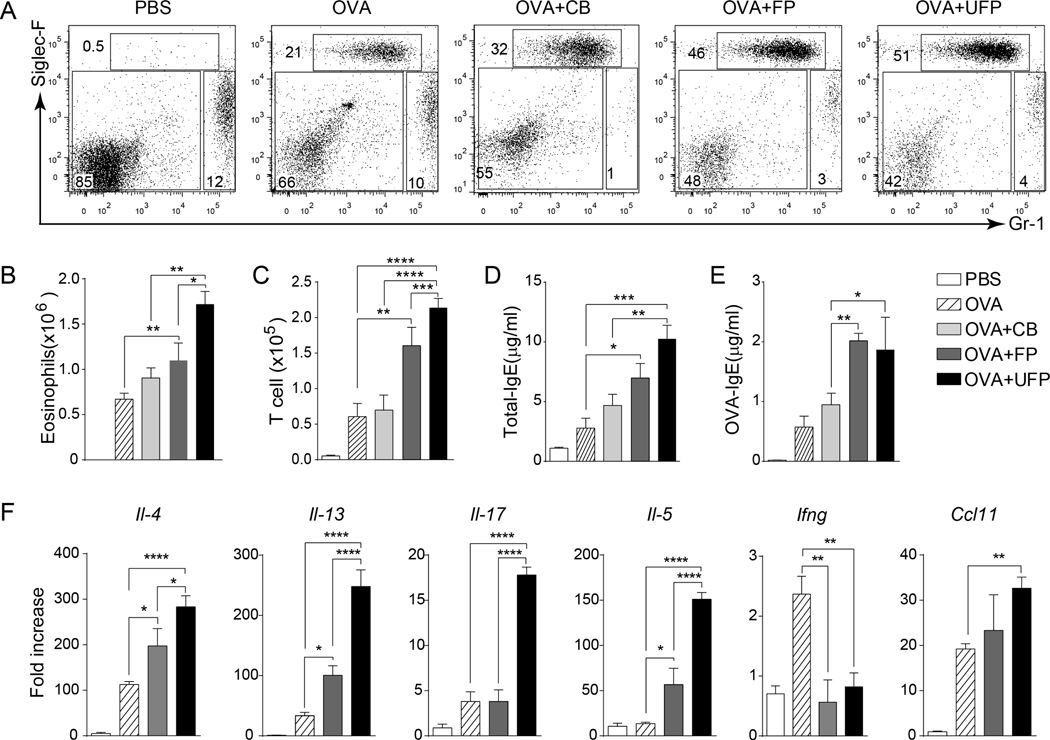
To elucidate the impact of different PMs in allergic airway response, Il4raR576 mice were sensitized and challenged with OVA alone or together with FP, UFP or CB. In line with their respective abilities to upregulate Jag1 expression, UFP and FP but not CB upregulated allergic airway inflammation in OVA-sensitized Il4raR576 mice (Fig. 4A–F). UFP were more effective on mass basis than FP in enhancing OVA-induced airway inflammation, consistent with their increased surface area/weight ratio and higher penetrance into the airways35.
Fig 4.
UFP and FP but not CB upregulated allergic airway inflammation in OVA-sensitized Il4raR576 mice. (A) Flow cytometric analysis of eosinophils and neutrophils in the BAL fluids of Il4raR576 mice in PBS, OVA, OVA+CB, OVA+FP or OVA+ UFP groups. (B and C) Absolute numbers of eosinophils (B) and T cells (C) in the BAL fluids of the mouse groups from panel (A). (D and E) Total (D) and OVA-specific (E) IgE levels in the serum the mouse groups from panel (A). (F) Il4, Il13, Il17, Il5, Ifng and Ccl11 mRNA expression in in the lung tissues of the mouse groups from panel (A). Results are representative of 2 independent experiments. N=4–5/group. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by two way ANOVA with Bonferroni post test analysis.
