Abstract
Background
The mechanisms underlying glucocorticoid responsiveness are largely unknown. Although redox regulation of the glucocorticoid receptor (GR) has been reported, it has not been studied in asthma.
Objective
We characterized systemic cysteine oxidation and its association with inflammatory and clinical features in healthy children and children with difficult-to-treat asthma. We hypothesized that cysteine oxidation would be associated with increased markers of oxidative stress and inflammation, increased features of asthma severity, decreased clinically defined glucocorticoid responsiveness, and impaired GR function.
Methods
Peripheral blood mononuclear cells were collected from healthy children (n = 16) and children with asthma (n = 118) age 6-17 years. Difficult-to-treat asthmatic children underwent glucocorticoid responsiveness testing with intramuscular triamcinolone. Cysteine, cystine, and inflammatory chemokines and reactive oxygen species (ROS) generation were quantified and expression and activity of the GR was assessed.
Results
Cysteine oxidation was present in children with difficult-to-treat asthma and was accompanied by increased ROS generation and increased CCL3 and CXCL1 mRNA expression. Children with the greatest extent of cysteine oxidation had more features of asthma severity including poorer symptom control, greater medication usage and less glucocorticoid responsiveness despite inhaled glucocorticoid therapy. Cysteine oxidation also modified the GR protein by decreasing available sulfhydryl groups and decreasing nuclear GR expression and activity.
Conclusions
A highly oxidized cysteine redox state promotes a post-translational modification of the GR that may inhibit its function. Given that cysteine oxidation is prevalent in children with difficult-to-treat asthma, the cysteine redox state may represent a potential therapeutic target for the restoration of glucocorticoid responsiveness in this population.
Keywords: Biomarker, Childhood asthma, Treatment response, Cysteine, Inflammation, Oxidative stress, Refractory asthma, Severe asthma
INTRODUCTION
Glucocorticoids are the cornerstone of treatment for persistent asthma,1 but the response to these medications is highly variable, particularly when administered at moderate-to-high-dosages.2 Whereas several large studies have shown that doubling the dose of inhaled glucocorticoids is of limited efficacy in asthmatic patients already receiving low-dose inhaled glucocorticoid therapy,3, 4 other studies suggest that quadrupling the dose may improve measures of asthma impairment and prevent exacerbations.5 However, chronic inflammation may still persist in a subset of patients with more severe disease6 and the mechanisms underlying decreased glucocorticoid responsiveness in this population remain largely unknown.
Although the biology of the glucocorticoid receptor (GR) is complex, post-translational modifications of the GR and associated downstream effects on glucocorticoid signaling have been described.7 While much of the literature has focused on serine phosphorylation, the function of the GR is also regulated by redox-dependent mechanisms8, 9 which may be of relevance in asthma. Indeed, we have previously reported marked redox abnormalities in the airways and systemic circulation of children with asthma that worsen with increased asthma severity.10-15 Notably, in children with symptomatic asthma despite glucocorticoid therapy, the presence of reactive oxygen species promotes oxidation of the amino acid thiol, cysteine, to its disulfide form (i.e., cystine) and further depletes the pool of cysteine available for signal transduction and other cellular functions.12, 13 Given that cysteine oxidation also stimulates cytokine secretion16 and promotes pathways associated with cellular stress and death,17 we sought to: 1) characterize systemic cysteine oxidation and its intracellular, inflammatory and clinical features (including glucocorticoid responsiveness) in children with difficult-to-treat asthma, and 2) explore the role of the cysteine oxidation in GR function. We hypothesized that in children with difficult-to-treat asthma, greater cysteine oxidation would be associated with increased intracellular and extracellular oxidative stress and inflammation, increased features of asthma severity, and decreased glucocorticoid responsiveness. We further hypothesized that cysteine oxidation would impair nuclear GR expression and consensus binding.
METHODS
Participants
Children 6 to 17 years of age with physician-diagnosed asthma who were receiving current treatment for asthma were enrolled from a difficult-to-treat asthma clinic at Emory University in Atlanta, Georgia. Asthmatic children had a history of ≥ 12% reversibility in the forced expiratory volume in one second (FEV1) after short-acting bronchodilator administration or airway hyperresponsiveness to methacholine evidenced by a methacholine PC20 ≤16 mg/mL. Healthy children without asthma were also enrolled for comparison. Exclusion criteria for all children included premature birth before 35 weeks gestation, current smoking, immunodeficiency, pulmonary aspiration disorders or vocal cord dysfunction. The Emory University Institutional Review Board granted approval for this study. Written informed consent was obtained from the parents or legal guardians. Children 12 to 17 years provided written informed assent, whereas children 6 to 11 years provided verbal assent.
Participant characterization procedures
Children were evaluated during two visits separated by two weeks. The baseline characterization visit was rescheduled if upper respiratory viral symptoms, acute worsening of asthma symptoms, antibiotic use, or systemic glucocorticoid use was reported within the preceding two weeks. Parents completed medical history questionnaires and children completed the Asthma Control Questionnaire (ACQ) and the Pediatric Asthma Quality of Life Questionnaire (PAQLQ).18, 19 Spirometry (KoKo PDS, Ferraris, Louisville, CO) was performed at baseline and after receipt of up to eight inhalations of albuterol sulfate (90 μg/actuation). The best of three forced vital capacity (FVC) maneuvers was interpreted.20 Exhaled nitric oxide concentrations were determined using online methods (NIOX MINO®, Aerocrine, Morrisville, NC).21 Whole blood (up to 25 mL) from venipuncture was collected into serum separation tubes and heparinized tubes containing a density gradient for peripheral blood mononuclear cell (PBMC) isolation (Vacutainer® CPT™, Becton, Dickinson and Company, Franklin Lakes, NJ). Total serum immunoglobulin E (IgE) was quantified with an assay kit according to the manufacturer’s instructions (Calbiotech, Spring Valley, CA).
