Abstract
The hedgehog (Hh) signaling pathway is crucial for pattern formation during metazoan development. Although originially characterized in Drosophila, vertebrate homologs have been identified for several, but not all, genes in the pathway. Analysis of mutants in Drosophila demonstrates that Suppressor of fused [Su(fu)] interacts genetically with genes encoding proteins in the Hh signal transduction pathway, and its protein product physically interacts with two of the proteins in the Hh pathway. We report here the molecular cloning and characterization of chicken and mouse homologs of Su(fu). The chick and mouse proteins are 27% identical and 53% similar at the amino acid level to the Drosophila melanogaster and Drosophila virilis proteins. Vertebrate Su(fu) is widely expressed in the developing embryo with higher levels in tissues that are known to be patterned by Hh signaling. The chick Su(fu) protein can physically interact with factors known to function in Hh signal transduction including the Drosophila serine/threonine kinase, Fused, and the vertebrate transcriptional regulators Gli1 and Gli3. This interaction may be significant for transcriptional regulation, as recombinant Su(fu) enhances the ability of Gli proteins to bind DNA in electrophoretic mobility shift assays.
INTRODUCTION
The vertebrate hedgehog genes, Sonic (Shh), Indian (Ihh), and Desert hedgehog (Dhh), encode secreted signaling molecules that are critical for the patterning of many tissues during development and for regulation of cell growth and homeostasis in adult organisms. For example, during development Shh is expressed in the zone of polarizing activity (ZPA) of the limb bud, the notochord, the floor plate of the neural tube, regions of the ventral diencephalon, the branchial arches, the gut, and hair follicles in the skin (Echelard et al., 1993; Riddle et al., 1993; Bitgood and Mc-Mahon, 1995). Ihh is expressed predominantly in the proliferating and prehypertrophic chondrocytes of the developing bones (Bitgood and McMahon, 1995; Vortkamp et al., 1996) and in the endoderm of the midgut (Bitgood and McMahon, 1995). Dhh is expressed in the cranial ganglia and in the Sertoli cells of the testes (Bitgood et al., 1996). Although each of the vertebrate hedgehog genes is expressed in a unique pattern, all three appear to act through the same signal transduction pathway.
Despite the importance of this signal transduction pathway in vertebrates (reviewed in Hammerschmidt et al., 1997; Goodrich and Scott, 1998), most of our knowledge of the molecular mechanism of vertebrate hedgehog (Hh) signaling is inferred from studies in Drosophila (reviewed in Ingham, 1998). The Drosophila hh gene was originally identified in a screen for genetic mutations that affect patterning in larvae (Nüsslein-Volhard and Wieschaus, 1980). A number of other genes functionally related to the hh gene emerged from this screen. Several of these have been shown to encode members of the Hh signal transduction pathway including the following: the 12-transmembrane domain protein Patched (Ptc), which can physically interact with Hh protein (Hooper and Scott, 1989; Nakano et al., 1989; Marigo et al., 1996a; Stone et al., 1996); the 7-transmembrane domain protein Smoothened (Smo), which is a member of the serpentine G-protein-coupled receptor superfamily (Alcedo et al., 1996; van den Heuvel and Ingham, 1996); the serine/threonine (ser/thr) kinase Fused (Fu; Preat et al., 1990); the kinesin-like cytoplasmic protein Costal-2 (Cos2; Sisson et al., 1997); and the zinc-finger class transcriptional regulator Cubitus interruptus (Ci; Orenic et al., 1990). Of these, Smo, Fu, full-length Ci, and Hh itself are necessary to activate target gene transcription, while Ptc, Cos2, and a proteolytically processed form of Ci repress target gene transcription.
In Drosophila, the earliest detectable event occurring after Hh binding to Ptc is the phosphorylation of Fu by an unknown factor, presumably resulting in activation of Fu (Therond et al., 1996). Fu activity is critical for Hh signaling as null mutations of fu severely inhibit Hh signal transduction (Ingham, 1993; Preat et al., 1993). Based upon early genetic dissections (Preat et al., 1993) and recent biochemical studies (Robbins et al., 1997), Fu has been subdivided into two major domains. The amino-terminus (~292 amino acids) contains a domain with homology to all known ser/thr kinases, while the longer carboxy-terminus (~580 amino acids) has been labeled the extracatalytic or regulatory domain.
One key tool for the analysis of Fu function came with the identification of a semidominant suppressor of the fu mutant phenotype, Suppressor of fused [Su(fu)] (Preat, 1992). While Su(fu) encodes a protein with no obvious homologies to known proteins (Pham et al., 1995), it does contain a putative PEST sequence, a feature thought to be involved in rapid protein degradation (Rogers et al., 1986). Paradoxically, flies lacking all Su(fu) function are viable, fertile, and display only very subtle developmental defects (Preat, 1992; Ohlmeyer and Kalderon, 1998), yet are phenotypically resistant to loss of fu function (Preat, 1992). This suggests that the Su(fu) gene product functions as an inhibitor to Hh signaling and that the fu gene product is required to bypass the inhibition. Consistent with this interpretation, extra copies of the Su(fu) gene enhance the phenotype of a weak fu allele (Pham et al., 1995).
While many fu alleles are fully suppressed by loss of Su(fu) function, mutations that alter only the carboxy-terminal regulatory domain of Fu in a Su(fu) null background result in a phenotype similar to cos2, i.e., equivalent to eliminating transcriptional repression of hh target genes (Preat et al., 1993). Furthermore, Su(fu) acts as a dominant enhancer of cos2 mutations. For example, the cos23 allele is heterozygous viable, with no obvious phenotype, while the loss of Su(fu) function in a (cos23/+) background results in larval lethality (Preat et al., 1993).
In addition to the genetic interactions between Su(fu), fu, and cos2, biochemical studies have shown that Drosophila Su(fu) protein can physically interact with Fu and Ci proteins both in the yeast two-hybrid system and in the GST pull-down assay (Monnier et al., 1998). The cos2 gene encodes a kinesin-like protein that is found in a complex with Fu and Ci proteins in Drosophila embryonic extracts (Aza-Blanc et al., 1997; Robbins et al., 1997; Sisson et al., 1997). These genetic and biochemical observations for Su(fu), fu, and cos2 are consistent with the model that their three protein products interact to regulate signaling by Hh, with Fu acting as an activator of Hh target gene transcription and both Su(fu) and Cos2 acting as repressors.
In addition to vertebrate homologs described for Drosophila Hedgehog (Fietz et al., 1994), vertebrate homologs have also been identified for the Ptc and Smo receptors (Marigo et al., 1996a; Stone et al., 1996; Akiyama et al., 1997; Carpenter et al., 1998; Motoyama et al., 1998) and for the transcriptional regulator Ci (Kinzler et al., 1987; Ruppert et al., 1990; Aruga et al., 1994, 1996a, 1996b; Hui et al., 1994; Nagai et al., 1997; Nakata et al., 1998). However, vertebrate homologs of the intermediate cytoplasmic factors involved in the transduction of the Hh signal from the cell surface to the nucleus [Fu, Su(fu), and Cos2] have not been described.
Although the mechanism of Hh signal transduction in Drosophila and that in vertebrates appear to be very similar, it is clear that some differences do exist. In Drosophila both the positive regulation and the negative regulation of target genes by Hh appear to occur predominantly through the Ci protein, while in vertebrates, there appears to be a complex interplay between the various members of the Gli and Zic families of transcriptional regulators (Brewster et al., 1998; Brown et al., 1998). Since this pathway plays such critical roles in vertebrate embryonic development as well as in cellular homeostasis in the adult, it is important to more clearly understand the molecular nature of the vertebrate signaling pathway. To this end, we have cloned and completed an initial characterization of Su(fu) from the chicken and mouse.
MATERIALS AND METHODS
Cloning of mouse and chicken Su(fu)
Electronic screening of the NCBI expressed sequence tag (EST) database revealed a clone with homology to Drosophila Su(fu). The EST clone ID 513730 (GenBank Accession No. AA061391) was obtained from Genome Systems, Inc. This clone was originally isolated from a mouse testis cDNA library and contains an insert of approximately 950 bp. The chicken Su(fu) cDNA clone was obtained using the Su(fu) homologous region of the mouse EST clone to probe a cDNA library under high-stringency conditions (random primed Lambda ZAP II library from stage 18–24 chicken limb bud RNA). This screen yielded 87 positive clones of varying intensity. Of these positives, 10 were carried to plaque purity and analyzed. The longest clone isolated from the chicken library contained a 3.0-kb insert with a 1452-bp open reading frame, a 35-bp 5’UTR, and a 1.5-kb 3’UTR. The mouse partial cDNA was obtained by high-stringency screening of an 8.5-dpc whole mouse embryo λ-gt10 library (a gift from Dr. B. Hogan, Vanderbilt University, Nashville, TN) with the radiolabeled (Prime-It II, Stratagene) 420-bp EcoRI fragment from the mouse Su(fu) EST. The 5′ end of the 1.4-kb insert is equivalent to base 236 in the published sequence of the mouse Su(fu) EST. 5′ RACE (Gibco) was performed according to the manufacturers directions using 17.5-dpc mouse skin RNA. Primer sequences used for RACE were as follows: 5′-TCT-GGAGGAAAGTCACTACCCC-3′, 5′-TGCAGACACCAACAATC-TGG-3′, and 5′-TCCACTGTTGGGCTGAATG-3′. RACE products were cloned using the pCR2.1 TA cloning kit (Invitrogen). All sequence was obtained by ABI automated sequencing.