UFP upregulates allergic airway inflammation in a Jag1-dependent manner
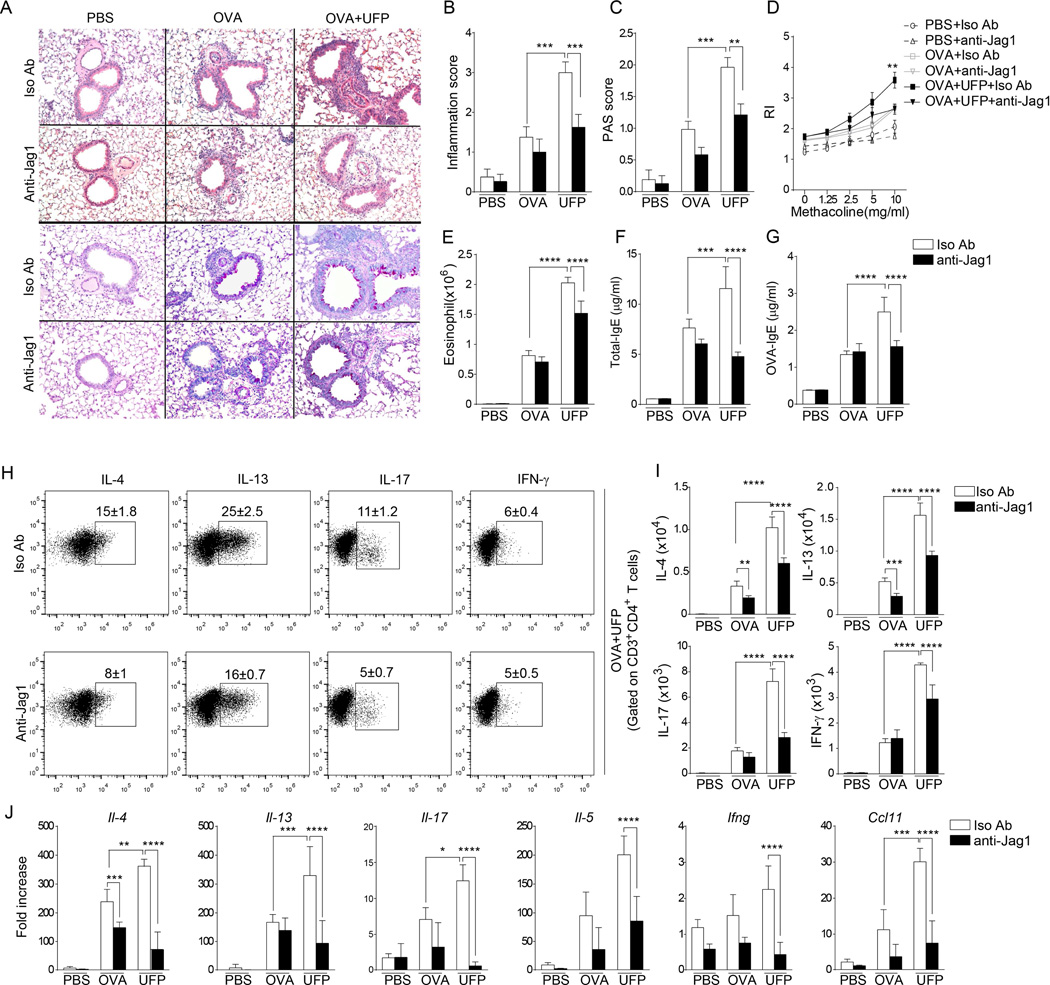
We next examined the impact of treatment with anti-Jag1 or an isotype control antibody on the airway inflammatory response in Il4raR576 mice sensitized and challenged with OVA alone or together with UFP. Anti-Jag1 antibody treatment had little or no effect on the OVA challenge response in terms of tissue inflammation, goblet cell metaplasia airway hyperresponsiveness, airway eosinophilia and IgE antibody response. In contrast, it inhibited the potentiation of the parameters induced by UFP (Fig. 5A–G). Similarly, anti-Jag1 antibody treatment inhibited the upregulation in cytokine production induced by UFP treatment of OVA-sensitized and challenged mice, including IL-4, IL-5, IL-13, IL-17 and IFN-γ as well as the eosinophil chemotactic factor CCL11 (Fig. 5H–J). In a separate set of experiments, treatment of Il4raR576 mice with a gamma secretase inhibitor (GSI), a Notch pathway inhibitor, also reversed the upregulation by PM of these parameters of allergic airway inflammation, consistent with a role for this pathway in disease promotion by PM (Fig. 6).
Fig 5.
UFP enhances OVA-induced airway inflammation in a Jag1-dependent manner. (A) Representative H&E staining (upper panels) and PAS (lower panels) staining of lung tissues isolated from Il4raR576 mice sensitized and challenged with OVA alone, or together with UFP, in the presence of anti-Jag1 or an isotype control mAb (200× magnification). (B and C) Inflammation (B) and PAS scores (C) in lung tissues of the mouse groups described in panel (A). (D–G) Airway hyper-responsiveness in response to methacoline (D), Absolute numbers of eosinophils in the BAL fluids (E), total (F) and OVA-specific (G) levels in the serum of the mouse groups described in panel (A). (H) Flow cytometric analysis of cytokine expression by CD4+ T cells present in the BAL fluids of Il4raR576 mice sensitized with OVA together with UFP. (I) Absolute numbers of lung CD4+T cells producing IL-4, IL13, IL-17 and IFN-γ in the BAL fluids of the mouse groups described in panel (A). (J) Il4, Il13, Il17, Il5, Ifng and Ccl11 mRNA expression in in the lung tissues of Il4raR576 in PBS, OVA or OVA-UFP groups treated with either isotype control or anti-Jag1 mAb. Results are representative of 2 independent experiments. N=5 mice/group. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by two way ANOVA with Bonferroni post test analysis.