Glucocorticoid responsiveness testing
At the completion of baseline visit, a subset (n = 57) of participants with symptomatic asthma despite moderate-to-high dose inhaled glucocorticoid therapy (i.e., >200 μg fluticasone equivalent for children 6-11 years and >500 μg fluticasone equivalent for children 12 years and older) received intramuscular triamcinolone (1 mg/kg, 60 mg maximum dose). Symptomatic asthma was defined according to available treatment guidelines1 as self-reported asthma symptoms more than twice weekly or nocturnal awakenings from asthma at least 2 nights per month. Responsiveness to triamcinolone was assessed after two weeks and was defined as an ACQ score < 0.75, which corresponds to “well-controlled asthma” with a positive predictive value and negative predictive value of 0.73 and 0.85, respectively.22
Cysteine and cystine determination
To prevent auto-oxidation prior to analysis, aliquots were preserved for in a 5% perchloric acid solution containing iodoacetic acid (6.7 μmol/L) and boric acid (0.1 mol/L) with 5 μmol/L γ-glutamyl-glutamate internal standard.23 Cysteine and cystine were quantified relative to γ-glutamyl-glutamate by reverse-phase high-performance liquid chromatography as described previously.12 Samples were derivatized with dansyl chloride and separated on a 10 μm Ultrasil amino-acid column (Waters Alliance 2690, Waters Corporation, Milford, MA). Fluorescence was detected at 365 and recorded by two detectors (Waters 474, Waters Corporation, and Gilson 121, Gilson Inc., Middletown, WI). The redox potential (Eh) of the cysteine/cystine thiol pair was calculated with the Nernst equation, Eh = Eo + RT/nF ln [disulfide]/([thiol1][thiol2]), where Eo is the standard potential for the redox couple (−250 mV), R is the gas constant, T is the absolute temperature, n is the number of electrons transferred, and F is the Faraday constant.
Cellular viability and reactive oxygen species generation
Cellular viability was determined using an automatic cell counter (Countess®, Invitrogen, Grand Island, NY) after staining with 0.4% trypan blue. Intracellular reactive oxygen species (ROS) generation was assessed after incubation with 5 μM 2′,7′-Dichlorodihydrofluorescin diacetate for 45 minutes. Relative fluorescent units (RFU) were quantified in 5-10 separate visual fields with FluoView software (Olympus; Center Valley, PA) after correction for background autofluorescence.
Real-time polymerase chain reaction
Cells were added to 5 volumes of RNAlater® (Life Technologies, Grand Island, NY) and RNA was extracted using a commercial kit (RNeasy® Mini kit RNeasy Mini, Qiagen, Valencia, CA). 10ng of total RNA per sample was reverse transcribed using MultiScribe® Reverse Transcriptase (62.5U/50ul reaction), RNase Inhibitor, oligoT primers and MgCl2 at a concentration of 5.5mM (Life Technologies). cDNA aliquots were preamplified for genes of interest using TaqMan PreAmp Master Mix Kit (Life Technologies). The pre-amplified cDNA was used to quantitate relative levels of CCL3 (Hs00234142_m1) and CXCL1 (Hs00236937_m1) in a 96-well assay system (StepOnePlus™ real-time PCR assay) with TaqMan® primer pairs and probes (Life Technologies). Data were normalized to B2M (4333766F), GAPDH (4333764F), ACTB (4333762F), PGK1 (4333765F), and PPIA (43337663F) housekeeping genes. Net cycle threshold (CT) values were used to calculate ΔCT values for each subject, using the average of the five housekeeping genes as a reference. mRNA gene expression was analyzed relative to the control group and was expressed as fold change values.
Cell culture
Cell culture experiments were performed with THP-1 monocytes (ATCC®, Manassas, VA), MM.1S B lymphoblasts (ATCC®) and primary PBMCs from healthy donors (AllCells, Alameda, CA). Reduced and oxidized conditions were created by adding 300 μL of 10 mM cysteine and 150 μL of 10 mM cystine (Sigma-Aldrich, St. Louis, MO), respectively, to 15 mL DMEM/F12 media without methionione, cysteine, cystine and glutamine (HyClone, Life Technologies). Media was supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Cellgro, Corning Life Sciences, Corning, NY), and 50 mg/mL gentamicin sulfate (Cellgro). Cells were re-suspended to a concentration of 1 million cells/mL and cultured for 4 hours at 37°C with 5% CO2. In selected experiments, cells were also exposed to 100 nM dexamethasone for 1 hour (Sigma-Aldrich), added at hour 3 of incubation.
Western blotting
Cells were suspended in 50 mM Bis-Tris-HCL lysis buffer, pH=6.5, containing 0.5% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 150mM NaCl, 1 mM EDTA and 0.1 mM PMSF. Protein sulfhydryl (−SH) group protein residues were labeled with biotinylated iodoacetamide using the methods of Go et al.24 at a final concentration of 20 μM for 15 minutes, after which iodoacetamide was added to a final concentration of 5 mM. The GR was immunoprecipated using an anti-GR receptor antibody (Santa Cruz Biotechnology, Dallas, TX) and the Protein G Immunoprecipitation Kit (Sigma-Aldrich). Eluted sample was divided and run on two 10% SDS-PAGE gels. Proteins were transferred overnight to nitrocellulose membranes and biotinylated iodoacetamine binding was visualized on an Odyssey® Classic Infrared Imaging System (LI-COR, Lincoln, NE) by incubating with streptavidin-conjugated IRDye® 680RD (LI-COR) for one hour. Even loading of samples was determined by incubating the second membrane overnight at 4°C with human GR antibody raised in rabbit (Santa Cruz Biotechnology). Visualization was performed after secondary incubation with anti-rabbit IgG conjugated to IRDye® 680RD (LI-COR).