Expression analysis
For whole-mount in situ hybridizations of chicken embryos, we generated a probe for Su(fu) expression by subcloning an 800-bp 5′ fragment (from EcoRI in the multiple cloning sequence to the SalI site in the open reading frame) into pBS SK(−). The template for the probe was generated by amplifying the insert of this SalI 800-bp clone by PCR using M13 forward and reverse universal primers and the AdvantageTaq polymerase system (Stratagene) (PCR cycle consists of 94°C for 30 s, 52°C for 30 s, 72°C for 1 min ×20 cycles). The resulting PCR product was gel purified and 1.0 µg of template was transcribed in the presence of digoxigenin-11–UTP and T7 RNA polymerase to generate a digoxigenin antisense riboprobe. The integrity of riboprobes was checked by analysis on a 1.5% agarose gel in 0.5× Tris-Borate EDTA (TBE) buffer. The probe was added to hybridization buffer (hybridization buffer: 50% formamide, 5× SSC, pH 4.5, 50 µg/ml yeast tRNA, 1% SDS, 50 µg/ml heparin).
Chick embryos were obtained by incubating fertilized eggs from white Leghorn chickens (Spafas) at 37°C in a rotating egg incubator (Petersime). Eggs were removed from incubation at varying times and staged according to described criteria (Hamburger and Hamilton, 1992). Embryos were dissected, rinsed, fixed with 4% paraformaldehyde, and subsequently permeabilized with 10 µg/ml proteinase K for 15 min at room temperature, with the exception of stage 30–32 embryos, which were permeabilized with 60 µg/ml proteinase K for 15 min. Subsequent steps were performed according to a described protocol (Riddle et al., 1993).
For mouse whole-mount in situ hybridization, the 420-bp EcoRI fragment from the mouse EST was used in run-off transcription reactions to generate digoxigenin-11–UTP-labeled riboprobes (Boehringer Mannheim). Digoxigenin incorporation was tested by dot-blotting probes on nitrocellulose, probing with anti-digoxigenin alkaline phosphatase-coupled antibodies, and analyzing for alkaline phosphatase activity using NBT and BCIP. Probes were dissolved in 100 µl DEPC-treated water and added to hybridization buffer at 5 µl/ml.
Mouse embryos were obtained from B6/CBA F1 females mated to B6/CBA F1 males. Embryos were staged by defining noon on the day following copulation as 0.5 dpc. Mouse whole-mount in situ hybridizations were performed as described (Wilkinson, 1992). Proteinase K treatment was varied with age (15 min for 10.5 dpc, 7 min for 9.5 dpc, and 5 min for 8.75 dpc). After alkaline phosphatase activity was visualized with NBT and BCIP for 1 h, embryos were fixed overnight in 4% paraformaldehyde in PBS, dehydrated in methanol, and rehydrated before storing in PBT. For Northern analysis, a mouse multiple-tissue RNA blot (Clontech) was probed with the radiolabeled approximately 950-bp XhoI/PstI fragment from the mouse EST. As a loading control, the blot was subsequently probed with the human β-actin probe supplied by the manufacturer. Hybridization and wash conditions were as described by Clontech.
GST protein:protein interaction assays
The GST-Su(fu) construct was generated by using the bacterial expression vector pGEX-KG to fuse glutathione S-transferase in-frame with a 2.0-kb NcoI chick Su(fu) fragment extending from the initial methionine (the ATG in the 5’ NcoI site) to 600 bp past the stop codon. Transformed DH5α bacteria were grown to OD600nm = 1.0 and induced with 3 mM isopropyl β-d-thiogalactopyranside for 3 h. Expressing bacteria were pelleted and lysed by sonication in lysis buffer (0.4 M NaCl, 50 mM Tris, pH 7.6, 1 mM EDTA, 1 mM DTT, 10% glycerol, and the Boehringer Complete mini protease inhibitor cocktail tablets). Recombinant protein was bound to glutathione agarose beads in lysis buffer and washed. Protein purity was assessed on SDS-PAGE gels. By staining the SDS-PAGE gels with Coomassie brilliant blue it is estimated that all proteins used in these assays were greater than 90% pure. Prior to the interaction assay the protein bound glutathione beads were equilibrated with protein:protein interaction buffer (PPI: 20 mM Tris, pH 7.9, 200 mM NaCl, 1 mM EDTA, 4 mM MgCl2, 0.02% NP-40, 10% glycerol, protease inhibitors, and 1.0 mg/ml BSA). Constructs for producing labeled binding proteins were generated by standard subcloning techniques and in vitro translated in either reticulocyte lysate or wheat germ extract using the TnT-coupled transcription/ translation system (Promega) in the presence of Redivue [35S]methionine (Amersham). The r1-only, Zn-only, NcoI-Zn3′, and Drosophila Fu-reg (aa 306–805) translation constructs were generated by PCR using oligonucleotides that flank the domains of interest and adding an initial methionine and Kozak sequence to the 5′ end of each domain. All TnT translated proteins were analyzed for protein integrity by SDS–PAGE and normalized with other reactions based on TCA precipitable counts.
To test interactions, 50,000 TCA precipitable cpm of 35S-labeled protein were added to 50 µl of glutathione agarose beads (50:50 slurry in PPI buffer +BSA) bound to either an excess of control (GST) or experimental GST–Su(fu) fusion proteins. The reaction was incubated at 25°C for 20 min (vortexed every 5 min). Beads were washed four to six times with 1.0 ml of PPI buffer. Any 35S-labeled protein bound to the beads after the washes was eluted by boiling in 25 µl of 2× protein loading buffer (2% SDS, 10% glycerol, 100 mM Tris, pH 6.8, 0.7 M β-mercaptoethanol, 0.002% bromophenol blue) for 1–2 min. After boiling, 20 µl of denatured eluate was loaded onto a 12% SDS–PAGE polyacrylamide protein gel for analysis. For quantitative analysis, 2 µl (10%) of the eluate was spotted onto a glass fiber filter and counted in a liquid scintillation counter.
Two-hybrid analysis
Protein:protein interactions were detected using plasmids from the MATCHMAKER GAL4 System 2 (Clontech). pAS2-1 is the “bait” plasmid in this system and allows expression of the protein of interest as a fusion with the GAL4 DNA binding domain. pACT2 is the “prey” plasmid in this system and allows expression of the protein of interest as a fusion with the transcriptional activation domain of GAL4. The regulatory domain of Drosophila Fu (Fu-reg) (amino acids 306–805) containing an EcoRI linker was excised from LexA-Fu-reg (Monnier et al., 1998) and subcloned into the EcoRI site of pAS2-1. The 2-kb NcoI chick Su(fu) fragment extending from the initial methionine (the ATG in the 5′ NcoI site) to 600 bp past the stop codon was cloned into the NcoI site of pACT2. pVA3-1 and pTD1-1 (Clontech) were used as control plasmids and contain amino acids 72–390 of p53 in pAS2-1 and amino acids 84–708 of the SV40 large T antigen in pACT2, respectively.
Yeast were transfected by a modified version (Gietz et al., 1992) of the lithium acetate method as described in the Hybrid Hunter instruction manual (Invitrogen). Yeast strain PJ69-4A (James et al., 1996) was chosen to detect protein:protein interactions because it contains three reporters: ADE2 under control of the GAL2 promoter, HIS3 under control of the GAL1 promoter, and β-galactosidase under control of the GAL7 promoter. Yeast were cotransfected with the following pairs of plasmids: pVA3-1 plus pTD1-1; pAS2-1 Fu-reg plus pTD1-1; pAS2-1 Fu-reg plus pACT2 Su(fu); and pVA3-1 plus pACT2 Su(fu). After transformation, yeast were plated onto trp-, leu-deficient (trp-leu) medium for several days to select for the presence of the pAS2-1 and pACT2 derivatives. Selected yeast were replica-plated onto medium lacking ade, trp, and leu to test for reporter activation and allowed to grow for 5 days. Yeast grown on -trp, -leu were then assayed by the X-gal colony filter lift method for β-galactosidase activity as described by Clontech (Breeden and Nasmyth, 1985).
