Abstract
A large and growing proportion of the global population rely on shared sanitation facilities despite evidence of a potential increased risk of adverse health outcomes compared with individual household latrines (IHLs). We sought to explore differences between households relying on shared sanitation versus IHLs in terms of demographics, sanitation facilities, and fecal exposure. We surveyed 570 households from 30 slums in Orissa, India, to obtain data on demographics, water, sanitation, and hygiene. Latrine spot-checks were conducted to collect data on indicators of use, privacy, and cleanliness. We collected samples of drinking water and hand rinses to assess fecal contamination. Households relying on shared sanitation were poorer and less educated than those accessing IHLs. Individuals in sharing households were more likely to practice open defecation. Shared facilities were less likely to be functional, less clean, and more likely to have feces and flies. No differences in fecal contamination of drinking water or hand-rinse samples were found. Important differences exist among households accessing shared facilities versus IHLs that may partly explain the apparent adverse health outcomes associated with shared sanitation. As these factors may capture differences in risk and promote sanitary improvements, they should be considered in future policy.
Introduction
Inadequate sanitation is associated with diarrhea, soil-transmitted helminths, trachoma, and schistosomiasis.1 Recent figures indicate that 280,000 deaths could be attributed to inadequate sanitation in low- and middle-income settings.2
Globally, ∼2.5 billion people lack access to improved sanitation.3 India represents a particular challenge, where 792 million people lack access to an improved sanitation facility, and an additional 597 million people practice open defecation, representing nearly two-thirds of the global estimate for open defection.3
Public and other “shared facilities”—those used by two or more households—have been excluded from the definition of “improved sanitation” used to monitor progress toward international targets.4 The reason stems from concerns that shared facilities may be unacceptable in terms of cleanliness and accessibility.5
Nevertheless, shared facilities represent a large and growing proportion of sanitation options available in low-income countries, with ∼784 million users of a shared sanitation facility of an otherwise improved type.3 In India, 9% of the overall population accesses some form of shared sanitation, which has steadily increased from 5% in 1990.3 In urban areas, 20% of the Indian population is reported to access shared sanitation.3 Shared facilities are considered by some to be the only realistic option for high-density populations in many urban slums.5–7 With the development of new targets and indicators for the Sustainable Development Goals, it has been proposed to include shared facilities as “improved” based on the number of users and whether they are known to each other.8
Recent evidence on shared sanitation and health, however, raises questions about this proposed change in policy.9–11 A systematic review found that persons relying on shared sanitation had an increased risk of diarrhea, though the methodological quality of the included studies varied considerably.9 An analysis of data on shared sanitation and diarrhea from 51 Demographic and Health Surveys (DHS) reported that sharing sanitation facilities was a risk factor for diarrhea, though differences in socioeconomic status were important.10
We hypothesize that the apparent increased risk may not be inherent in shared sanitation, but due to differences in the population itself or facilities that could potentially be addressed. This is based in part on a more detailed analysis of DHS and other household survey data showing that people who rely on shared sanitation tend to be poorer, have less access to improved water supplies, live in households with more young children, and are managed by people with no formal education.10 We undertook this study to assess whether these demographic differences were present in a population of slum dwellers in India, and to investigate any differences in facilities and maintenance of household versus shared facilities that may be associated with increased exposure and health risks.
Methods
Study design and setting.