Nuclear isolation, total protein measurement and nuclear GR expression and activation
Nuclei were isolated using a commercial nuclear extract kit (Active Motif, Carlsbad, CA). Protein concentrations were measured at 750nm using bovine serum albumin as the standard (DC™ Protein Assay Kit, Bio-Rad, Hercules, CA). Equal nuclear protein volumes were added to a TransAM™ assay plate (Active Motif) coated with immobilized oligonucleotide containing the GR consensus binding site (5′-GGTACAnnnTGTTCT-3′). GR activity was assessed per the manufacturer’s instructions with an optical density of 450 nm. SDS-PAGE was performed using 30 μg of nuclear lysate loaded onto a precast gradient (4-20%) gel (Bio-Rad). Proteins were transferred onto a nitrocellulose membrane (Bio-Rad) and incubated with human GR antibody raised in rabbit (Santa Cruz) and IRDye 800CW Donkey anti-Rabbit IgG and IRDye 680RD Donkey anti-Goat IgG (Li-Cor Biosciences, Lincoln, NE). Membranes were visualized on an Odyssey Classic Infrared Imaging System and densitometry performed using Image Studio Lite version 4.0 (Li-Cor Biosciences).
Statistical analyses
Statistical analyses were performed with IBM® SPSS® Statistics software (Version 22, SPSS Inc, Chicago, Illinois). Chi-square tests were used for dichotomous variables. T-tests and analysis of variance with Fishers Least Significance Difference post-hoc tests were used for variables that were normally distributed. For skewed variables, statistical significance testing was performed with non-parametric Mann-Whitney U tests or Kruskal-Wallis tests, as appropriate. Bivariate Pearson correlations were used to examine associations between linear variables. Logistic regression analyses were performed to determine the associations between tertiles of plasma cysteine/cystine redox potentials and clinical outcomes. Univariate models were first used to narrow the list of covariates (statistically significant at p < 0.05) to be incorporated into the final multivariate model. Significance was defined as α < 0.05 using two-tailed tests.
RESULTS
One hundred thirty four children were enrolled for this study but 20 were excluded due to an inadequate sample, leaving 114 children (healthy control, n = 15; asthma, n = 99) in the final analysis (Online Repository, Figure E1). Features of the excluded children were not significantly different (12 ± 4 years; 15% White; 45% with BMI percentile <85%; 93 ± 13% predicted FEV1, p > 0.30 for each). Features of the included participants are shown in Table 1. Children with asthma were more likely to be non-white males and were further characterized by greater allergic sensitization, more airflow limitation, and increased airway inflammation as reflected by exhaled nitric oxide values, consistent with their asthma diagnosis. Children with asthma were also more obese with higher body mass index percentiles.
Table 1.
Features of the included participants. Data represent the median (IQR) or the number of participants (%).
| Feature | Healthy controls N = 15 |
Asthmatics N = 99 |
p-value |
|---|---|---|---|
|
| |||
| Age (years) | 10 (9, 13) | 12 (10, 15) | 0.175 |
|
| |||
| Sex | |||
| Male | 3 (20) | 66 (67) | 0.001 |
| Female | 12 (80) | 33 (33) | |
|
| |||
| Race | |||
| White | 3 (20) | 16 (16) | 0.001 |
| Black | 9 (60) | 69 (70) | |
| Asian | 3 (20) | 1 (1) | |
| More than one race | 0 | 13 (11) | |
|
| |||
| Body mass index | |||
| < 85th percentile (normal weight) | 9 (82) | 43 (43) | 0.036 |
| 85th to 95th percentile (overweight) | 2 (18) | 26 (26) | |
| ≥ 95th percentile (obese) | 0 | 30 (30) | |
|
| |||