Band shift assays
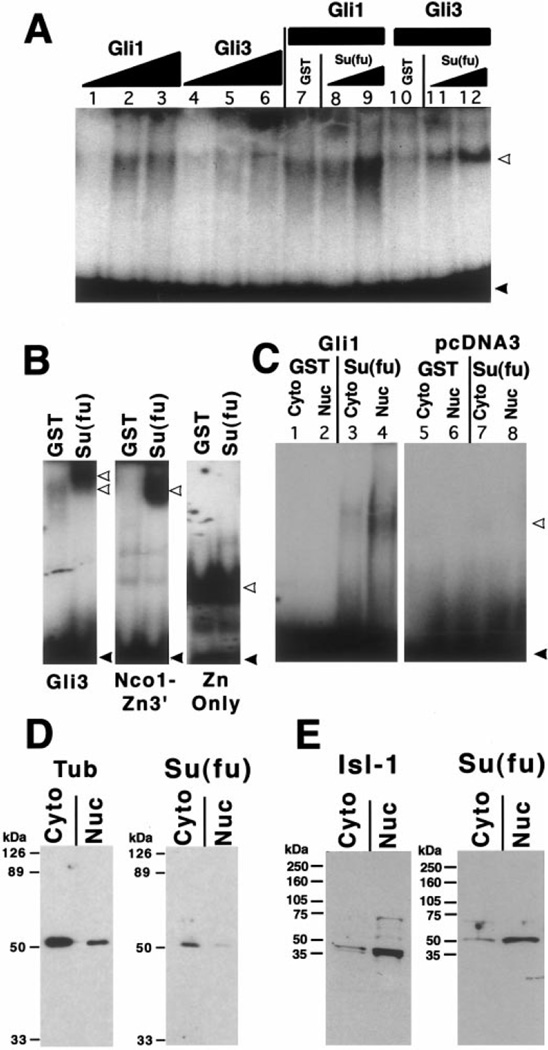
Two Gli DNA binding sites were synthesized that correspond to the natural human Gli-B and Gli-C binding sites (Kinzler and Vogelstein, 1990). Band shifts with the Gli-B binding site are shown, but indistinguishable results were obtained with the Gli-C site. The sequence of one strand of the Gli-B site is 5′AACCAGACGCGTGGACCACCCAAGACGAAATTCACACTC-3′ and the Gli-C binding site is 5′-AGGCACACAGATAGACCACCCAGCTTCAGGTGGGGGACC-3′. The residues in boldface type are the core binding sequence in each case. The two strands of the binding site were annealed, radiolabeled by phosphorylation with polynucleotide kinase in the presence of [γ-32P]ATP, and purified with a 6% nondenaturing polyacrylamide gel or over a Sephadex G25 spin column. Band shift binding buffer (1×) contained 10% glycerol, 10 mM Tris, pH 7.5, 50 mM KCl, 10 µM ZnCl2, 2 mM spermidine, 0.5 mg/ml ssDNA, 1 mM DTT, 0.5 mg/ml BSA. Five microliters of nuclear or cytoplasmic extracts or of nonradiolabeled, in vitro translated human Gli1 or Gli3 was added to the binding buffer together with 1–5 µl of either GST or GST–Su(fu) bacterially expressed fusion proteins (total volume of the binding reaction was 20 µl). The proteins were incubated at 37°C for 30 min prior to adding 50,000 cpm of the 32P labeled, purified, Gli-B binding site. After incubation for 20 min at room temperature with the binding site, complexes were analyzed by electrophoresis through a 4.5% nondenaturing polyacrylamide gel in 0.25× TBE buffer (Fig. 5A and 5C) or over a 6% nondenaturing polyacrylamide gel in 0.5× TBE buffer (Fig. 5B).
FIG. 5.
Su(fu) protein enhances the binding of Gli proteins to their consensus DNA binding site. (A) Gel mobility shift assay shows the binding of unlabeled in vitro translated human Gli1 or Gli3 proteins to a 32P-labeled Gli-B DNA binding site. Lanes 1–3 show the Gli binding activity in 1, 5, or 10 µl of pGli1 programmed reticulocyte lysate. Lanes 4–6 show the binding of 1, 5, or 10 µl of pGli3 programmed lysate. All reactions with less than 10 µl of programmed lysate were balanced up to 10 µl with unprogrammed lysate. Lanes 7–9 show the effects of adding GST alone (approx. 100 µg; lane 7) or of adding 1 or 5 µl of GST-Su(fu) fusion protein (approx. 2 or 10 µg; lanes 8–9, respectively) to 5 µl of pGli1 programmed lysate. Lanes 10–12 show the same reactions as in lanes 7–9 except that 5 µl of in vitro translated Gli3 was added to each binding reaction rather than Gli1. Gel shown in (A) is 4.5% acrylamide in 0.25× TBE running buffer. White arrows, probe protein complexes; black arrows, free probe. (B) Mapping of the DNA binding inhibitory domain on Gli3. Three versions of Gli3 were analyzed for the ability to bind DNA in the presence or in the absence of Su(fu) (Gli3, full length; NcoI-Zn3’, Zn finger + portion of the amino terminus; and Zn only, DNA binding domain only; see Fig. 3B for schematics). All lanes were run on the same gel and are shown correctly proportioned to one another, but the Zn only lanes shown were exposed for a shorter period for clarity. Band shift conditions in B were 6% acrylamide in 0.5× TBE running buffer. (C) Gel mobility shift assay showing the binding activity in 5 µl of cytoplasmic or nuclear extract from DF-1 cells transfected with vector alone or with an expression construct of human Gli1. Lanes 1–4 show the effect of 5.0 µl of either eluted GST alone (lanes 1, 2) or GST-Su(fu) (lanes 3, 4) on the formation of the binding complex in this assay. (D, E) Western blot analysis of cytoplasmic or nuclear protein extracts from Drosophila embryos (D) or stage 31 chick embryos (E) probed with an antibody raised against the amino-terminus of Drosophila Su(fu) (SF57). Blots shows a predominant band of approximately 50 kDa that is stronger in cytoplasmic fractions from Drosophila embryos (D) and in nuclear fractions of chicken embryos. Fractionation controls (anti-tubulin for Drosophila extracts and anti-Isl-1 for chick extracts) illustrate the degree of separation of cytoplasmic and nuclear proteins in the respective extracts.
Western blot analysis
Rat polyclonal antisera were prepared against Drosophila melanogaster Su(fu). Bases 22–1015 of D. melanogaster Su(fu) were cloned into the pATH10 vector (Rimm and Pollard, 1989) that results in the fusion of amino acids 8–338 of Su(fu) with Trp E. The Su(fu)–Trp E fusion was expressed in DH5α cells. The fusion protein was isolated from cell extracts by cutting a preparative SDS-PAGE gel and was used to immunize rats (Josman Labs). Nuclear and cytoplasmic extracts were made from either dechorionated Drosophila embryos or stage 30–31 chick embryonic tissue by dissociating embryos into single cells in a dounce homogenizer and extracting cytoplasmic and nuclear protein according to a previously described protocol (Schreiber et al., 1989). Extracts were denatured and separated over a 10 or a 4–12% gradient SDS-PAGE gel and blotted onto nitrocellulose membrane. Membranes were blocked and subsequently probed with the anti-Drosophila Su(fu) antibody described above (SF57) or the control antibodies monoclonal mouse anti-Isl-1 or anti-tubulin antibody. Antibodies were detected using HRP anti-rat or HRP anti-mouse antibodies and were developed with ECL detection reagents (Amersham).
RESULTS AND DISCUSSION
Isolation and Expression of Vertebrate Homologs of Drosophila Suppressor of Fused
Scanning of the NCBI EST database revealed a mouse sequence annotated as bearing homology to Drosophila Su(fu). A cDNA fragment containing the Su(fu) EST was used to screen a random-primed stage 18–22 chicken limb-bud library yielding a 3-kb clone that encodes a protein whose closest relative is Drosophila Su(fu). Because the mouse EST does not contain a complete cDNA [it does not contain an initiation ATG, a translational stop, or a poly(A) tract], a mouse 8.5-dpc whole embryo λgt10 library was screened with the Su(fu) homologous region of the EST. The mouse clone identified in this screen begins at base 236 of the published sequence of the EST. 5′ RACE was used to obtain 41 additional bases of sequence 5′ to the start of the EST, but failed to reach the ATG. The 3′ end of the cDNA does contain a translational stop followed by additional stop codons in every frame.
