Abstract
In cardiovascular (CV) diabetology a “one-size fits-all” approach needs caution as vasculopathy and CV manifestations in patients with type 2 diabetes (T2D) with short disease duration are different as compared to those with longer duration. This is of relevance when interpreting results of CV outcome trials as responses to any intervention aimed to reduce CV risk might be different in patients with established vasculopathy as compared to those without, where also the duration of the intervention may play a role. Additionally, the mode-of-action of the intervention and its assumed time to peak CV risk modulation need to be taken into account: an intervention with possibly immediate effects, like on blood pressure or other direct functional dynamic parameters such as endothelial function or renal hemodynamics, could likely provide a meaningful impact on CV outcomes over a shorter time span than interventions that primarily target pathways that work on atherosclerotic processes, organ-remodelling, or vessel integrity. We are now faced with CV outcome results to interpret from a plethora of outcomes trials in T2D, some of which are testing the CV risk modulation predominantly beyond glucose lowering, e.g., as is the case for several trials testing the newer therapy classes di-peptidyl peptidase-4 inhibitors, glucagon-like protein-1 receptor analogues and sodium glucose co-transporter-2 inhibitors, and this paper reviews the data that support a call for a multiaxial approach to interpret these results.
Keywords: Type 2 diabetes, Pharmaceutical, Risk reduction, Outcomes, Cardiovascular
Core tip: Vasculopathy and cardiovascular (CV) manifestations in patients with type 2 diabetes differ dependent on disease duration. This literature review supports that it is necessary to contextualize results of CV outcome trials in diabetes to diabetes duration as well as duration and mode of action of the intervention, which may be of particular relevance for those interventions that primarily target pathways related to atherosclerotic processes, organ-remodelling, or vessel integrity. Several CV outcome trials testing newer therapy classes (i.e., di-peptidyl peptidase-4 inhibitors, glucagon-like protein-1 receptor analogues and sodium glucose co-transporter-2 inhibitors) are now due to report and a multiaxial approach to interpret these results is needed.
INTERPRETATION OF CARDIOVASCULAR OUTCOME TRIALS IN TYPE 2 DIABETES
The human mind is a master in pattern recognitions. A flip-side to this profound ability in predicting cause-and-effects surfaces however in dealing with complex questions where a “one-size fits-all” approach not necessarily longer applies. Cardiovascular (CV) diabetology is one example of a complex system where a “one-size fits-all” approach needs caution. For example, vasculopathy and CV manifestations in patients with type 2 diabetes (T2D) with short disease duration are different as compared to those with longer T2D duration. Further, the response to any intervention aimed to reduce CV risk might be different in patients with established vasculopathy as compared to those without, where also the duration of the intervention may play a role for a successful risk reduction. The last point is however also dependent on the mode-of-action of the intervention, since an intervention with possibly immediate effects, like on blood pressure or other direct functional dynamic parameters such as endothelial function or renal hemodynamics, likely could provide a meaningful impact on outcomes over a shorter time span than interventions that primarily targets pathways that work on atherosclerotic processes, organ-remodelling, or vessel integrity. These are all important considerations that need to be taken into account when we soon will be faced with results to interpret from a plethora of outcomes trials in T2D, some of which are testing the CV risk modulation potential predominantly beyond glucose lowering, e.g., as is the case for the newer therapy classes di-peptidyl peptidase (DPP)-4 inhibitors, glucagon-like protein-1 receptor analogues and sodium glucose co-transporter-2 inhibitors (Figure 1).
Figure 1.