| Self-reported allergic rhinitis | 10 (67) | 91 (92) | 0.004 |
|
| |||
| Serum IgE (kU/L) | 61 (30, 94) | 218 (40, 503) | 0.011 |
|
| |||
| Exhaled nitric oxide (ppb) | 12 (9, 14) | 29 (17, 51) | < 0.001 |
|
| |||
| Baseline lung function | |||
| FVC (% predicted) | 102 (88, 110) | 105 (98, 115) | 0.652 |
| FEV1 (% predicted) | 98 (89, 108) | 93 (81, 105) | 0.046 |
| FEV1/FVC | 0.87 (0.83, 0.93) | 0.78 (0.70, 0.83) | < 0.001 |
| FEV1/FVC (% predicted) | 101 (95, 109) | 90 (81, 95) | < 0.001 |
| FEF25-75 (% predicted) | 92 (74, 112) | 71 (56, 86) | < 0.001 |
|
| |||
| Post-bronchodilator lung function | |||
| FVC (% predicted) | 104 (90, 121) | 110 (102, 120) | 0.294 |
| FEV1 (% predicted) | 108 (93, 118) | 105 (93, 115) | 0.705 |
| FEV1/FVC | 0.90 (0.83, 0.96) | 0.84 (0.78, 0.88) | 0.004 |
| FEV1/FVC (% predicted) | 104 (94, 110) | 97(91, 101) | 0.010 |
| FEF25-75 (% predicted) | 108 (91, 137) | 94 (81, 110) | 0.161 |
Characterization of systemic cysteine oxidation
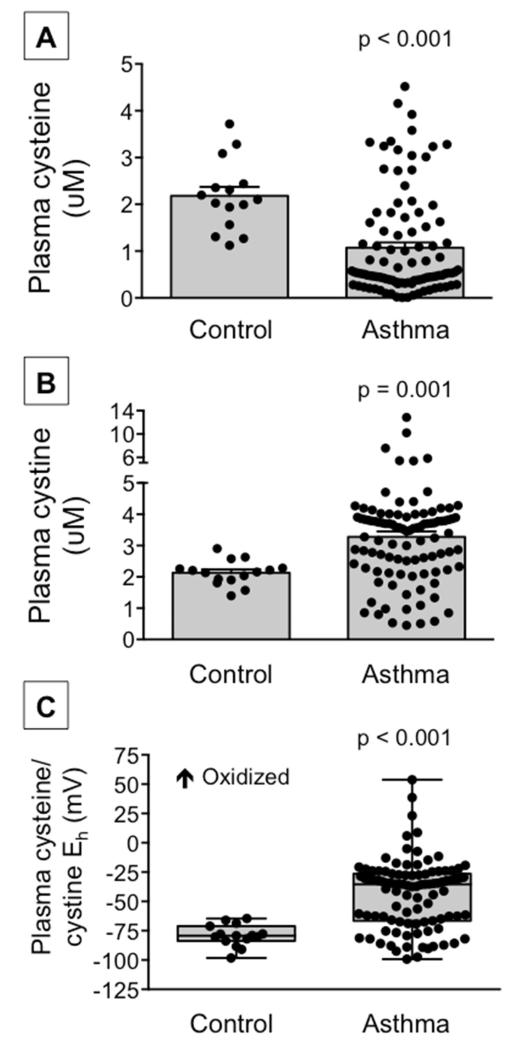
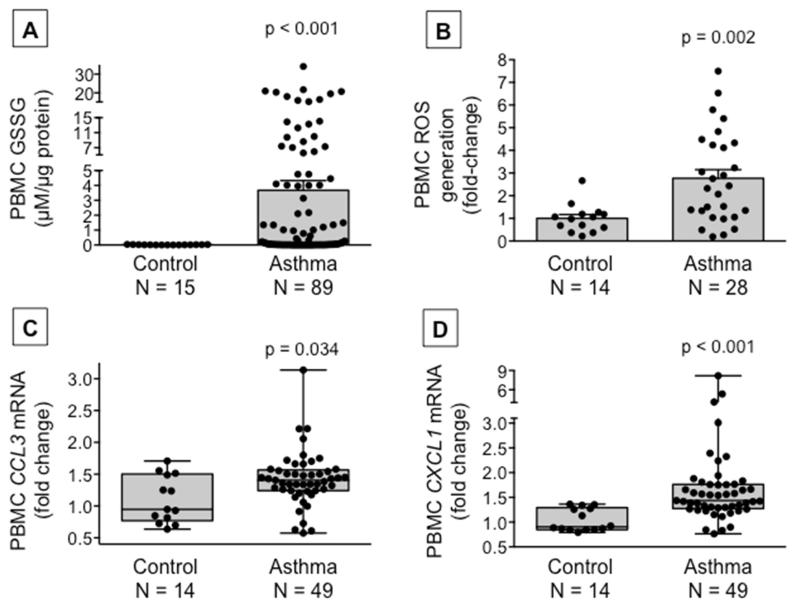
Compared to healthy non-asthmatic children, children with asthma had lower baseline plasma concentrations of cysteine and higher plasma concentrations of the disulfide, cystine, resulting in a more oxidized cysteine/cystine redox potential (Figure 1). Extracellular cysteine oxidation in the asthmatic participants was further accompanied by increased intracellular glutathione disulfide formation, increased reactive oxygen species generation and increased mRNA expression of the pro-inflammatory genes, CCL3 and CXCL1 (Figure 2). Although all children with asthma were difficult-to-treat, children with severe asthma defined by the need for high-dose inhaled glucocorticoids plus an additional controller medication to maintain asthma control25 had the lowest plasma cysteine concentrations and the most oxidized cysteine/cystine redox potential (Online repository, Figure E2).
Figure 1.
Plasma (A) cysteine and (B) cystine concentrations and (C) the cysteine/cystine redox potential (Eh) in healthy control children and children with asthma. Cysteine and cystine data are presented as the mean ± SEM and the cysteine/cystine Eh boxplots are shown with minimum and maximum values. Dots represent individual participants. Control: n = 15, asthma: n = 99 for each panel.
Figure 2.
Peripheral blood mononuclear cell (A) glutathione disulfide (GSSG) concentrations, (B) reactive oxygen species (ROS) generation, and (C) CCL3 and (D) CXCL1 mRNA gene expression in healthy control children and children with asthma. GSSG and ROS data are presented as the mean ± SEM and CCL3 and CXCL1 mRNA data are presented as boxplots with minimum and maximum values. Dots represent individual values.
Associations between systemic cysteine oxidation and asthma clinical features
Given the heterogeneity in extracellular cysteine/cystine redox potentials observed within the group of asthmatic participants, associations between tertiles of plasma cysteine/cystine redox potentials and asthma clinical features were explored. Asthmatic children with plasma cysteine/cystine redox potentials in the highest (i.e., most oxidized) tertile had over three-fold increased odds of high-dose inhaled glucocorticoid therapy and daily short-acting beta-agonist therapy compared to children in the lowest (i.e., more reduced) tertile. Moreover, children in the highest tertile also had evidence of poorer current and historical asthma control, reflected by ACQ scores greater than 1.5 at the characterization visit22 and more frequent emergency department visits and intubations (Online Repository, Table E1).