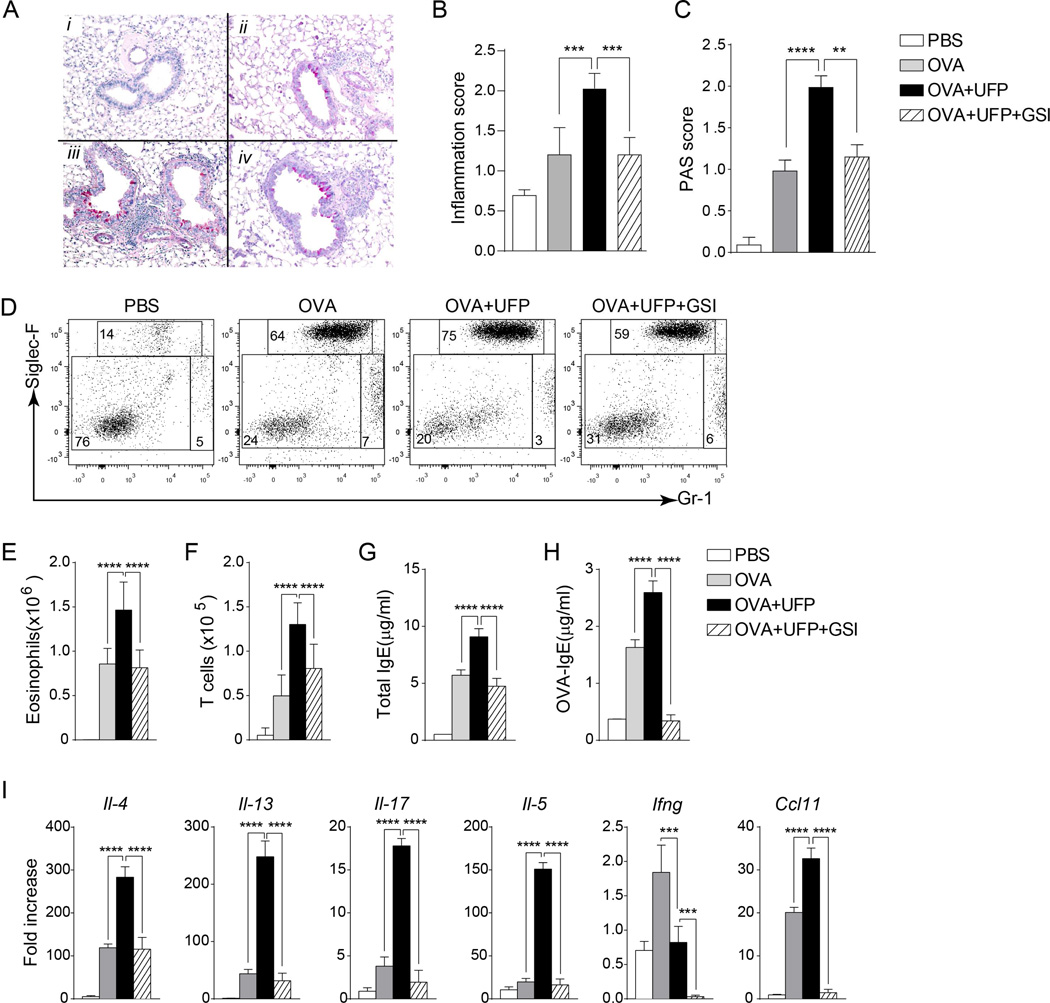
Fig 6.