We conducted a cross-sectional study in a convenience sample of 30 informal settlements (slums), half in Bhubaneshwar and half in Cuttack, two cities in Orissa. Eligible slums required a minimum of 10 households accessing a shared, communal, or public sanitation facility (hereafter referred to as “shared”) and 10 households accessing individual household latrines (IHLs) that were not reported to be shared with other households. All sanitation facilities observed in this study were of a pour-flush technology. Working from lists of slums provided by municipal authorities, we visited all 33 potentially eligible slums in Bhubaneshwar and an additional six slums identified through local contacts and randomly selected 15 for inclusion. In Cuttack, for which lists did not identify shared facilities versus IHLs, we visited 84 slums before identifying 15 that met the inclusion criteria. Within each slum, we targeted a total of 20 households, half accessing an IHL and half a shared sanitation facility. An adapted Expanded Program on Immunization (EPI) approach12 was chosen as no accurate population data were available for the 30 slums. This approach prescribed coin tossing or bottle spinning at every crossing in the slum to ensure random direction, and selection of every second household on the left for possible inclusion in the sample. Sampling continued in the same slum until 20 questionnaires were completed with a maximum imbalance of 12-8 (i.e., 12 households accessing a shared facility and eight accessing an IHL, or the reverse).
Household questionnaire and latrine spot-checks.
Trained field staff used a pre-piloted structured household questionnaire to collect demographic and socioeconomic data. The questionnaires were conducted in the local language Oriya by native speakers who were extensively trained on such data collection methods, and the tools were piloted extensively to ensure the questions were asked in a consistent manner. During the household questionnaires, respondents were asked if anyone in the household had suffered from diarrhea in the past week (7-day recall). Diarrhea was defined using the World Health Organization definition of three or more loose stools in 24 hours.13
Field staff conducted spot-checks of the latrines that householders identified as their primary sanitation facility. During the spot-check, observations on the functionality of the cubicle were recorded—if the cubicle was blocked in a way that prevented use, if there were leaves or rubbish blocking the squatting pan or if the pan was broken, the cubicle was considered nonfunctional. In addition, information on indicators of use,14 perceived cleanliness (presence of fecal matter, number of flies, and smell in cubicle), and whether the facility was shared or not was collected. Use of the cubicle was determined based on whether it had a wet floor or if there was a color change or standing water in the pan. Data on hand-washing facilities were collected during the latrine spot-checks.
Microbiological methods.
A sample of the drinking water used in the household was collected for assessment of fecal contamination. Samples were collected directly from the drinking water vessel or from the water source used for drinking, if no water was stored in the home, using sterile 125 mL Whirl-Pak bags (Nasco, Fort Atkinson, WI) containing sodium thiosulphate to neutralize any halogen disinfectant. A “hand-rinse” sample of both hands of the primary caretaker of the house/children was taken using the methods described previously.15 The respondent was asked to rub their fingers and thumb together for 15 seconds inside a 2L Whirl-Pak bag containing 350 mL distilled water, after which the enumerator massaged the hand through the outside of the bag for an additional 15 seconds. The process was repeated with the other hand. Both the hand-rinse and the drinking water sample were stored on ice and transported to the laboratory within 4 hours to be assayed for thermotolerant coliforms (TTC), an indicator of fecal contamination.16 The samples were processed using the membrane filtration technique on 0.45-micron membrane (Millipore Corporation, Billerica, MA), cultured on membrane lauryl sulphate medium (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) and incubated at 44°C.17 The number of yellow colonies were counted and recorded as individual TTCs and reported as the number of colony forming units (CFUs) per 100 mL of analyzed sample water. Plates that yielded CFUs that were too numerous to count were reported as 300 TTC/100 mL.
Activities undertaken by the caretaker 30 minutes before the hand-rinse sample were recorded.
Statistical analyses.
All data were double entered into Epi-Info 3.5.4 (Epi Info™, CDC Atlanta, GA) and were analyzed using Stata 12 (StataCorp LP, College Station, TX).
To generate a relative asset index, we combined household-level information on type of cooking fuel and ownership of specific items (i.e., fridge, bicycle, etc.) using principal component analysis to define the summed weights.18 This score was then categorized into “poor,” “middle,” and “least poor.”