Associations between systemic cysteine oxidation and glucocorticoid responsiveness
To further understand associations between systemic cysteine oxidation and features of asthma severity in children, we determined whether the baseline plasma cysteine/cystine redox potential was also associated with clinical responsiveness to systemic glucocorticoids. For this analysis, a subset of asthmatic participants with poor asthma control despite treatment with moderate-to-high doses of inhaled glucocorticoids (n = 57) received intramuscular triamcinolone and returned to the clinic for re-evaluation in two weeks. Features of the children who received triamcinolone are presented in Table E2 (Online Repository).
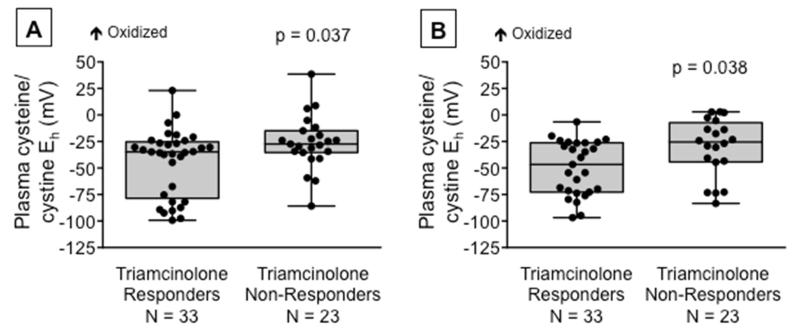
At the two-week visit, the prevalence of study-defined triamcinolone responsiveness and non-responsiveness was 59% (n = 33) and 41% (n = 23), respectively. In addition to having poorer asthma control, asthmatic children who did not respond to triamcinolone also had more impaired asthma-related quality of life, increased exhaled nitric oxide concentrations, and greater pre-bronchodilator airflow limitation after the triamcinolone injection (Table 2). Furthermore, the plasma cysteine/cystine redox potential was higher (i.e., more oxidized) in triamcinolone non-responders at both the baseline visit and the two-week characterization visit (Figure 3) due to lower cysteine concentrations in the non-responders at both time points (responders vs. non-responders, baseline cysteine: 1.32 ± 1.50 vs. 0.48 ± 0.52 μM, p = 0.013; 2-week cysteine: 1.33 ± 1.31 vs. 0.58 ± 0.79 μM, p =0.40). Plasma concentrations of cystine, mixed disulfides, glutathione and glutathione disulfide did not differ between groups (data not shown). Stratification of participants by baseline tertiles of plasma cysteine/cystine redox potentials also demonstrated increased odds of triamcinolone non-responsiveness at the follow-up visit in the most oxidized group (OR for highest versus lowest tertile: 5.83; 95% CI: 1.06 – 32.02, p = 0.042). Overall, the plasma cysteine/cysteine redox potential for each participant, including healthy controls and children who did not receive triamcinolone, was relatively stable (Online Repository, Figure E4). mRNA gene expression of CCL3 and CXCL1was also increased in triamcinolone non-responders compared to triamcinolone responders (Online Repository, Figure E5), consistent with the clinical observations of increased inflammation in these children (Table 2).
Table 2.
Features of triamcinolone responders versus non-responders at the two-week follow-up visit.1 Triamcinolone response was defined as an Asthma Control Questionnaire score < 0.75.22 Data represent the median (IQR).
| Feature | Triamcinolone responder N = 33 |
Triamcinolone non-responder N = 23 |
p-value |
|---|---|---|---|
|
| |||
| Pediatric Asthma Quality of Life Questionnaire total score2 |
6.56 (6.25, 6.83) | 5.41 (4.45, 6.01) | < 0.001 |
|
| |||
| Exhaled nitric oxide (ppb)3 | 14 (6, 25) | 26 (12, 45) | 0.040 |
|
| |||
| Baseline lung function | |||
| FVC (% predicted) | 105 (99, 115) | 92 (84, 117) | 0.038 |
| FEV1 (% predicted) | 96 (87, 102) | 80 (69, 101) | 0.007 |
| FEV1/FVC | 0.78 (0.72, 0.87) | 0.75 (0.67, 0.80) | 0.207 |
| FEV1/FVC (% predicted) | 90 (85, 101) | 87 (78, 92) | 0.093 |
| FEF25-75 (% predicted) | 75 (61,91) | 56 (45, 76) | 0.016 |
|
| |||
| Post-bronchodilator lung function | |||
| FVC (% predicted) | 109 (103, 120) | 101 (93, 117) | 0.111 |
| FEV1 (% predicted) | 105 (98, 114) | 96 (88, 114) | 0.085 |
| FEV1/FVC | 0.85 (0.79, 0.91) | 0.85 (0.81, 0.87) | 0.692 |
| FEV1/FVC (% predicted) | 98 (92, 106) | 98 (95, 100) | 0.523 |
| FEF25-75 (% predicted) | 99 (83, 113) | 94 (72, 109) | 0.157 |
One participant did not complete the follow up visit and was excluded from this comparison.
Scores on the Pediatric Asthma Quality of Life Questionnaire range from 0 to 7, with higher scores indicating better quality of life.
Data were logarithmically transformed prior to statistical analysis.
Figure 3.