UFP promotes OVA-induced airway inflammation in a Notch-dependent mechanism. (A). Representative PAS staining of lung tissues of Il4raR576 mice in PBS (i), OVA (ii), OVA+UFP (iii) and OVA+UFP+GSI (iv) groups (200× magnification). (B and C) Inflammation and PAS scores of the mouse groups shown in panel (A). (D) Flow cytometric analysis of eosinophils and neutrophils in the BAL fluids isolated form the mouse groups described in panel (A). (E and F) Absolute numbers of eosinophils (E) and T cells (F) in the BAL fluids of the respective mouse groups. (G and H) Total (G) and OVA-specific (H) levels in the serum of the mouse groups described in panel (A). (I) Il4, Il13, Il17, Il5, Ifng and Ccl11 mRNA expression in in the lung tissues of Il4raR576 mice in PBS, OVA, OVA+UFP or OVA+UFP+GSI groups. Results are representative of 2 independent experiments. N=5/group. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by one and two way ANOVA with Bonferroni post test analysis.
The role of Jag1 in mediating the upregulation by UFP of allergic airway inflammation was also verified using house dust mites (D. Pteronyssinus) as an allergen. As in the OVA model. UFP augmented several parameters of airway inflammation induced by D. Pteronyssinus, including airway eosinophilia and Th cytokine production, in a Jag1-dependent manner. These results identified Jag1 induction as a common mechanism by which UFP upregulates airway inflammation by different allergens (Fig. E2 in this article's Online Repository).
UFP upregulates Jag1 by an AhR-dependent mechanism
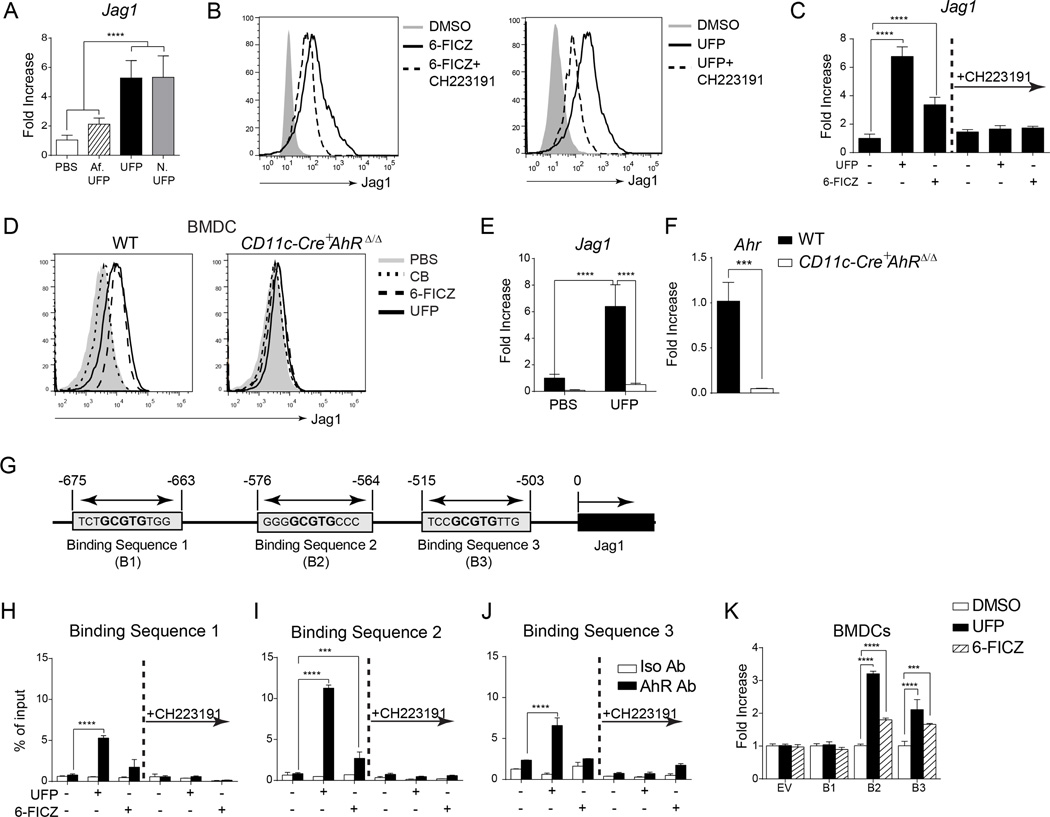
PM, particularly UFP, contain polycyclic aromatic hydrocarbons that act in part via the AhR receptor to induce cellular responses42, 43. UFP collected at night and in the early morning, which are enriched in PAH and other semi-volatile hydrocarbons, were more effective in inducing Jag1 expression in mouse BMDCs than those collected in the afternoon, which are depleted of those compounds (Fig. 7A). An obligate role for the AhR in the induction by UFP of Jag1 expression was confirmed by several approaches. The AhR agonist 6-FICZ recapitulated the capacity of UFP to induce Jag1 protein and transcript expression in BMDCs as determined by flow cytometry and real time PCR analysis, respectively. Reciprocally, the selective AHR receptor antagonist CH223191 inhibited the capacities of UFP and 6-FICZ to induce Jag1 expression (Fig. 7B–C). Importantly, deletion of a floxed AhR allele in BMDCs by co-expression of a CD11c-driven Cre recombinase transgene (CD11c-Cre+AhRΔ/Δ) abolished Jag1 induction by UFP and 6-FICZ (Fig. 7D). This was associated with profoundly decreased expression of mRNA encoding Jag1 and Ahr as determined by real time PCR (Fig. 7E–F).