Descriptive measures in the form of geometric means and 95% confidence intervals (CI) were prepared for the microbiological count data. Further analyses of microbiological data were conducted after log10 transformation of TTC counts to account for the skewed distribution. Means of the log-transformed values were compared between the IHL and shared households, using non-parametric tests. We also used ordinal logistic regression to explore associations between covariates and drinking water and hand contamination. For this purpose, the microbiological results were converted to a binary variable (presence or absence of fecal contamination). Two sample t tests and χ2 tests were used to assess differences between the sharing and IHL households.
Ethical approval and consent.
The study was approved by the Ethics Committee of the London School of Hygiene and Tropical Medicine and the Ethics Committee of Xavier University. Household questionnaire participants signed a consent form covering the questionnaire, as well as the hand-rinse and water samples.
Results
Household questionnaires.
Overall characteristics of the included households can be seen in Table 1. A total of 570 households were visited, covering 3,022 individuals. Roughly half of the households reported relying on shared latrines (52.3%), with the balance having IHLs. While users of shared sanitation and IHLs were similar on most demographics, the caretakers in households relying on shared sanitation were almost twice as likely to lack any formal education (P < 0.001) and these households were almost three times more likely to be in the poorest wealth tertile (73.2% sharing versus 26.8% IHL). Households in the “least poor” category were less likely to access shared sanitation than those in the “poorest” category (P < 0.001). Households with IHLs had more members than those accessing shared sanitation (average 5.7 versus 4.9, P < 0.001), and had more rooms used for sleeping (average 2.1 versus 1.5 rooms, P < 0.001). Households with IHL were more likely to live in a house with a cement wall and roof (pucca) (60.4% versus 39.6%). Households relying on shared sanitation were more than twice as likely to collect their water from a source outside of their dwelling (P < 0.001). In addition, these households were more than twice as likely to have a household member reporting open defecation.
Table 1.
Basic characteristics of study households (N = 570)
| Characteristics | Total | IHL | Shared facility | Significance test | ||
|---|---|---|---|---|---|---|
| N | Percentage | N | Percentage | (χ2 test unless indicated otherwise) | ||
| Total number of households | 570 | 272 | 47.7 | 298 | 52.3 | – |
| Total number of individuals (reported) in households | 3,022 | 1,555 | 51.5 | 1,467 | 48.5 | – |
| Sex head of household | ||||||
| Male | 476 | 225 | 47.3 | 251 | 52.7 | P = 0.628 |
| Female | 94 | 47 | 50.0 | 47 | 50.0 | |
| Education level of the household caretaker | ||||||
| No formal education | 100 | 35 | 35.0 | 65 | 65.0 | P < 0.001 |
| Some/complete primary | 133 | 52 | 39.1 | 81 | 60.1 | |
| Some secondary | 241 | 120 | 49.8 | 121 | 50.2 | |
| Secondary and higher | 74 | 50 | 67.6 | 24 | 32.4 | |
| Years in house | ||||||
| < 5 years | 59 | 22 | 37.3 | 37 | 62.7 | P = 0.09 |
| > 5 years | 511 | 250 | 48.9 | 261 | 51.1 | |
| Average no. of individuals in household Mean (SD) | 5.7 (2.7) | 4.9 (2.1) | Two sample t test P < 0.001 | |||