The plasma cysteine/cystine redox potential (Eh) in triamcinolone responders and triamcinolone non-responders at (A) the baseline visit prior to triamcinolone receipt and (B) two weeks after triamcinolone administration. Triamcinolone “response” was defined as an Asthma Questionnaire Control Score <0.75 at the two-week visit.22 Boxplots are shown with minimum and maximum values. Dots represent individual values.
Proof-of-concept mechanistic studies
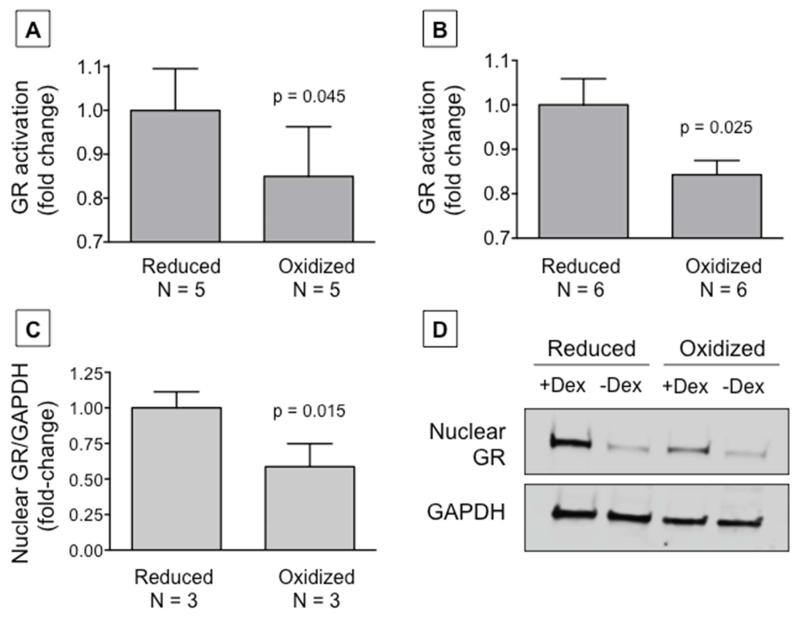
Because the volume of samples available from children was limited, proof-of-concept experimentation was also performed using THP-1 monocytes and primary PBMCs from healthy donors. Exposure of these cells to extracellular oxidizing versus reducing cysteine/cystine environments resulted in physiologically relevant redox potentials and differences in intracellular glutathione disulfide and ROS generation (Online Repository, Figure E6) compared what we previously observed in our pediatric participants. Oxidizing extracellular conditions were also associated with decreased availability of GR protein −SH groups for binding (Online Repository, Figure E7, A-B). Similar decreases in GR protein −SH group availability were observed samples from difficult-to-treat asthmatic children (Online Repository, Figure E7, C-D). Furthermore, GR activity, reflected by decreased GR consensus binding, was blunted in response to extracellular oxidizing conditions (Figure 4, A-B). Additional experiments with MM.1S cells, which naturally over-express the GR, further revealed decreased expression of the GR in the nucleus with extracellular cysteine/cystine oxidation.
Figure 4.
Glucocorticoid receptor (GR) activation in (A) THP-1 monocyes and (B) human primary PBMCs and (C) nuclear GR expression in MM.1S cells after exposure to extracellular oxidizing versus reduced conditions. GR densitometry is normalized to glyceraldehyde-3-phosphate (GAPDH). Representative images of nuclear GR expression with (+) and without (−) dexamethasone (Dex) treatment are shown in panel (D).
DISCUSSION
To our knowledge, this is the first study to characterize cysteine oxidation and its association with glucocorticoid responsiveness in patients with difficult-to-treat asthma. Although heterogeneity in our results was noted, a significant degree of cysteine oxidation was noted in a subset of children with difficult-to-treat asthma and was accompanied by increased ROS generation and increased mRNA expression of CCL3 and CXCL1. This heterogeneity was not clearly attributable to obesity or inhaled glucocorticoid exposure, although children with the greatest extent of cysteine oxidation did have more features of asthma severity including poorer symptom control, greater medication usage including use of high-dose inhaled glucocorticoids, and decreased clinically defined glucocorticoid responsiveness. While the precise mechanisms underlying these observations are unclear, our proof-of-concept experiments suggest that cysteine oxidation promotes a post-translational modification of the GR that impairs its nuclear expression and binding to the glucocorticoid consensus.
Clinically-defined impairments in glucocorticoid responsiveness have been previously reported in patients with asthma, albeit there are no gold standard definitions for glucocorticoid “response” and methodologies vary widely across studies.26 A recent unbiased cluster analysis of children enrolled in the Childhood Asthma Management Research Program identified two clusters of childhood asthma with positive versus minimal responses to inhaled budesonide that differed significantly with regard to the rate of exacerbations, the number of controller medications for asthma, and longitudinal differences in pulmonary function.27 A separate study of children with difficult-to-treat asthma similarly identified several groups of patients with varying degrees of responsiveness to intramuscular triamcinolone.28 In that study, only 11% of enrolled children had “complete” responsiveness to triamcinolone as determined by cut-points of symptoms, FEV1, bronchodilator reversibility and exhaled nitric oxide.28 Rather, the majority of children were “partial” or “incomplete” responders,28 highlighting the heterogeneity of the disorder as it is currently defined. It is also not clear how clinical end points such as lung function or symptoms associate with airway inflammation or other biological mechanisms that may underlie asthma phenotypes.