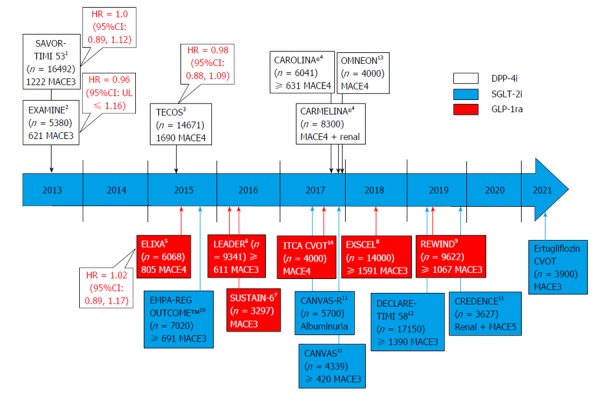
Anticipated ending of outcome trials in type 2 diabetes and their primary outcomes and patient/event numbers involving di-peptidyl peptidase-4 inhibitors, glucagon-like protein-1 receptor analogues and sodium glucose co-transporter-2 inhibitors. Superscript note indicate study drug(s) in testing. All trials are placebo controlled except CAROLINA® that compared vs the sulfonylurea glimepiride. 1Saxagliptin, Astra Zeneca; 2Alogliptin, Takeda; 3Sitagliptin, Merck; 4Linagliptin, Boehringer Ingelheim/Eli Lilly; 5Lixisenatide, Sanofi Aventis; 6Liraglutide, Novo Nordisk; 7Semaglutide, Novo Nordisk; 8Exenatide, Astra Zeneca; 9Dulaglutide, Eli Lilly; 10Empagliflozin, Boehringer Ingelheim/Eli Lilly; 11Canagliflozin, J and J; 12Dapagliflozin, Astra Zeneca; 13Omarigliptin (once weekly tablet), Merck; 14ITCA 650 [once/twice yearly exenatide via subcutaneous mini-pump (Duros device)], Intarcia Therapeutics. DPP-4: Di-peptidyl peptidase-4; GLP-1: Glucagon-like protein-1; SGLT-2: Sodium glucose co-transporter-2; MACE3: Composite endpoint of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke; MACE4: MACE3 plus hospitalized unstable angina pectoris; MACE5: MACE4 plus hospitalized congestive heart failure.
THE RELEVANCE OF CONTEXTUALIZING OUTCOME TRIAL RESULTS TO T2D DURATION AND PRESENCE OR ABSENCE OF CV COMPLICATIONS?
T2D is a progressive complex metabolic disease[1] leading to disturbances in several pathways (e.g., hyperglycemia, insulin resistance, inflammation, oxidation, endothelial dysfunction, dysfunctional adiposity) involved in vasculo-biopathology and CV complications[2]. With this in mind, what could possibly explain differing impact on CV risk of an intervention given early in the T2D disease course vs late? One element relates to that longer-standing T2D is associated with silent vasculopathy, as illustrated by e.g., approximately 20% of clinically asymptomatic patients with T2D having significant coronary artery disease, either by invasive coronary angiography[3] or by photon emission-computed tomography myocardial perfusion imaging[4].
Further, since longer duration of the disease and advancing age typically lead to an accumulation of subclinical [such as vascular stiffness[5], coronary artery calcifications (CAC)[6], or myocardial dysfunction[7]] or clinical manifestations of CV complications (i.e., myocardial infarction), or microvascular complications (which is an emerging risk factor for CV complications[8]), it might be conceivable that if the patient population being studied has advanced vasculopathy, the likelihood to influence the disease course could be lower. In particular if end-stage complications have manifested, e.g., as observed in patients on dialysis where statins apparently do not reduce CV risk[9], since these patients may be less sensitive to improvement in CV risk factors.