| Average no. of children under five in household Mean (SD) | 0.61 (1.0) | 0.61 (0.9) | Two sample t test | |||
| P = 0.50 | ||||||
| Average no. of rooms used for sleeping in household Mean (SD) | 2.11 (1.2) | 1.50 (0.7) | Two sample T-test | |||
| P < 0.001 | ||||||
| Has BPL* card | ||||||
| Yes, verified | 152 | 67 | 44.1 | 85 | 55.9 | P = 0.546 |
| Yes, reported | 47 | 23 | 48.9 | 24 | 51.1 | |
| No | 371 | 183 | 49.3 | 188 | 50.7 | |
| Open defecation practiced (at least one member of household, on some occasions) | 63 | 20 | 32.3 | 42 | 67.7 | P = 0.01 |
| Diarrhea† (at individual level) | 24 | 12 | 0.77 | 12 | 0.82 | Two sample t test P = 0.001 |
| Water source (drinking water) | ||||||
| Piped water | 460 | 219 | 47.6 | 241 | 52.4 | P = 0.914 |
| Non-piped water | 110 | 53 | 48.2 | 57 | 51.8 | |
| Location of (drinking) water source | ||||||
| In own dwelling | 166 | 92 | 55.4 | 74 | 44.6 | P < 0.001 |
| In own yard/compound | 190 | 114 | 60.0 | 76 | 40.0 | |
| Outside of dwelling | 213 | 65 | 30.5 | 148 | 69.5 | |
| House structure | ||||||
| Cement wall and roof (pucca) | 217 | 131 | 60.4 | 86 | 39.6 | P < 0.001 |
| Cement wall (semi pucca) | 296 | 123 | 41.6 | 173 | 58.5 | |
| No cement (kucha) | 56 | 18 | 32.1 | 38 | 67.4 | |
| Wealth tertile | ||||||
| Poor | 190 | 51 | 26.8 | 139 | 73.2 | P < 0.001 |
| Middle | 193 | 94 | 48.7 | 99 | 51.3 | |
| Least poor | 128 | 128 | 68.5 | 59 | 31.6 | |
BPL = below poverty line card, provided by the Government indicating financial disadvantage and identifies households and individuals in need of assistance.
No. of individuals reporting diarrhea, can be different from households as several individuals reporting diarrhea may reside in the same place.
A total of 24 individuals—half accessing IHLs and half accessing shared sanitation—reported suffering from diarrhea at any time in the 7 days before the questionnaire, indicating a total period prevalence of 0.79%. The period prevalence for individuals accessing shared sanitation was slightly higher (0.82%) than that for individuals accessing IHLs (0.77%) (P = 0.001). Overall, more women than men suffered from diarrhea (nine out of 12 women in IHLs and seven out of 12 women in sharing households). In the sharing households, only one diarrhea case was reported in children under five, as compared with three cases in the IHL households (data not shown).
Latrine spot-checks.
Overall, 273 IHLs and 197 shared facilities were spot-checked. All were pour-flush latrines with ceramic or tiled squatting pans. While 250 of the 273 (91.6%) IHLs were functional, only 142 of 197 (72.1%) of the shared facilities were functional (blocked or broken squatting pans, etc.) (data not shown). These non-functional cubicles were excluded from further analysis in Table 2.
Table 2.
Descriptive data on functional IHLs and shared latrines from latrine spot-checks. Data exclude 23 IHLs and 55 shared facilities that were not functional
| IHL | Shared facility | Two sample test of proportions | |
|---|---|---|---|
| Total no. of facilities assessed | 273 | 197 | |
| Total no. of cubicles assessed | 304 | 460 | |
| Average number of cubicles per facility, (min, max) | 1.11 (1, 5) | 2.34 (1, 42) | |
| No. and percentage of functional cubicles | 226 (74.3%) | 277 (60.2%) | P < 0.001 |