Fig 7.
UFP activates AhR to induce Jag1 expression. (A) Fold change of Jag1 transcripts in BMDCs treated with PBS, Af-UFP, UFP and N-UFP (10 µg/ml). (B and C) Flow cytometric analysis of Jag1 expression (B) and Jag1 mRNA levels (C) in BMDCs treated with DMSO (sham), UFP, AhR agonist 6-FICZ, in the presence or absence of AhR antagonist CH223191. (D–F) Flow cytometric analysis of Jag1 expression (D) and Jag1 (E) and Ahr (F) mRNA levels in WT or CD11c-Cre+AhRΔ/Δ BMDCs. (G) Schematic representation of candidate AhR bindings sites in the proximal Jag1 promoter. (H–J) ChIP analysis of AhR binding to candidate response elements in the Jag1 promoter in BMDCs treated with the indicated reagents. (K) Luciferase activity in BMDCs transfected with luciferase reporters driven by individual candidate Jag1 AhR response elements then treated with vehicle (DMSO), UFP or 6-FICZ. Results are representative of 3 independent experiments. N=3–7/group. p***<0.001, ****<0.0001 by one and two way ANOVA with Bonferroni post test analysis.
We analyzed the murine Jag1 promoter for evidence of AhR response elements, and identified candidate binding sites that incorporate the conserved central GCGTG core of the consensus AhR response element (T/GNNGCGTGA/CG/CA) in the proximal promoter region at positions −675, −575 and −515 (Fig. 7G)44. Chromatin immunopercipitation (ChIP) assays confirmed that stimulation with UFP, and to lesser extent 6-FICZ, resulted in the immunoprecipitation of all three sites with an AHR antibody, with site 2 being the most active (Fig. 7H–J). We further analyzed the capacity of the respective sites to drive expression of a luciferase reporter gene under control of a minimal promoter in primary BMDCs. Stimulation of transfected cells with UFP or 6-FICZ resulted in increased luciferase expression driven by site 2, and to a lesser extent site 3, while site 1 was inactive (Fig. 7K). A luciferase reporter driven by a Jag1 promoter sequence spanning all 3 sites exhibited similar activity to one driven by site 2, indicating that the latter is the main target of AhR in the proximal Jag1 promoter (data not shown). These results establish that UFP activates AhR to induce Jag1 expression by a mechanism involving direct transcriptional activation of the Jag1 promoter.
We analyzed the role of AhR in the upregulation by UFP of Th cytokine expression in naïve DO11.10+CD4+ Il4raR576 T cells activated by OVA323–339 pulsed BMDCs using the AhR antagonist CH223191. Results revealed that treatment with CH223191 inhibited the upregulation by UFP of Th cytokine expression in activated DO11.10+ T cells (Fig. E3A in this article's Online Repository). Treatment with the AhR antagonist decreased the expression of Notch1c and the transcription of the Notch target gene Hes1 in activated DO11.10+ T cells (Fig. E3B–D in this article's Online Repository). These results, together with those of Fig. 5, are consistent with the upregulation by UFP of Th cytokine expression in DO11.10+ T cells/OVA323–339-pulsed DC cultures by an AhR-Jag1-mediated mechanism.