The chicken and mouse proteins are 27% identical and 53% similar at the amino acid level to both D. melanogaster and Drosophila virilis Su(fu) proteins (Fig. 1). The similarity is strongest between the amino-terminal regions of the predicted Drosophila and vertebrate proteins. The only notable feature of Drosophila Su(fu) is a possible PEST sequence that resides in the carboxy-terminal portion of the protein. PEST sequences have been identified in proteins such as Cactus and Notch and are believed to be involved in rapid protein degradation (Rogers et al., 1986). We analyzed the Drosophila and vertebrate Su(fu) proteins using the computer algorithm PESTfind (http://www.at.embnet.org/htbin/embnet/PESTfind) for potential PEST sequences. The algorithm searches proteins for potential PEST sequences and assigns a score of −50 to +50. A score greater than zero indicates a possible PEST sequence, while scores above 5 are considered more likely to function as bona fide PEST domains. Using PESTfind, amino acids 309–326 of D. melanogaster and the corresponding amino acids (313–330) of D. virilis Su(fu) both score +1.48. Interestingly, it is just prior to this potential PEST sequence that the Drosophila and vertebrate Su(fu) protein sequences diverge. When chick and mouse Su(fu) proteins were analyzed by PESTfind, amino acids 440–455 of cSu(fu) and the corresponding amino acids of mSu(fu) score +1.51 and +4.07, respectively. Although both vertebrate proteins contain possible PEST sequences, the actual sequences are totally different from the Drosophila Su(fu) putative PEST sequence and are located in a different region of the protein, so they do not appear to be derived from a common ancestral domain. We therefore question whether the fly or vertebrate PEST-like sequences will actually affect Su(fu) protein stability.
FIG. 1.
Sequence comparison between Drosophila and vertebrate Su(fu) proteins. Chicken and mouse amino acid sequences were aligned with the published Su(fu) sequences for D. melanogaster and D. virilis. Amino acids with identity to chick Su(fu) are boxed. Chick Su(fu) sequence extends from the initial methionine to the stop codon and contains an additional 15 amino acids at the amino-terminus compared to the Drosophila proteins. Numbers on the right indicate the position of the last amino acid in that row with the exception of mouse Su(fu), where we have not identified the initial methionine. For mouse Su(fu) position 1 is the most amino-terminal amino acid. The first shaded box illustrates the previously identified PEST sequences in Drosophila, and the second shaded box illustrates the amino acid sequences in chick and mouse Su(fu) that scored +1.51 and +4.07, respectively, using the PESTfind algorithm (http://www.at.embnet.org/htbin/embnet/PESTfind). The PEST sequences in Drosophila share no similarity to those in the vertebrate proteins.
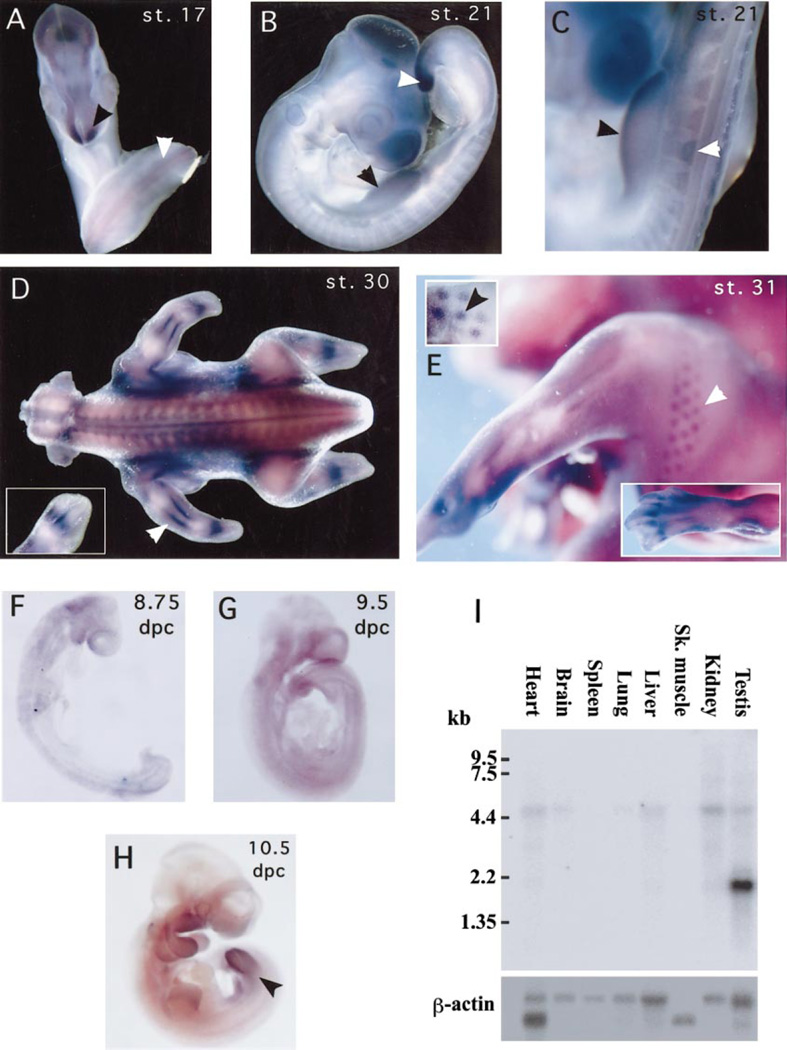
To analyze Su(fu) expression in vertebrates, probes were hybridized in situ to chicken embryos of various stages. Although low levels of Su(fu) message appear to be in virtually all tissues, there are notable locations where Su(fu) appears to be expressed at higher levels. At stage 17 (29–32 somites), there is slightly higher expression in the neural tube and a significantly higher abundance of mRNA in the anterior tip of the developing forebrain (Fig. 2A). By stage 21, slightly higher expression can be seen in the limb buds, somites, and tail bud (Figs. 2B and 2C). By stage 30 we observe the most dramatic Su(fu) expression in a pattern similar to the induction of Ptch1 in the perichondrium of the developing bones, adjacent to the domain where Ihh is expressed in the prehypertrophic chondrocytes (Vortkamp et al., 1996). Additionally, while at stage 31 Su(fu) message appears to be highly abundant in many tissues, we can clearly see higher expression of Su(fu) in the developing feather buds (Fig. 2E). This is reminiscent of the previously described induction of Ptch1 by Shh in the feather buds (Marigo and Tabin, 1996).
FIG. 2.
Expression patterns of chick and mouse Su(fu). Su(fu) expression in the chick (A, B, C, D, E) and in mouse (F, G, H) embryos and in mouse adult tissues (I). (A) Hamburger–Hamilton stage 17 chick embryo showing Su(fu) expression at low levels in the neural tube (white arrow) with higher expression in the anterior tip of the neural epithelium (black arrow). (B) Stage 21 embryo showing slightly higher Su(fu) expression in the developing limb buds (black arrow) and in the tail bud (white arrow). (C) Higher magnification of limb bud (black arrow) and somite (white arrow) expression in stage 21 embryos. (D) Stage 30 chick embryo showing high levels of Su(fu) expression in the regions of the developing perichondrium. White arrow shows staining in the developing stylopod (forearm) in the region of the perichondrium and inset shows staining in the developing autopod (wrist and hand). (E) Stage 31 embryo showing higher expression in the developing feather buds (white arrow). The inset (upper left) shows staining in the feather buds in epithelium that has been dissected away from muscle tissue to clarify the specificity of the staining. Second inset (lower right) shows strong staining in the developing bones of the hind limb autopod. (F) 8.75-dpc mouse embryo showing diffuse, ubiquitous Su(fu) mRNA (G) 9.5-dpc mouse embryo showing the persisting ubiquitous expression of Su(fu). (H) 10.5-dpc mouse embryo shows slightly higher Su(fu) expression in limbs (I) Multiple mouse tissue Northern hybridization with mouse Su(fu) probe. One large transcript (5.4 kb) is seen at low levels in many adult tissues including heart, brain, liver, kidney, testis, and to a lesser extent, in lung. A smaller transcript (2.0 kb) is highly expressed in the testis.
Although the higher expression of Su(fu) in the developing bones and feather buds appears similar to the induction of Ptch1 by Ihh and Shh, respectively, Su(fu) is not ex- pressed at an elevated level in other cells that receive Hh signals. For example, the expression of Su(fu) is no higher in the posterior limb bud mesenchyme, which is exposed to endogenous Shh, than it is in the anterior mesenchyme. Moreover, Shh-soaked beads are unable to induce higher expression of Su(fu) in the anterior wing bud (data not shown). In the early developing mouse, Su(fu) mRNA is also widespread. The mRNA is ubiquitous at 8.75 dpc (post-turning) and 9.5 dpc (Figs. 2F and 2G). However, as in the chick, by 10.5 dpc, both the neural epithelium and the limb buds express higher levels of Su(fu) (Fig. 2H).