| Average number of functional cubicles per functional facility (min, max) | 0.90 (1, 5) | 1.95 (1, 25) | |
| Does the facility have space for bathing? n (%) | 211 (84.4) | 114 (39.4) | P = 0.30 |
| Is the pipe from the pan to the pit intact? n (%) | N = 249 | N = 142 | |
| Yes | 44 (17.7) | 25 (17.6) | P = 0.99 |
| No | 3 (1.2) | 0 (0) | P = 0.19 |
| Not Visible | 175 (70.3) | 107 (75.4) | P = 0.28 |
| Not Applicable | 33 (13.3) | 10 (7.0) | P = 0.06 |
| Is there a cover over the pit? n (%) | |||
| Yes | 79 (31.7) | 58 (40.8) | P = 0.07 |
| No | 10 (4.1) | 2 (1.4) | P = 0.15 |
| Not Visible | 133 (53.4) | 72 (50.7) | P = 0.61 |
| Not Applicable | 27 (10.8) | 10 (7.0) | P = 0.21 |
| Does the facility have a place for hand-washing? n (%) | 199 (79.6) | 95 (66.9) | P = 0.01 |
| For all proportions below, only the functional cubicles are used | |||
| No. of cubicles with water inside n (%) | 196 (86.7) | 154 (55.6) | P < 0.001 |
| No. of cubicles with a door or screen up to 1 m n (%) | 214 (94.7) | 262 (94.6) | P = 0.96 |
| No. of cubicles with a roof n (%) | 216 (95.6) | 268 (96.8) | P = 0.49 |
| No. of cubicles where the floor is wet (n, %) | 211 (93.4) | 253 (91.3) | P = 0.39 |
| Is there color change in pan? n (%) | 60 (26.5) | 198 (71.5) | P < 0.001 |
| Is there standing water in pan? n (%) | 221 (97.8) | 272 (98.2) | P = 0.75 |
| Are there feces in cubicle? n (%) | 5 (2.2) | 66 (23.8) | P < 0.001 |
| Flies in cubicle n (%) | N = 224 | N = 277 | |
| None | 85 (37.9) | 20 (7.2) | P < 0.001 |
| Some | 120 (53.6) | 75 (27.1) | P < 0.001 |
| Many | 19 (8.5) | 182 (65.7) | P < 0.001 |
| Smell in cubicle n (%) | N = 224 | N = 277 | |
| No detectable smell | 92 (41.1) | 26 (9.4) | P < 0.001 |
| Some detectable smell | 131 (58.5) | 114 (41.2) | P < 0.001 |
| Strong detectable smell | 1 (0.4) | 137 (49.5) | P < 0.001 |
IHLs = individual household latrines
The shared facilities ranged from 1 to 25 functional cubicles (Table 2). Significantly, only 60.2% of all shared cubicles were deemed functional compared with 74.3% of cubicles in IHLs (P < 0.001). Among households that rely on shared latrines, most (60.3%) report sharing with neighboring households or their landlord, with the balance relying on communal other or pay-per-use facilities (data not shown). Similar numbers of squatting pans in shared facilities and IHLs had standing water and a wet floor, both of which may be indicators of use. Significantly more IHL cubicles had water for cleansing available inside compared with shared cubicles (86.7% versus 55.6%, P < 0.001). The shared cubicles had significantly more feces visible in and around the squatting pan (23.8% shared versus 2.2% IHL, P < 0.001), a stronger smell, and larger numbers of flies than IHL cubicles (Table 2).
Hand-washing stations.
The households accessing IHLs were more likely to have a place near the sanitation facility for hand washing (79.6%) than households accessing shared sanitation (66.9%, P < 0.001) (Table 2). Households with water inside the dwelling or yard were more likely to have a place for hand washing (P < 0.001) (data not shown). Soap was observed in 89.8% of the households accessing IHLs versus 59.0% in the sharing households (P < 0.001). Just under half (47.5%) of all the shared facilities had a place where hands could be washed and 59.9% of these had soap present (data not shown).
Drinking water samples and hand rinses.