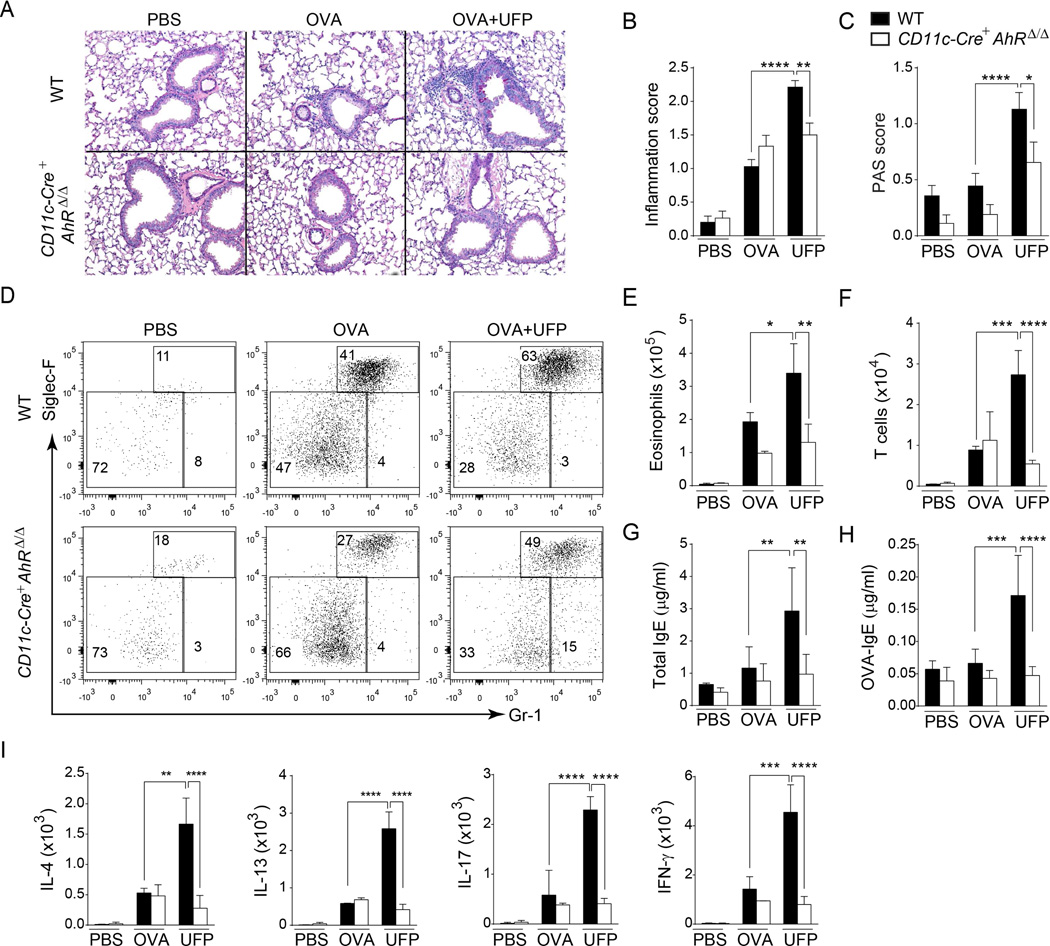
We determined the impact of lineage-specific deletion of AhR in CD11c+ cells on the airway inflammation in mice sensitized and challenged with OVA alone or with UFP. CD11c cell-specific deletion of AhR in CD11c-Cre+AhRΔ/Δ mice had a modest effect on the OVA challenge response in terms of airway eosinophilia, IgE antibody generation and Th cytokine production. In contrast, it inhibited the potentiation of these parameters induced by UFP (Fig. 8). We further confirmed these results using the AhR antagonist CH223191, given during the challenge phase to Il4raR576 mice sensitized and challenged with OVA or D. Pteronyssinus alone or in the present of UFP. CH223191 treatment had a modest effect on OVA or D. Pteronyssinus challenge responses in terms of airway eosinophilia, IgE antibody generation and Th cytokine production, but inhibited the potentiation of these parameters induced by UFP (Fig. E4 and E5 in this article's Online Repository). Overall, these results supported a central role for AhR activation in mediating the trophic effects of UFP on allergic airway inflammation.
Fig 8.
AhR lineage-specific deletion in CD11c+ cells confers protection against OVA and OVA with UFP induced airway inflammation. (A) Representative PAS-stained sections of lung isolated from WT and CD11c-Cre+AhRΔ/Δ mice in PBS, OVA or OVA+UFP groups (200× magnification). (A and B) Histological inflammation (B) and PAS scores (C) in the lung tissues isolated from the mouse groups described in panel (A). (D) Flow cytometric analysis of eosinophils and neutrophils in the BAL fluids in of WT and CD11c-Cre+AhRΔ/Δ mice in PBS, OVA or OVA+UFP groups. (E and F) Eosinophils (E) and T cells (F) absolute numbers in the BAL fluids of WT and CD11c-Cre+AhRΔ/Δ mice in PBS, OVA or OVA+UFP groups. (G and H) Total (G) and OVA-specific (H) IgE levels in the serum of the mouse groups described in panel (A). (I) Absolute numbers of lung CD4+T cells secreting IL-4, IL13, IL-17 and IFN-γ in the BAL fluids of the mouse groups described in panel (A). Results are representative of 2 independent experiments. N=5 mice/group. *p<0.05, **<0.01, ***<0.001, ****<0.0001 by two way ANOVA with Bonferroni post test analysis.