While the biological mechanisms that regulate the response to glucocorticoids are not entirely clear, other studies have described reduced binding of glucocorticoid to its receptor and reduced GR consensus binding in these patients29, 30 that might be related to persistent activation of p38 mitogen-activated protein kinase.31, 32 Other studies have reported increased expression of the GR beta isoform in airway cells,33 immune cells34 and airway tissue35 of patients with severe asthma that may act as a negative inhibitor and compete for binding of GR consensus. Abnormal histone acetylation has also been reported in patients with severe asthma36 and COPD37 and is characterized by a reduction in histone deacetylase 2 expression and activity, which may be induced by oxidative and nitrosative stress.38, 39
Like any protein, the GR contains a number of cysteine residues that maintain its structure and function. The hormone binding domain of the GR (residues 529-777 in humans) contains 13 methionines but only 5 cysteines (Cys) at residues 622, 638, 643, 665, and 736 that are sensitive to changes in redox status.40 Under oxidizing conditions, Cys-622, 638, and 643 form disulfide bonds41-43 that may cause the GR to assume a folded conformation.44 Additionally, disulfide bonds with Cys-481 in the DNA binding domain of the GR have been shown to inhibit nuclear translocation, presumably through altered GR protein conformation.9 Thus, redox regulation of the GR is most likely due to functional alteration of the cysteine residues of the GR in the presence of reactive oxygen species, and not to a decrease in expression of the GR protein.45-47
This study does have a number of limitations. Although adherence to inhaled glucocorticoids cannot be guaranteed in our participants with difficult-to-treat asthma, intramuscular triamcinolone removes some issues with inhaled glucocorticoid delivery given its depot effect.26 While previous studies utilizing triamcinolone in children have demonstrated overall reductions in inflammatory biomarkers and asthma symptoms after the injection, variability in the clinical response was noted.28, 48 Although a higher dose of triamcinolone may have conferred additional benefit,49 these benefits are likely to be outweighed by the systemic side effects of the medication in school age children who are still undergoing linear growth. Furthermore, while the use of PBMCs can be criticized, our previous work12 and that of others34 has shown similarities between PBMCs and airway cells in patients with severe asthma whose inflammation is not limited to the airways. The use of PBMCs also allowed for a control group that otherwise could not be obtained due to ethical reasons.
In conclusion, we have shown that oxidation of the amino acid, cysteine, is associated with decreased responsiveness to systemic glucocorticoids in children with difficult-to-treat asthma. While additional mechanistic studies are warranted, our findings also suggest that redox-related mechanisms can impair GR function in asthmatic patients. While glucocorticoid responsiveness is likely associated with multiple mechanisms (including mechanisms independent of oxidative stress), the cysteine redox state may represent one of several potential therapeutic targets for the restoration of glucocorticoid responsiveness in this population.
Supplementary Material
CLINICAL IMPLICATIONS.
Systemic oxidative stress, reflected by oxidation of the amino acid, cysteine, is associated with poor asthma control and decreased responsiveness to systemic glucocorticoids in children with difficult-to-treat asthma.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Emory+Children’s Pediatric Research Center Biomarkers Core, the staff members at the Emory Children’s Center asthma clinic, and the staff members at the Children’s Healthcare of Atlanta Pediatric Research Center for their assistance with this project.
This study was funded by RO1 NR012021 and was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health, award number UL1 TR000454.
ABBREVIATIONS
- ACQ
Asthma Control Questionnaire
- CT
Cycle threshold
- Cys
Cysteine residue
- Eh
Redox potential
- FEF25-75
Mid-expiratory flow rate at 25-75% of vital capacity
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GR
Glucocorticoid receptor
- IgE
Immunoglobulin E
- PAQLQ
Pediatric Asthma Quality of Life Questionnaire
- PBMC
Peripheral blood mononuclear cell
- PC20
Provocative concentration of methacholine resulting in a 20% decline in FEV1
- RFU
Relative fluorescent units
- ROS
Reactive oxygen species
- −SH
Sulfhydryl group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Asthma E, Prevention P Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Kelly HW. Inhaled corticosteroid dosing: double for nothing? J Allergy Clin Immunol. 2011;128:278–81 e2. doi: 10.1016/j.jaci.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–7. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 4.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 6.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033–44. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H, Makino Y, Okamoto K, Iida T, Yan K, Yoshikawa N. Redox regulation of the glucocorticoid receptor. Antioxid Redox Signal. 1999;1:403–23. doi: 10.1089/ars.1999.1.4-403. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Tanaka H, Ogawa H, Makino Y, Eguchi H, Hayashi S, et al. Redox-dependent regulation of nuclear import of the glucocorticoid receptor. J Biol Chem. 1999;274:10363–71. doi: 10.1074/jbc.274.15.10363. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Severe Asthma Research P. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–52 e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AM, Teague WG, Burwell L, Brown MS, Brown LA. Program NNSAR. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154–9. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AM, Stephenson ST, Hadley GR, Burwell L, Penugonda M, Simon DM, et al. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol. 2011;127:1604–11. doi: 10.1016/j.jaci.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick AM, Park Y, Brown LA, Jones DP. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J Allergy Clin Immunol. 