The widespread distribution of Su(fu) mRNA in the developing vertebrate is not surprising, because in Drosophila, Su(fu)mRNA is ubiquitous throughout embryonic development (Pham et al., 1995). Additionally, cos2 and fu mRNA expression patterns also contrast with that of hh itself, which is spatially controlled, and ptc, which is transcriptionally induced in response to Hh signaling (Hidalgo and Ingham, 1990; Lee et al., 1992; Taylor et al., 1993; Tabata and Kornberg, 1994; Sisson et al., 1997).
To determine whether Su(fu) mRNA is produced in adult tissues, a mouse multiple-tissue RNA blot was probed with the mouse Su(fu) EST clone. Identical results were obtained by probing the blot with the 1.2-kb EcoRI fragment from the mouse Su(fu) clone identified in the λ library (data not shown). Two transcripts were detected. The larger, 5.4-kb transcript was present at low levels in testis, kidney, heart, liver, and brain and at very low levels in lung (Fig. 2I). The smaller, more abundant 2.0-kb transcript was testis specific. It is possible that the high Su(fu) expression in the testis relates to the presence of Dhh signaling in Sertoli/Leydig cell communication (Bitgood et al., 1996; Carpenter et al., 1998). However, at the present time it is not known how the testis-specific transcript differs from the larger transcript. Expression of Su(fu) in adult tissue is not unexpected, as Ptch1 mRNA has been detected (at least at low levels) in all adult tissues examined (Goodrich et al., 1996). It is also known that vertebrate Hh signaling plays a role in cellular homeostasis, as constitutive activation of vertebrate Hh signaling in the adult can lead to certain types of tumors (Chidambaram et al., 1996; Hahn et al., 1996; Unden et al., 1996; Dahmane et al., 1997; Fan et al., 1997; Oro et al., 1997; Raffel et al., 1997; Vorechovsky et al., 1997; Xie et al., 1998). Therefore, it is possible that Su(fu) also plays a role in cellular homeostasis in the adult.
Protein:Protein Interactions between Su(fu) and Gli1, Gli3, and Fu
If the vertebrate and Drosophila Su(fu) proteins play similar roles in regulating Hh signal transduction, we would expect them to exhibit similar biochemical interactions. Insight into the Drosophila Hh signaling pathway came with the observation that the Fu, Ci, and Cos2 proteins exist in a large cytoplasmic complex that is bound to microtubules in the absence of Hh signaling (Robbins et al., 1997; Sisson et al., 1997). Upon binding of Hh to Ptc, this cytoplasmic complex is released from the microtubules but appears to remain otherwise intact. Although it has not been shown biochemically that Su(fu) is present in such complexes, it has been shown that Su(fu) interacts physically with both the carboxy-terminal regulatory domain of Fu and the amino-terminus of Ci (Monnier et al., 1998). To determine whether the vertebrate Su(fu) protein exhibits similar biochemical interactions we investigated the ability of chick Su(fu) to physically interact with the vertebrate homologs of Ci and with Drosophila Fu, since a vertebrate homolog of Fu has not yet been identified.
The closest vertebrate relatives of Ci are the Gli family of Zn finger transcriptional regulators. Gli1 was originally identified based on its amplification in glioblastoma cell lines (Kinzler et al., 1987). Gli2 and Gli3 genes were subsequently isolated by their homology to human Gli1 (Ruppert et al., 1990; Grindley et al., 1997; Hughes et al., 1997; Mo et al., 1997). In the chick, Gli1 expression is induced in the anterior limb bud mesenchyme in response to Shh-soaked beads while Gli3 is repressed (Marigo et al., 1996b), indicating that Gli1 may be an activator of Shh target genes while Gli3 may repress genes in the absence of Shh. This hypothesis is further supported by mutations of Gli3 in mice, which result in a preaxial polydactyly due to ectopic Shh expression in the anterior limb bud (Hui and Joyner, 1993; Buscher et al., 1997). However, it has recently been reported that Gli3 is able to bind the transcriptional coactivator CBP and that Gli3 may function as both a positive and a negative regulator of transcription (Dai et al., 1999).
The notion that Gli proteins may both positively and negatively regulate Shh target genes is supported by studies in Drosophila. In the absence of Hh signaling, Ci protein is proteolytically processed into a repressor form (Ci75) that contains only the amino-terminal and DNA binding domains (Aza-Blanc et al., 1997). Although it has been recently reported that in mouse embryos Gli3, and not Gli1, is proteolytically processed (Dai et al., 1999), it is not clear whether this processing results in a conversion from an activator to a repressor form of Gli3 or whether each Gli factor exerts a distinct positive or negative influence on the signaling pathway. Based on the similarity of Gli proteins to Ci, it seems likely that Gli proteins will exert both positive and negative control over gene expression by some mechanism. Recent genetic data indicate that Zic2, a Zn finger transcriptional regulator that is more distantly related to Ci than the Gli genes, may also play an important role in Shh signal transduction (Brewster et al., 1998; Brown et al., 1998).
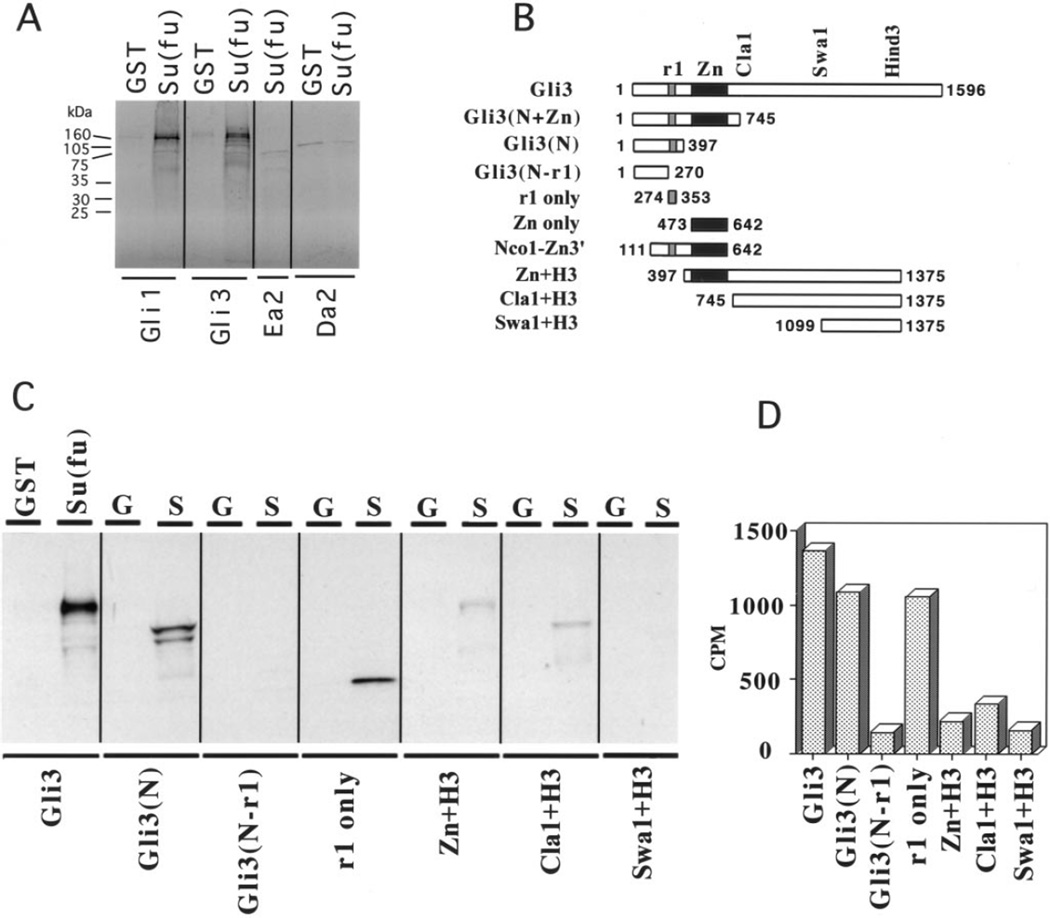
To investigate the ability of Su(fu) to interact directly with Gli proteins, we performed in vitro GST protein: protein interaction assays using a chick GST–Su(fu) fusion and 35S-labeled human Gli1 and Gli3. Both Gli1 and Gli3 interact with Su(fu) by this assay (Fig. 3A). To map the domain of interaction on Gli3 we made a deletion series of human Gli3 protein for use in the GST pull-down assay (Fig. 3B). Gli1 and Gli3 share seven regions of similarity to each other (Ruppert et al., 1990). Among these domains, region 2 (the Zn finger DNA binding domain) and region 1 (r1), have the highest similarity to the equivalent regions in the Drosophila Ci protein. Since Drosophila Su(fu) interacts with the amino-terminal part of Ci (without the Zn finger or the carboxy-terminus), we predicted that Su(fu) would interact specifically with the r1 domain in Gli1 and Gli3. Indeed, while Su(fu) interacts with either full-length Gli3 or Gli3 lacking either the carboxy-terminus or the Zn finger domain, the interaction is lost when the protein is truncated just aminoterminal to the Gli3 r1 domain. A small radiolabeled peptide containing only the Gli3 r1 domain is also sufficient for the interaction with Su(fu). Gli3 deletion proteins containing only the sequence carboxy-terminal to the r1 domain interact only very weakly, the signal beng slightly above background (Figs. 3B–3D). Thus, r1, a domain that is conserved between Drosophila Ci and all known human Gli proteins, appears to be involved in protein: protein interactions with Su(fu) proteins.