In longer-term outcome trials in T2D, where different strategies to intensively improve glucose control were tested, this point, to a certain degree, was illustrated by different results on outcomes as observed in the United Kingdom Prospective Diabetes Study (UKPDS); a study[10] that recruited newly diagnosed patients with T2D with a low CV disease burden, and the ORIGIN trial[11], which recruited patients with 5-6 years of diabetes duration of whom approximately 60% had prior CV complications (Figure 2). Although both studies achieved meaningful differences in glucose control between treatment arms, only those patients with newly diagnosed T2D without prevalent CV disease in UKPDS, achieved outcome benefits. Whether this was related to the short diabetes duration and low vasculopathy burden at the start of the intervention, a long treatment duration, or mode of action of the different interventions, is not known. The potential differing response to preventive therapies in patients with short vs long standing diabetes was also illustrated in a subanalysis of the recent CV outcome trial comparing outcomes of placebo or alogliptin superimposed on standard of care in patients with T2D and acute coronary syndrome (the EXAMINE trial)[12]. Overall the glycemic differences between the treatment arms were small and the primary outcome was neutral, however, patients with shorter diabetes duration (i.e., less than 5 years) had reduced risk [hazard ratio (HR) = 0.74 (95%CI: 0.54, 1.01)] for the composite primary CV endpoint as compared to those with longer disease duration [5-10 years HR = 0.81 (95%CI: 0.58, 1.13); > 10 years HR = 1.22 (95%CI: 0.98, 1.53); interaction with treatment P-value 0.014]. Another interesting observation in the context of degree of vasculopathy as a potential determinant for the effect of an intervention stems from the veterans affairs diabetes trial (VADT)[13]. The VADT tested whether intensive glucose control (targeted/achieved HbA1c < 6.0%/6.9%) vs conventional (targeted/achieved HbA1c < 9.0%/8.9%) could reduce CV risk in 1791 patients with long-standing T2D[13]. Although intensive glucose-lowering therapy did not significantly reduce CV events in the study cohort as a whole, there was evidence that the response was modified by baseline CAC. They observed, e.g., that among those randomized to intensive treatment, in the subgroup with CAC > 100, 11 of 62 individuals had events, while only 1 of 52 individuals with CAC ≤ 100 had an event (significant risk reduction), indicating that intensive glucose lowering reduced CV events only in those with less extensive calcified coronary atherosclerosis[14].
Figure 2.
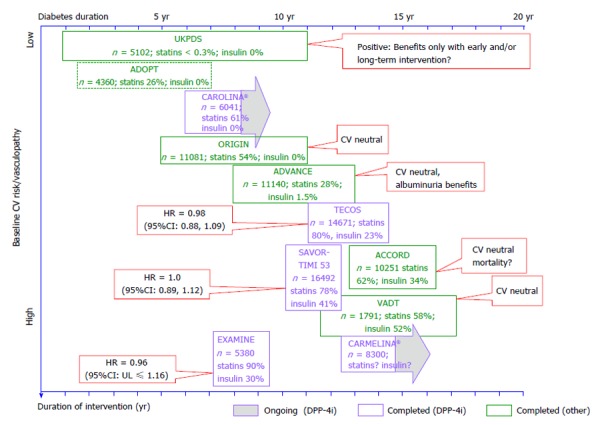
Selected outcome trials in type 2 diabetes with a focus on di-peptidyl peptidase-4 inhibitor studies, and their results, in the context of the duration of intervention and the study population’s diabetes duration and baseline cardiovascular risk. CV: Cardiovascular; UKPDS: United Kingdom Prospective Diabetes Study; VADT: Veterans affairs diabetes trial.
WHY IS IT IMPORTANT TO CONTEXTUALIZE OUTCOME TRIAL RESULTS TO DURATION OF INTERVENTION?
In order for an intervention to reduce CV risk it has to interfere with the cascade of events that lead to complications. Since T2D is a CV risk entity by itself, where CV risk typically is further magnified in the presence of CV complications, any intervention that targets outcomes like myocardial infarction or hospitalization for angina pectoris primary related to atherosclerosis, likely have to be of sufficient duration since the biopathological processes typically might evolve over decades[15,16]. Although the targeted study outcome as well as the mode of action of the intervention certainly plays an important role here, one important question is when the effects of an intervention are assumed to peak. This was illustrated, for example, by the PRO active trial[17], comparing pioglitazone vs placebo as secondary CV prevention: at study end the primary endpoint just missed the significance level, but as the survival curves separated in favour of pioglitazone towards study end, it was speculated that the trial result could have looked different if the trial had run longer[18]. At this point it is only speculations if the two other recent neutral outcome trials involving DPP-4 is, a class that in animal studies have been implied to reduce several pathways leading to atherosclerosis[19], namely SAVOR-TIMI53[20], EXAMINE[12], and TECOS[21] would have showed different results if ran longer than their median duration of respectively 2.1, 1.5 and 2.8 years. Obviously this needs further clarification in trials of longer duration.