2014;133:258–61. e1–8. doi: 10.1016/j.jaci.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick AM, Brown LA, Holguin F, Teague WG, National Institutes of Health. National Heart L. Blood Institute Severe Asthma Research P Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol. 2009;124:990–6. e1–9. doi: 10.1016/j.jaci.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012;129:388–96. 96 e1–8. doi: 10.1016/j.jaci.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer SS, Accardi CJ, Ziegler TR, Blanco RA, Ritzenthaler JD, Rojas M, et al. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS One. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go YM, Craige SE, Orr M, Gernert KM, Jones DP. Gene and protein responses of human monocytes to extracellular cysteine redox potential. Toxicol Sci. 2009;112:354–62. doi: 10.1093/toxsci/kfp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–6. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic S. European Respiratory S ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–21. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr., Mody VC, Jr., Reed RL, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 24.Go YM, Pohl J, Jones DP. Quantification of redox conditions in the nucleus. Methods Mol Biol. 2009;464:303–17. doi: 10.1007/978-1-60327-461-6_17. [DOI] [PubMed] [Google Scholar]
- 25.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636–45. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 27.Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133:1289–300. 300 e1–12. doi: 10.1016/j.jaci.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossley CJ, Saglani S, Kavanagh C, Payne DN, Wilson N, Tsartsali L, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J. 2009;34:1052–9. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adcock IM, Lane SJ, Brown CR, Peters MJ, Lee TH, Barnes PJ. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol. 1995;154:3500–5. [PubMed] [Google Scholar]
- 30.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–8. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Bhavsar P, Khorasani N, Hew M, Johnson M, Chung KF. Effect of p38 MAPK inhibition on corticosteroid suppression of cytokine release in severe asthma. Eur Respir J. 2010;35:750–6. doi: 10.1183/09031936.00071309. [DOI] [PubMed] [Google Scholar]
- 32.Mercado N, Hakim A, Kobayashi Y, Meah S, Usmani OS, Chung KF, et al. Restoration of corticosteroid sensitivity by p38 mitogen activated protein kinase inhibition in peripheral blood mononuclear cells from severe asthma. PLoS One. 2012;7:e41582. doi: 10.1371/journal.pone.0041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 2006;173:607–16. doi: 10.1164/rccm.200507-1046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goleva E, Jackson LP, Gleason M, Leung DY. Usefulness of PBMCs to predict clinical response to corticosteroids in asthmatic patients. J Allergy Clin Immunol. 2012;129:687–93 e1. doi: 10.1016/j.jaci.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergeron C, Fukakusa M, Olivenstein R, Lemiere C, Shannon J, Ernst P, et al. Increased glucocorticoid receptor-beta expression, but not decreased histone deacetylase 2, in severe asthma. J Allergy Clin Immunol. 2006;117:703–5. doi: 10.1016/j.jaci.2005.12.1344. [DOI] [PubMed] [Google Scholar]
- 36.Butler CA, McQuaid S, Taggart CC, Weldon S, Carter R, Skibinski G, et al. Glucocorticoid receptor beta and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax. 2012;67:392–8. doi: 10.1136/thoraxjnl-2011-200760. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–76. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–5. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Osoata GO, Yamamura S, Ito M, Vuppusetty C, Adcock IM, Barnes PJ, et al. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun. 2009;384:366–71. doi: 10.1016/j.bbrc.2009.04.128. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Stallcup MR. The hormone-binding role of 2 cysteines near the C terminus of the mouse glucocorticoid receptor. J Biol Chem. 1994;269:7914–8. [PubMed] [Google Scholar]
- 41.Chakraborti PK, Garabedian MJ, Yamamoto KR, Simons SS., Jr. Role of cysteines 640, 656, and 661 in steroid binding to rat glucocorticoid receptors. J Biol Chem. 1992;267:11366–73. [PubMed] [Google Scholar]
- 42.Granberg JP, Ballard PL. The role of sulfhydryl groups in the binding of glucocorticoids by cytoplasmic receptors of lung and other mammalian tissues. Endocrinology. 1977;100:1160–8. doi: 10.1210/endo-100-4-1160. [DOI] [PubMed] [Google Scholar]
- 43.Simons SS, Jr., Pratt WB. Glucocorticoid receptor thiols and steroid-binding activity. Methods Enzymol. 1995;251:406–22. doi: 10.1016/0076-6879(95)51144-x. [DOI] [PubMed] [Google Scholar]
- 44.Silva CM, Cidlowski JA. Direct evidence for intra- and intermolecular disulfide bond formation in the human glucocorticoid receptor. Inhibition of DNA binding and identification of a new receptor-associated protein. J Biol Chem. 1989;264:6638–47. [PubMed] [Google Scholar]
- 45.Bodwell JE, Holbrook NJ, Munck A. Sulfhydryl-modifying reagents reversibly inhibit binding of glucocorticoid-receptor complexes to DNA-cellulose. Biochemistry. 1984;23:1392–8. doi: 10.1021/bi00302a009. [DOI] [PubMed] [Google Scholar]
- 46.Esposito F, Cuccovillo F, Morra F, Russo T, Cimino F. DNA binding activity of the glucocorticoid receptor is sensitive to redox changes in intact cells. Biochim Biophys Acta. 1995;1260:308–14. doi: 10.1016/0167-4781(94)00209-l. [DOI] [PubMed] [Google Scholar]
- 47.Makino Y, Okamoto K, Yoshikawa N, Aoshima M, Hirota K, Yodoi J, et al. Thioredoxin: a redox-regulating cellular cofactor for glucocorticoid hormone action. Cross talk between endocrine control of stress response and cellular antioxidant defense system. J Clin Invest. 1996;98:2469–77. doi: 10.1172/JCI119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panickar JR, Bhatnagar N, Grigg J. Exhaled nitric oxide after a single dose of intramuscular triamcinolone in children with difficult to control asthma. Pediatr Pulmonol. 2007;42:573–8. doi: 10.1002/ppul.20583. [DOI] [PubMed] [Google Scholar]
- 49.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004;170:601–5. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.