FIG. 3.
Su(fu) interacts with the r1 domain of the Gli proteins. (A) GST protein:protein interaction assay showing interactions between GST–Su(fu) bound to glutathione agarose beads and 35S-labeled in vitro translated proteins. Labels at the top of the lanes designate the glutathione agarose bound protein used in the assay: GST, GST alone; Su(fu), GST–Su(fu). Labels at the bottom of each lane indicate the in vitro translated radiolabeled protein. Radiolabeled Gli1 and Gli3 that remained bound to GST–Su(fu) bait are equivalent to 10% input (data not shown). Ea2 and Da2 are 35S-labeled negative controls. (B) Illustration of all Gli3 deletion constructs used in this figure and in Fig. 5. r1 is one domain of homology between all Gli proteins and Ci; Zn is the Zn finger DNA binding domain; and Cla1, Swa1, and Hind3 mark the positions of restriction sites in human Gli3. Numbers flanking the schematics indicate the amino acid boundaries of each construct. (C) GST protein:protein interaction assays mapping the Su(fu) interaction domain on Gli3 to the r1 domain. The GST fusion bait construct used in each assay is indicated at the top of each lane. GST or G, GST alone; Su(fu) or S, GST–Su(fu) fusion protein. 35S-labeled prey constructs are labeled at the bottom of each lane and are schematized in B. (D) Quantitation of the interactions between GST–Su(fu) and each 35S-labeled prey construct. The graph shows, on the Y-axis, 10% of the total cpm specifically binding to the GST–Su(fu) bait minus the cpm that nonspecifically bound to the GST-only control bait.
In light of the regulation of Ci by proteolytic processing, it has been proposed that some of the Gli proteins, specifically Gli3, may be similarly processed (Dai et al., 1999). If, as in Drosophila, amino-terminal processed forms of the Gli proteins act as repressors, this could explain the dominant phenotypes that result from mutations producing truncated forms of Gli proteins. Three dominant human mutations of Gli3 result in truncations of the protein, causing varying degrees of polydactyly depending on the location of the mutation in heterozygous individuals (Vortkamp et al., 1991; Radhakrishna et al., 1997). Two of the mutations retain both the r1 domain and the Zn finger domain, while one of the mutations, identified in patients with Greig cephalopolysyndactyly syndrome (GCPS), results in a protein that contains only the amino-terminus without the Zn finger. In the GCPS mutant the only defined domain remaining in the Gli3 protein is r1. Although it is quite possible that other factors interact with the amino-terminus of Gli3 and perhaps even compete with Su(fu) for interaction with r1, it is tempting to speculate that the interaction between the truncated Gli3 (lacking a DNA binding domain) and Su(fu) may result in a dominant abnormal regulation of the Shh signaling pathway in these individuals. While we have not tested the ability of the Zic family of transcriptional regulators to interact with Su(fu), they do not contain any region similar to r1.
Drosophila Su(fu) was originally identified in a screen for suppressors of the fused phenotype and has since been shown to interact physically with the Fu regulatory domain (Preat, 1992; Preat et al, 1993; Alves et al., 1998; Monnier et al., 1998; Ohlmeyer and Kalderon, 1998). As a vertebrate homolog of fused has yet to be identified, we investigated the ability of chick Su(fu) to interact with the regulatory domain (amino acids 306–805) of Drosophila Fu (Fu-reg) in the both the yeast two-hybrid system and the GST pulldown assay. Yeast strain PJ69-4A, a strain with two auxotrophic nutritional reporter genes, ADE and HIS, was used to test the Su(fu):Fu-reg interaction. In addition, the strain contains a β-galactosidase reporter. In the absence of interacting proteins, the ADE reporter in this strain is fully repressed. Growth on ADE-deficient medium is readily detectable when interacting proteins are present (James et al., 1996). We chose to assay for ADE and β-galactosidase reporter activity, and not for HIS function, due to the intrinsic low level of background activity of the HIS reporter.
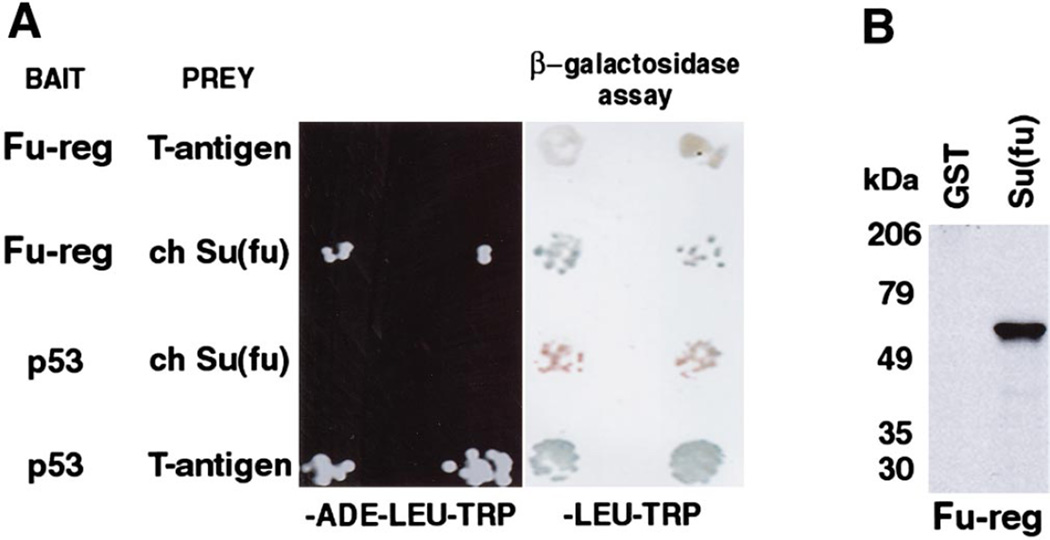
The Fu-reg–DNA binding domain fusion was tested for its ability to interact with Su(fu)–activation domain fusions. The two proteins do interact, based on colony growth that is dependent upon transcription of the ADE reporter. In contrast, Fu-reg–DNA binding domain fusion did not interact with the control prey, the SV40 large T antigen– activation domain fusion. The Su(fu)–activation domain fusion did not interact with a control bait, the p53–DNA binding domain fusion. However, the well-documented interaction between SV40 large T antigen and p53 was readily detected, indicating that both proteins are expressed in yeast. Only the Fu-reg:Su(fu) and T antigen:p53 combinations were able to activate transcription of the β-galactosidase reporter, as detected by the X-gal filter lift assay (Fig. 4A).
FIG. 4.
Su(fu) interacts with the regulatory domain of Drosophila Fused in the yeast two-hybrid system. (A) Strain PJ 69-4A yeast were cotransformed with the indicated bait (in pAS2-1) and prey (in pACT2) constructs. Bait and prey constructs were maintained in yeast by selection on trp- and leu-deficient media (trp and -leu), respectively. After transformation, yeast were grown on -ade-leu-trp medium (left) to assay for ADE reporter activity and on -leu-trp medium (right) to assay for β-galactosidase activity. Drosophila Fu regulatory domain (Fu-reg) and chicken Su(fu) are able to interact and activate reporter gene transcription as demonstrated by growth on medium lacking ADE and by β-galactosidase activity. This interaction is specific as Fu-reg does not interact with the SV40 large T antigen and Su(fu) does not interact with p53. However, the well-documented interaction between p53 and the SV40 large T antigen is readily detected. Two representative transformants for each pair of bait and prey plasmids are shown. (B) GST-protein:protein interaction assay showing the direct interaction between chick Su(fu) and Drosophila Fu-reg. GST-Su(fu) but not GST alone binds 35S-labeled protein in vitro translated Fu-reg. Size of molecular weight standards are indicated in kDa. In vitro translated Fu-reg is predicted to be 55 kDa.