RESULTS OF CV OUTCOME TRIALS IN T2D NEED TO BE INTERPRETED IN A MULTIDIMENSIONAL FRAME
Over the next years, with several CV outcome trials due to report (Figure 1)[22-29], an opportunity for great learnings is at our doorsteps. Since some trials might even contribute to paradigm shifts in our approach to T2D management, it is important to contextualize the results to the study populations in scope taking into account T2D disease duration, degree of vasculopathy, duration of the intervention, and the mode of action of the intervention (Figure 2). Only this will fully support and facilitate an optimized patient centered approach to T2D care and CV risk management[30].
Footnotes
P- Reviewer: Gómez-Sáez J, Pirola L S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: I am employed by Boehringer Ingelheim Norway KS.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 22, 2015
First decision: March 6, 2015
Article in press: July 23, 2015
References
- 1.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen OE. Cardiovascular disease and type 2 diabetes mellitus: a multifaceted symbiosis. Scand J Clin Lab Invest. 2007;67:786–800. doi: 10.1080/00365510701408558. [DOI] [PubMed] [Google Scholar]
- 3.Johansen OE, Birkeland KI, Orvik E, Flesland Ø, Wergeland R, Ueland T, Smith C, Endresen K, Aukrust P, Gullestad L. Inflammation and coronary angiography in asymptomatic type 2 diabetic subjects. Scand J Clin Lab Invest. 2007;67:306–316. doi: 10.1080/00365510601045088. [DOI] [PubMed] [Google Scholar]
- 4.Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 5.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370–379. doi: 10.1016/j.atherosclerosis.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Cox AJ, Hsu FC, Freedman BI, Herrington DM, Criqui MH, Carr JJ, Bowden DW. Contributors to mortality in high-risk diabetic patients in the Diabetes Heart Study. Diabetes Care. 2014;37:2798–2803. doi: 10.2337/dc14-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ofstad AP, Urheim S, Dalen H, Orvik E, Birkeland KI, Gullestad L, W Fagerland M, Johansen OE, Aakhus S. Identification of a definite diabetic cardiomyopathy in type 2 diabetes by comprehensive echocardiographic evaluation: A cross-sectional comparison with non-diabetic weight-matched controls. J Diabetes. 2014:Epub ahead of print. doi: 10.1111/1753-0407.12239. [DOI] [PubMed] [Google Scholar]
- 8.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30:292–299. doi: 10.2337/dc06-1747. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, Hegbrant J, Strippoli GF. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;9:CD004289. doi: 10.1002/14651858.CD004289.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 12.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 13.Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, Emanuele N, Kayshap M, Marks J, Mudaliar S, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58:2642–2648. doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 15.Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991;121:1244–1263. doi: 10.1016/0002-8703(91)90694-d. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310:2609–2610. doi: 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 18.Erdmann E, Dormandy J, Wilcox R, Massi-Benedetti M, Charbonnel B. PROactive 07: pioglitazone in the treatment of type 2 diabetes: results of the PROactive study. Vasc Health Risk Manag. 2007;3:355–370. [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 21.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 22.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Espeland MA, Bluhmki E, Mattheus M, Ryckaert B, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®) Diab Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) [Accessed 2015 Mar 21] Available from: https://www.clinicaltrials.gov/ct2/show/NCT01897532?term=carmelina&rank=1.
- 25.Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, Pocock S, Steinberg WM, Bergenstal RM, Mann JF, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823–30.e5. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Result presentation of evaluation of LIXisenatide in acute coronary syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. American Diabetes Association annual meeting. United States: Boston; 2015. [Google Scholar]
- 27.Exenatide study of cardiovascular event lowering trial (EXSCEL): A trial to evaluate cardiovascular outcomes after treatment with exenatide onceweekly in patients with type 2 diabetes mellitus. [Accessed 2015 Mar 21] Available from: https://www.clinicaltrials.gov/ct2/show/NCT01144338?term=exenatide cardiovascular&rank=1.
- 28.Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) [Accessed 2015 Mar 21] Available from: https://clinicaltrials.gov/ct2/show/NCT01394952?term=NCT01394952&rank=1. [DOI] [PubMed]
- 29.A study to evaluate cardiovascular outcomes in patients with type 2 diabetes treated with ITCA 650. [Accessed 2015 Mar 21] Available from: https://www.clinicaltrials.gov/ct2/show/NCT01455896?term=itca&rank=3.
- 30.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]