DISCUSSION
In this report, we provide evidence that traffic-related PM, a major environmental pollutant implicated in increased incidence and severity of asthma, acts to promote allergic airway inflammation by modulating the expression of components of the Notch pathway. Studies on human monocytes and murine DCs confirmed the upregulation by PM of specific components of the Notch pathway, including Jag1, Notch1 and Fringe enzymes, as well as down-stream targets of the Notch pathway. Furthermore, upregulation by PM of allergic airway inflammation was dependent on the induction of Jag1 expression, which proceeded by an AhR-dependent mechanism. These results outline a mechanistic cascade by which PM modulate the function of key developmental and regulatory pathways implicated in the differentiation of Th subsets to promote disease.
The functional significance of the modulation of Notch pathway component expression by PM was revealed in both in vitro and in vivo studies. In vitro, PM augmented the capacity of OVA323–339 peptide-presenting BMDCs to induce Th1, -2 and -17 cytokines in naïve DO11.10+ T cells in a Jag1-dependent fashion. These results are consistent with recently reported function of Notch as an unbiased amplifier of Th cell differentiation45. In the context of a pro-Th2 genetic element, the human Il4raR576 polymorphism, PM also upregulated production of the different Th cytokines, albeit with a Th2-skewed profile that reflected the inherent Th2 bias of the responding Il4raR576 T cells. In vivo, PM exposure also interacted with Il4raR576 to promote allergic airway inflammation in a Notch pathway-dependent manner. These findings implied that the inflammatory outcome of exposure to PM is orchestrated by Notch signaling under the influence of the prevailing Th bias of the responding host. Thus, PM may interact with host pro-allergic and asthmatic genetic factors to promote allergic diseases and asthma. Similarly, PM components may interact with other genetic host factors to promote different outcomes such as chronic obstructive lung disease and atherosclerosis.
The mechanism by which PM induced Notch pathway components involved activation of the AhR pathway. Treatment with a selective AhR inhibitor or deletion of AhR in CD11c+ DCs cells abrogated the induction by PM of Jag1 expression in BMDCs and suppressed their capacity to upregulate Th cytokine expression by antigen-specific naïve T cells. Pharmacological AhR inhibition or lineage-specific deletion of AhR in CD11c+ cells also abrogated the upregulation by PM of airway inflammation. Induction by AhR of Jag1 expression involved direct activation of Jag1 promoter at specific AhR response elements. PM, especially UFP, are enriched in polycyclic aryl hydrocarbons (PAH), agonistic ligands for the AhR, which have been previously tied to the adjuvant like-effect of these particles44, 46, 47. Our results provide a molecular basis for PM adjuvancy by linking AhR activation by PAH of PM to the induction of Jag1 expression on antigen presenting cells and consequently Notch signaling in T cells.
Unlike larger sized particles, UFP are not regulated by the US Environmental Protection Agency (EPA). Nevertheless, comparison of the different particles revealed that UFP were, per unit weight, the most effective among the particles tested in inducing Jag1-dependent airway inflammation. The smaller effective aerodynamic diameter of UFP enables deeper penetration into the airways as compared to larger particles. Also, the larger surface area per particle unit weight, greater PAH content and higher oxidant potential all enable more potent biological responses47. Aside from the respiratory system, UFP exposure has been associated with cardiovascular and neurological diseases, especially atherosclerosis48–50. The role of the AhR-Jag1/Notch pathway in mediating extra-pulmonary effects of UFP and other particles remains to be established.
Supplementary Material
Key messages.
-
–
Traffic related particulate matter activate the aryl hydrocarbon receptor to induce the expression of the Notch ligand Jagged 1 (Jag1) on antigen presenting cells.
-
–
Jagged-Notch interaction promotes allergic airway inflammation.