To eliminate the possibility that a yeast protein mediates the detected Fu-reg:Su(fu) interaction, in vitro GST protein: protein interaction assays were performed. Fu-reg was in vitro translated in the presence of 35S-labeled methionine and incubated with either GST alone as a control or GST–Su(fu). Only GST–Su(fu) was able to interact with Fu-reg in this assay (Fig. 4B), confirming the yeast two-hybrid data.
The ability of chick Su(fu) to interact with Drosophila Fu-reg indicates that the Fu-interacting domain has been functionally conserved over the wide evolutionary distance between Drosophila and vertebrates. Su(fu) can also interact with Gli1 and Gli3, so in vertebrates, as in flies, Su(fu) may link the cytoplasmic transducers of Hh signaling to nuclear transcription factors.
Localization of Su(fu) Protein
The Drosophila full-length Ci protein has been observed only in the cytoplasm, while a truncated repressor form of Ci is mostly nuclear. In vertebrates the Gli1 protein seems to be mostly nuclear, while there is evidence that Gli3 is mostly cytoplasmic (Kinzler and Vogelstein, 1990; Lee et al., 1997). Since the Gli proteins, like Ci, seem to reside in different cellular compartments, we investigated Su(fu) localization. The high degree of similarity in the amino-terminal regions of vertebrate and Drosophila Su(fu) proteins allows an antibody raised against the amino-terminus of Drosophila Su(fu) (SF57) to cross-react with chick Su(fu). The antibody recognizes both bacterially expressed and in vitro translated chicken Su(fu) (data not shown). We made cytoplasmic and nuclear extracts of stage 31 chicken embryonic tissue and Drosophila embryos. To test the separation between nuclear and cytoplasmic proteins the extracts were probed with control antibodies that recognize known nuclear antigens (Isl-1 and Pax6) for the chick extracts or for known cytoplasmic antigens (tubulin and kinesin heavy chain) for Drosophila embryo extracts. These control antibodies recognize appropriately sized bands predominantly in the nuclear and cytoplasmic extracts, respectively (Figs. 5D and 5E; and data not shown). The Su(fu) antibody recognizes one major band of approximately 50 kDa [the predicted size of Su(fu)] in both Drosophila and chick extracts. While in Drosophila this protein band is predominantly cytoplasmic, in chicken it is much more intense in the nuclear extract than in the cytoplasmic extract (Figs. 5D and 5E). While we cannot rule out the possibility that the SF57 antibody is cross-reacting in the chicken extracts with a non-Su(fu) 50-kDa protein in the nuclear extract, these results at face value show a contrast between the cytoplasmic Drosophila protein and the nuclear chick protein.
Su(fu) Protein Enhances the Ability of Gli and Gli3 to Bind Target DNA Sequences
Sequences within the human genome selected by their ability to bind Gli1 protein contain the core sequence 5′-GACCACCCA-3′ (Kinzler and Vogelstein, 1990). Remarkably, this consensus DNA sequence is perfectly conserved between human Gli and Drosophila Ci binding sites. The promoter of the Drosophila ptc gene contains three exact matches of the Gli binding site that are required for activation of ptc by hh (Alexandre et al., 1996). Since Su(fu) may act within the Shh signal transduction pathway by interacting with Gli1 and Gli3, and since chick Su(fu) protein appears to be in the nucleus where Gli proteins ultimately affect gene transcription, we tested whether Su(fu) can affect the binding of DNA by Gli proteins. We synthesized a site corresponding to a described natural Gli binding site within the human genome referred to as Gli-B (Kinzler and Vogelstein, 1990). This site contains the core 9-bp sequence as well as flanking sequence (see Materials and Methods for full sequence).
In vitro translated human Gli1 and Gli3 are both capable of binding to the Gli binding sites on their own. Strikingly, addition of bacterially expressed chicken Su(fu) protein results in a dose-dependent enhancement of the binding of both Gli1 and Gli3 (Fig. 5A). To further analyze this enhancement of DNA binding, we tested the ability of Su(fu) to enhance the binding of truncated Gli3 proteins. The Zn finger domain alone binds strongly to DNA and is unaffected by Su(fu). When the Zn finger domain is tethered to the r1-containing amino-terminal region (Gli3 NcoI-Zn3′) it does not bind efficiently to DNA until Su(fu) is added, behaving similarly to full-length Gli3 (Fig. 5B). Thus, the amino-terminus contains a domain that inhibits DNA binding in this assay and the effect of this domain can be overcome by the addition of Su(fu). Under the gel shift conditions used in Fig. 5A (4.5% acrylamide, 0.25× TBE) we never see a change in migration of the Gli1:DNA or Gli3:DNA band caused by the addition of Su(fu). However, under the conditions used in the mapping of the Su(fu)-affected domain in Fig. 5B (6% acrylamide, 0.5× TBE), we do see a slower mobility when Su(fu) is added to the gel shift reaction. These data, combined with the protein:protein interaction between Gli3 and Su(fu), suggest that Gli proteins and Su(fu) might bind together on DNA. In some, though not all, cases where proteins bind together on DNA the identities of the proteins in the complex can be verified by “supershifting” with specific antibodies. Attempts to supershift the Su(fu) enhanced band with anti-Su(fu) or anti-GST antibodies were unsuccessful.
To rule out the possibility that the enhancement of Gli binding is an artifactual effect of Su(fu) in the reticulocyte lysate of the in vitro translation mixture, we transfected a chicken mesodermal cell line (DF-1) with a plasmid expressing Gli1 protein and isolated both cytoplasmic and nuclear extracts from these cells. Nuclear extracts from these cells bind only very weakly to the Gli-B binding site. The addition of GST–Su(fu) fusion protein again causes a dramatic increase in the Gli binding activity of both cytoplasmic and nuclear extracts (Fig. 5C). Cells transfected with the pcDNA3 vector alone show no Gli-B DNA binding regardless of the presence of Su(fu) (Fig. 5C). Su(fu) alone displays no binding to the Gli site in any of these assays (data not shown) and the addition of GST protein without Su(fu) fails to alter Gli binding activity.
A recent paper proposed that in Drosophila full-length Ci protein must undergo an activation event to convert it into a labile transcriptional activator and that Su(fu) stabilizes full-length Ci in its preactivated state (Ohlmeyer and Kalderon, 1998). If Gli proteins are also stabilized by vertebrate Su(fu), then one interpretation of our band shift results is that labile Gli proteins in both the cellular extract and the reticulocyte lysate are rapidly degraded and Su(fu) results in enhanced binding due to Gli stabilization. However, we have followed the integrity of in vitro translated radiolabeled Gli proteins in the presence and in the absence of Su(fu) and see no significant effect of Su(fu) on the stability of Gli1 or Gli3 in the reticulocyte lysate environment (data not shown).
Either of two models could explain the observed effect of Su(fu) protein on Gli DNA binding. The r1 domain may inhibit DNA binding directly, and the binding of Su(fu) to this domain could release the inhibition by inducing a conformational change. This simple model is inconsistent with the role of Su(fu) in Drosophila as inferred from genetic analysis. Another explanation is that Su(fu) is naturally part of a ubiquitous repressor complex to which Gli proteins are tethered. The large excess of Su(fu) protein added to these band shift assays allows the possibility that exogenous Su(fu) may disrupt a naturally occurring complex within the protein mixtures. The disruption of this protein complex would then free Gli proteins for DNA binding. Since we cannot confirm that the slower migrating complexes in Fig. 5B actually contain Su(fu), Gli proteins may be reassociating with separate complexes that are permissive for DNA binding. While the effect of Su(fu) on Gli binding activity in the band shift experiments reflects a functional interaction between Su(fu) and Gli proteins, the biological significance of this interaction must be tested in vivo.
In summary, the two vertebrate homologs of the Drosophila gene Su(fu) are predicted to encode proteins highly similar to the Drosophila Su(fu) protein with the notable exception of the previously described PEST sequence. As in Drosophila, the vertebrate Su(fu) gene is widely expressed and does not appear to be induced in response to Hh signaling. Vertebrate Su(fu) seems to have retained some of the protein:protein interaction properties of its Drosophila counterpart, such as the interaction with Gli proteins in vitro and with Drosophila Fu protein both in yeast and in vitro. Drosophila Su(fu) protein appears to be predominantly cytoplasmic, while in chicken embryonic extracts an anti-Drosophila-Su(fu) antibody recognizes a single band that is enriched in nuclear fractions. Finally, the enhancement of the binding of Gli proteins to DNA by Su(fu) in electrophoretic mobility shift assays suggests that Su(fu) proteins may regulate Gli activity.