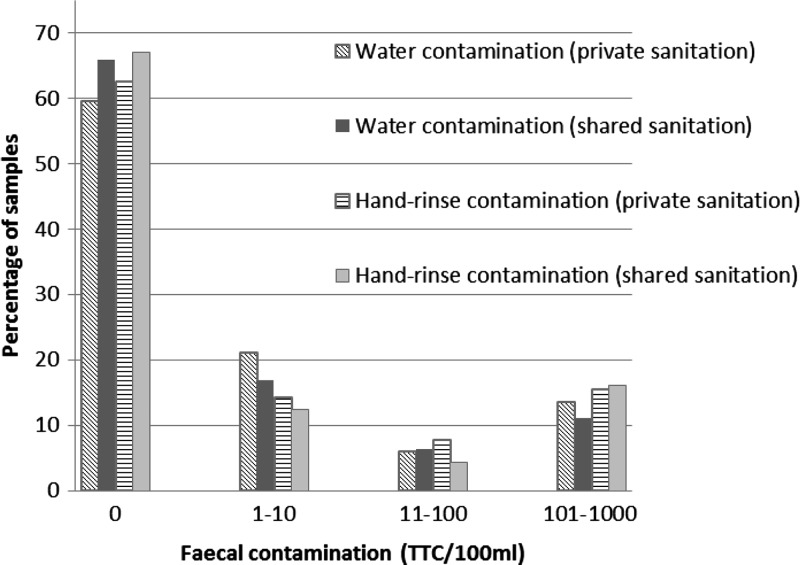
Drinking water and hand-rinses samples were equally contaminated among householders relying on shared facilities versus IHLs. The geometric mean TTC in the drinking water samples was 18.8 (95% CI: 12.4–28.5)/100 mL for households relying on shared latrines versus 18.3 (95% CI: 12.1–27.7) (P = 0.15) for households with IHLs. For hand-rinse samples, the geometric mean TTC was 35.9 (95% CI: 22.9–56.4)/100 mL for households accessing shared sanitation, compared with 27.6 (95% CI: 18.0–42.2) for households accessing IHLs (P = 0.37). No statistically significant association was found between the activity undertaken before hand rinse (i.e., cleaning, visiting latrine) and the level of hand-rinse contamination. Overall, 59.6% (162/272) of household water samples in IHL households had no detectable TTC, as compared with 65.8% (196/298) of the samples from the sharing households. Similarly, 62.6% (171/273) of the hand-rinse samples from IHL households had no detectable TTC, as compared with 67.1% (200/298) of the sharing households (Figure 1 ).
Figure 1.
Distribution of fecal contamination level in drinking water and on hands by sanitation type access.
As a result of the strong zero inflation of the microbiological data (64.9%) and right truncation of “too numerous to count” (15.7%), the outcome data was dichotomized into presence/absence of TTC for further analyses exploring the association with demographic factors. Overall, increased education was associated with the absence of TTC in the drinking water sample (OR: 0.55, 95% CI: 0.34–0.89 for some secondary education; OR: 0.51, 95% CI: 0.27–0.95 for secondary and higher) and the use of a borehole with pump as the main water source was associated with a higher risk of contaminated water, specifically in the shared sanitation group (OR: 3.93, 95% CI: 1.66–9.30). (Supplemental Table 1). For hand-rinse contamination, households in the middle-wealth tertile were more likely to have a contaminated hand-rinse sample than those in the poorest tertile (overall: OR: 0.59, 95% CI: 0.39–0.90; shared households: OR: 0.49, 95% CI: 0.28–0.87). In the households sharing sanitation, respondents with some or complete primary education were less likely to have a contaminated hand-rinse sample than those with no formal education (OR: 0.43, 95% CI: 0.22–0.87).
Discussion
Though several studies have indicated that the use of shared sanitation may be associated with adverse health outcomes, these have also highlighted that there is significant heterogeneity and use of shared sanitation may be a confounding factor.9–11 This study aimed to identify potential factors that could explain an association between shared sanitation access and increased risk of adverse health outcomes. We investigated differences in demographics of users and in the types, use, and maintenance of sanitation facilities. We also explored whether there were differences in hand-rinse or drinking water contamination that might indicate differences in exposure. Though this study was conducted in a small sample in a very specific setting, limiting generalizability, we believe that the results provide important direction for future research on shared sanitation facilities, as well as future policies on this type of sanitation.