-
–
The aryl-hydrocarbon receptor-Jag1-Notch cascade acts in concert with pro-asthmatic alleles to mediate heightened allergic airway inflammation.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant 2R01AI065617 (to T.A.C.). We thank Dr. Andre Nel for gift of reagents, and Schuyler A. Girion, Reuben Caja and Manjot Sadhu for providing technical support.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- AREs
AhR response elements
- BAL
bronchoalveolar lavage
- BMDCs
Bone marrow-derived Dendritic cells
- ChIP
Chromatin Immunoprecipitation
- DLL
Delta like ligand
- DEP
Diesel exhausted particulate
- FDR
False discovery rate
- FICZ
6-Formylindolo (3,2-b) carbazole
- FP
Fine particles
- GSI
gamma-secretase inhibitor
- Jag1
Jagged 1
- RL
Lung resistance
- LFNG
Lunatic Fringe
- MFNG
Manic Fringe
- PM
particulate matter
- TLR4
Toll like receptor 4
- UFP
Ultrafine particles
- UFP
ultrafine particles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vital signs: asthma prevalence, disease characteristics, and self-management education --- United States, 2001--2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 2.von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol. 2009;123:3–11. doi: 10.1016/j.jaci.2008.10.046. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 3.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dockery DW, Pope CA., 3rd Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 5.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 6.Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol. 2005;6:223–226. doi: 10.1038/ni0305-223. [DOI] [PubMed] [Google Scholar]
- 7.Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 8.Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;63:635–641. doi: 10.1136/thx.2007.091371. [DOI] [PubMed] [Google Scholar]
- 9.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 10.von Klot S, Wolke G, Tuch T, Heinrich J, Dockery DW, Schwartz J, et al. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur Respir J. 2002;20:691–702. doi: 10.1183/09031936.02.01402001. [DOI] [PubMed] [Google Scholar]
- 11.Knol AB, de Hartog JJ, Boogaard H, Slottje P, van der Sluijs JP, Lebret E, et al. Expert elicitation on ultrafine particles: likelihood of health effects and causal pathways. Part Fibre Toxicol. 2009;6:19. doi: 10.1186/1743-8977-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, et al. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010;299:L374–L383. doi: 10.1152/ajplung.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204. e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H, Khurana Hershey GK. Genetic and epigenetic influence on the response to environmental particulate matter. J Allergy Clin Immunol. 2012;129:33–41. doi: 10.1016/j.jaci.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999;104:1183–1188. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 16.Kang X, Li N, Wang M, Boontheung P, Sioutas C, Harkema JR, et al. Adjuvant effects of ambient particulate matter monitored by proteomics of bronchoalveolar lavage fluid. Proteomics. 2010;10:520–531. doi: 10.1002/pmic.200900573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, et al. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol. 2006;118:455–465. doi: 10.1016/j.jaci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, et al. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37:706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue K, Koike E, Takano H, Yanagisawa R, Ichinose T, Yoshikawa T. Effects of diesel exhaust particles on antigen-presenting cells and antigen-specific Th immunity in mice. Exp Biol Med (Maywood) 2009;234:200–209. doi: 10.3181/0809-RM-285. [DOI] [PubMed] [Google Scholar]
- 21.Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 184:426–432. doi: 10.4049/jimmunol.0902564. [DOI] [PubMed] [Google Scholar]
- 22.Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206:2191–2204. doi: 10.1084/jem.20091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- 25.Misra C, Kim S, Shen S, Sioutas C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. Journal of Aerosol Science. 2002;33:735–752. [Google Scholar]
- 26.Blaeser F, Bryce PJ, Ho N, Raman V, Dedeoglu F, Donaldson DD, et al. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198:1189–1200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, et al. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–1777. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 28.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 29.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 30.Gong KW, Zhao W, Li N, Barajas B, Kleinman M, Sioutas C, et al. Air-pollutant chemicals and oxidized lipids exhibit genome-wide synergistic effects on endothelial cells. Genome Biol. 2007;8:R149. doi: 10.1186/gb-2007-8-7-r149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 32.Stanley P. Regulation of Notch signaling by glycosylation. Curr Opin Struct Biol. 2007;17:530–535. doi: 10.1016/j.sbi.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amsen D, Spilianakis CG, Flavell RA. How are T(H)1 and T(H)2 effector cells made? Curr Opin Immunol. 2009;21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kittelson DB. Engines and nanoparticles: A review. Journal of Aerosol Science. 1998;29:575–588. [Google Scholar]
- 35.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 184:3535–3544. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak MK, et al. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal. 2010;3:ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provoost S, Maes T, Pauwels NS, Vanden Berghe T, Vandenabeele P, Lambrecht BN, et al. NLRP3/caspase-1-independent IL-1beta production mediates diesel exhaust particle-induced pulmonary inflammation. J Immunol. 2011;187:3331–3337. doi: 10.4049/jimmunol.1004062. [DOI] [PubMed] [Google Scholar]
- 40.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 41.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, et al. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 45.Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39:148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, et al. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117:1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S, Verma MK, Srivastava AK. Ultrafine particles in urban ambient air and their health perspectives. Rev Environ Health. 2013;28:117–128. doi: 10.1515/reveh-2013-0008. [DOI] [PubMed] [Google Scholar]
- 50.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.