ACKNOWLEDGMENTS
We thank L. Goodrich, K. Higgins, L. Milenkovic, J. Jin, and J. Hartford for help with embryo collections, sequencing and in situ procedures; K. Suyama for the anti-Su(fu) antibody and for help with Western blotting; K. Vogan for band shift help; and members of the Cepko, Scott, and Tabin labs for critically reading the manuscript. L.S.C. also thanks the Baxter foundation. L.S.C. is supported by a Howard Hughes Medical Institute predoctoral fellowship. R.V.P. is supported by a postdoctoral fellowship from the NIH. M.P.S. is an investigator with the Howard Hughes Medical Institute. C.J.T. is funded by a grant from the NIH.
REFERENCES
- Akiyama H, Shigeno C, Hiraki Y, Shukunami C, Kohno H, Akagi M, Konishi J, Nakamura T. Cloning of a mouse smoothened cDNA and expression patterns of hedgehog signalling molecules during chondrogenesis and cartilage differentiation in clonal mouse EC cells, ATDC5. Biochem. Biophys. Res. Commun. 1997;235:142–147. doi: 10.1006/bbrc.1997.6750. [DOI] [PubMed] [Google Scholar]
- Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–232. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- Alves G, Limbourg-Bouchon B, Tricoire H, Brissard-Zahraoui J, Lamour-Isnard C, Busson D. Modulation of hedgehog target gene expression by the fused serine-threonine kinase in wing imaginal discs. Mech. Dev. 1998;78:17–31. doi: 10.1016/s0925-4773(98)00130-0. [DOI] [PubMed] [Google Scholar]
- Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired. J. Biol. Chem. 1996a;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- Aruga J, Yozu A, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. Identification and characterization of Zic4, a new member of the mouse Zic gene family. Gene. 1996b;172:291–294. doi: 10.1016/0378-1119(96)00111-4. [DOI] [PubMed] [Google Scholar]
- Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by desert hedgehog regulates the male germline. Curr. Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Breeden L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harbor Symp. Quant. Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- Brewster R, Lee J, Ruiz i Altaba A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature. 1998;393:579–583. doi: 10.1038/31242. [DOI] [PubMed] [Google Scholar]
- Brown SA, Warburton D, Brown LY, Yu CY, Roeder ER, Stengel-Rutkowski S, Hennekam RC, Muenke M. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat. Genet. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 1997;62:175–182. doi: 10.1016/s0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc. Natl. Acad. Sci. USA. 1998;95:13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram A, Goldstein AM, Gailani MR, Gerrard B, Bale SJ, DiGiovanna JJ, Bale AE, Dean M. Mutations in the human homologue of the Drosophila patched gene in Caucasian and African-American nevoid basal cell carcinoma syndrome patients. Cancer Res. 1996;56:4599–4601. [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [Published erratum appears in Nature 1997 Dec 4;390(6659): 536]. [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic hedgehog-induced activation of the gli1 promoter is mediated by GLI3. J. Biol. Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nat. Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- Fietz MJ, Concordet JP, Barbosa R, Johnson R, Krauss S, McMahon AP, Tabin C, Ingham PW. The hedgehog gene family in Drosophila and vertebrate development. Dev. 1994;(Suppl):43–51. [PubMed] [Google Scholar]
- Gietz D, St. Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev. Biol. 1997;188:337–348. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13:14–21. doi: 10.1016/s0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Ingham P. Cell patterning in the Drosophila segment: Spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989;59:751–765. doi: 10.1016/0092-8674(89)90021-4. [DOI] [PubMed] [Google Scholar]
- Hughes DC, Allen J, Morley G, Sutherland K, Ahmed W, Prosser J, Lettice L, Allan G, Mattei MG, Farrall M, Hill RE. Cloning and sequencing of the mouse Gli2 gene: Localization to the Dominant hemimelia critical region. Genomics. 1997;39:205–215. doi: 10.1006/geno.1996.4468. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: The extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature. 1993;366:560–562. doi: 10.1038/366560a0. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Transducing hedgehog: The story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law MLSJOB, Wong AJ, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol. Cell. Biol. 1990;10:634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the hedgehog receptor. Nature. 1996a;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, Tabin CJ. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev. Biol. 1996b;180:273–283. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc. Natl. Acad. Sci. USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Monnier V, Dussillol F, Alves G, Lamour-Isnard C, Plessis A. Suppressor of fused links fused and cubitus interruptus on the hedgehog signalling pathway. Curr. Biol. 1998;8:583–586. doi: 10.1016/s0960-9822(98)70227-1. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Takabatake T, Takeshima K, Hui C. Ptch2, a second mouse patched gene is co-expressed with sonic hedgehog. Nat. Genet. 1998;18:104–106. doi: 10.1038/ng0298-104. [DOI] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Dev. Biol. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle JR, Ingham PW. A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature. 1989;341:508–513. doi: 10.1038/341508a0. [DOI] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus zic family and its role in neural and neural crest development. Mech. Dev. 1998;75:43–51. doi: 10.1016/s0925-4773(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila . Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer JT, Kalderon D. Hedgehog stimulates maturation of cubitus interruptus into a labile transcriptional activator. Nature. 1998;396:749–753. doi: 10.1038/25533. [DOI] [PubMed] [Google Scholar]
- Orenic TV, Slusarski DC, Kroll KL, Holmgren RA. Cloning and characterization of the segment polarity gene cubitus interruptus dominant of Drosophila . Genes Dev. 1990;4:1053–1067. doi: 10.1101/gad.4.6.1053. [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- Pham A, Therond P, Alves G, Tournier FB, Busson D, Lamour-Isnard C, Bouchon BL, Preat T, Tricoire H. The suppressor of fused gene encodes a novel PEST protein involved in Drosophila segment polarity establishment. Genetics. 1995;140:587–598. doi: 10.1093/genetics/140.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preat T. Characterization of suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster . Genetics. 1992;132:725–736. doi: 10.1093/genetics/132.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preat T, Therond P, Lamour-Isnard C, Limbourg-Bouchon B, Tricoire H, Erk I, Mariol MC, Busson D. A putative serine/threonine protein kinase encoded by the segment-polarity fused gene of Drosophila . Nature. 1990;347:87–89. doi: 10.1038/347087a0. [DOI] [PubMed] [Google Scholar]
- Preat T, Therond P, Limbourg-Bouchon B, Pham A, Tricoire H, Busson D, Lamour-Isnard C. Segmental polarity in Drosophila melanogaster: Genetic dissection of fused in a suppressor of fused background reveals interaction with costal-2. Genetics. 1993;135:1047–1062. doi: 10.1093/genetics/135.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna U, Wild A, Grzeschik KH, Antonarakis SE. Mutation in GLI3 in postaxial polydactyly type A. Nat. Genet. 1997;17:269–271. doi: 10.1038/ng1197-269. [DOI] [PubMed] [Google Scholar]
- Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, James CD. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Pollard TD. New plasmid vectors for high level synthesis of eukaryotic fusion proteins in Escherichia coli . Gene. 1989;75:323–327. doi: 10.1016/0378-1119(89)90278-3. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Ruppert JM, Vogelstein B, Arheden K, Kinzler KW. GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol. Cell. Biol. 1990;10:5408–5415. doi: 10.1128/mcb.10.10.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A. The tumour-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Nakano Y, Mohler J, Ingham PW. Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech. Dev. 1993;42:89–96. doi: 10.1016/0925-4773(93)90101-3. [DOI] [PubMed] [Google Scholar]
- Therond PP, Knight JD, Kornberg TB, Bishop JM. Phosphorylation of the fused protein kinase in response to signaling from hedgehog. Proc. Natl. Acad. Sci. USA. 1996;93:4224–4228. doi: 10.1073/pnas.93.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden AB, Holmberg E, Lundh-Rozell B, Stahle-Backdahl M, Zaphiropoulos PG, Toftgard R, Vorechovsky I. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: Different in vivo mechanisms of PTCH inactivation. Cancer Res. 1996;56:4562–4565. [PubMed] [Google Scholar]
- van den Heuvel M, Ingham PW. Smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- Vorechovsky I, Unden AB, Sandstedt B, Toftgard R, Stahle-Backdahl M. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: Support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677–4681. [PubMed] [Google Scholar]
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson D, editor. In Situ Hybridization: A Practical Approach. Press, New York: Oxford Univ; 1992. pp. 75–83. [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]