Overall, our results suggest that users of shared sanitation are poorer, less educated, and reside in households with fewer members. These results are consistent with a recent study assessing the scope of shared sanitation using DHS and other data.11 In addition, we found that more users of shared sanitation still practice open defecation. This has previously been reported in various other settings, where users were ashamed to be seen using shared latrines,19 or where users opted for open defecation because they found shared facilities were too filthy to use.20,21 The facilities may be used differently by different member of the household—in a study in India, twice as many men used the shared facilities as compared with women.22 In addition, long waiting times at shared facilities may compel users to defecate elsewhere.
Some potentially important differences were seen in the actual latrines. Shared facilities and cubicles were more likely to be nonfunctional. Water availability was significantly higher in the IHL cubicles compared with shared cubicles. Other studies have identified water availability as a factor affecting latrine use,23 which could also impact hand washing after latrine use.24 Shared sanitation facilities were less clean and more likely to have feces and flies—all factors associated with increased risk25 and decreased use.20,26
Despite this, we detected no differences in levels of fecal contamination of household drinking water or hands of the household caretakers. While we used these metrics to explore differences in fecal exposure, other studies have raised questions about their sensitivity and specificity.27,28 In addition, TTC are not direct indicators of contamination,29 and the indicator used did not allow us to distinguish if the contamination found in the drinking water was of human or animal origin, or if it was pathogenic. Moreover, the level of contamination of drinking water was significantly lower than that observed in previous studies in Orissa.30,31 Thus, our study may not have had the power to detect a difference between the groups.
The overall prevalence of diarrhea was lower than expected during the monsoon season. However, a significant difference was observed with individuals accessing shared sanitation reporting a higher period prevalence of diarrhea compared to those accessing IHLs.
Our study has several important limitations. First, the manner for selecting slums and households was purposely designed to achieve balance and internal validity and not external validity. While our approach allows us to make comparisons between householders in the same slums that rely on shared sanitation facilities or IHLs, our results should not be generalized beyond the slums comprising our study population. Second, as a cross-sectional study conducted over a period of 3 months, we had no ability to capture potentially important differences over time and seasons that a longitudinal study would reveal. Third, much of our data was self-reported and is subject to recall, courtesy, and other reporting biases. Lastly, no accurate data were collected on the number of households sharing a particular facility. Assumptions can be made on the basis of the type of sharing (i.e., smaller number of households using neighbor- or family-shared latrine versus larger households accessing communal or pay-per-use facilities) but additional data would have to be collected to justify these assumptions.
Despite these limitations, we identified important differences between users of shared sanitation facilities versus IHLs. Some of these, such as socioeconomic status and education, cannot be easily changed. However, they do point to vulnerable groups that can be targeted. Other differences, such as cleanliness of latrine facilities, presence of water and hand-washing facilities, and factors that may discourage use and contribute to open defecation, could be addressed through improved management and maintenance of latrine facilities and promotion of latrine use and hand washing. They are also factors that international monitoring may wish to consider rather than simply counting numbers of households with access in determining whether to designate a shared facility as improved. As shared sanitation is expected to increase globally, it is important to ensure that these facilities can be safe, acceptable, and sustainable for all users.
Supplementary Material
Footnotes
Authors' addresses: Marieke Heijnen, Parimita Routray, and Belen Torondel, Department of Disease Control, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mails: marieke.heijnen@gmail.com, parimita.routray@lshtm.ac.uk, and belen.torondel@lshtm.ac.uk. Thomas Clasen, Rollins School of Public Health, Emory University, Atlanta, GA, E-mail: thomas.f.clasen@emory.edu.
References
- 1.Pruss-Ustun A, Bos R, Gore F, Bartram J. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health. Geneva, Switzerland: World Health Organization; 2008. p. 60. [Google Scholar]
- 2.Prüss-Ustün A, Bartram J, Clasen T, Colford JM, Jr, Cummming O, Curtis V, Bonjour S, Dangour AD, De France J, Fewtrell L, Freeman MC, Gordon B, Hunter PR, Johnston RB, Mathers C, Mausezahl D, Medlicott K, Neira M, Stocks M, Wolf J, Cairncross S. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health. 2014;19:894–905. doi: 10.1111/tmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, United Nations Children's Fund . Progress on Sanitation and Drinking Water 2014 Update. Geneva, Switzerland: World Health Organization, United Nations Children's Fund; 2014. Joint Monitoring Programme. [Google Scholar]
- 4.World Health Organization, United Nations Children's Fund . Progress on Sanitation and Drinking Water: 2010 Update. Geneva, Switzerland: World Health Organization, United Nations Children's Fund; 2010. Joint Monitoring Programme. [Google Scholar]
- 5.World Health Organization, United Nations Children's Fund . Progress on Drinking Water and Sanitation: 2012 Update. Geneva, Switzerland: World Health Organization, United Nations Children's Fund; 2012. Joint Monitoring Programme. [Google Scholar]
- 6.Wegelin-Schuringa M, Kodo T. Tenancy and sanitation provision in informal settlements in Nairobi: revisiting the public latrine option. Environ Urban. 1997;9:181–190. [Google Scholar]
- 7.Nelson KL, Murray A. Sanitation for unserved populations: technologies, implementation challenges, and opportunities. Annu Rev Environ Resour. 2008;33:119–151. [Google Scholar]
- 8.Joint Monitoring Programme . Proposal for Consolidated Drinking Water, Sanitation and Hygiene Targets, Indicators and Definitions. The Hague, The Netherlands; 2012. December 3–5, 2012. [Google Scholar]
- 9.Heijnen M, Cumming O, Peletz R, Chan GK-S, Brown J, Baker K, Clasen T. Shared sanitation versus individual household latrines: a systematic review of health outcomes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller JA, Clasen T, Heijnen M, Eisenberg JNS. Shared sanitation and the prevalence of diarrhea in young children: evidence from 51 Countries, 2001–2011. Am J Trop Med Hyg. 2014;911:173–180. doi: 10.4269/ajtmh.13-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heijnen M, Rosa G, Fuller J, Eisenberg JNS, Clasen T. The geographic and demographic scope of shared sanitation: an analysis of national survey data from low- and middle-income countries. Trop Med Int Health. 2014;19:1334–1345. doi: 10.1111/tmi.12375. [DOI] [PubMed] [Google Scholar]
- 12.Barnard S, Routray P, Majorin F, Peletz R, Boisson S, Sinha A, Clasen T. Impact of Indian Total Sanitation Campaign on latrine coverage and use: a cross-sectional study in Orissa three years following programme implementation. PLoS One. 2013;8:e71438. doi: 10.1371/journal.pone.0071438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Diarrhoeal Disease—Fact Sheet No. 330. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 14.O'Loughlin R, Fentie G, Flannery B, Emerson PM. Follow-up of a low cost latrine promotion programme in one district of Amhara, Ethiopia: characteristics of early adopters and non-adopters. Trop Med Int Health. 2006;11:1406–1415. doi: 10.1111/j.1365-3156.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 15.Pickering AJ, Davis J, Walters SP, Horak HM, Keymer DP, Mushi D, Strickfaden R, Chynoweth JS, Liu J, Blum A, Rogers K, Boehm AB. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol. 2010;44:3267–3272. doi: 10.1021/es903524m. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Guidelines for Drinking Water Quality. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 17.American Public Health Association . Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association; 2005. [Google Scholar]
- 18.Filmer D, Pritchett LH. Estimating wealth effect without expenditure data or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee N, Robiarto A, Saputra E. Washington, DC: World Bank; 2012. Achieving and sustaining open defecation free communities: learning from east Java. Report from WSP. [Google Scholar]
- 20.Bapat M, Agarwal I. Our needs, our priorities; women and men from the ‘slums' in Mumbai and Pune talk about their needs for water and sanitation. Environ Urban. 2003;15:71–86. [Google Scholar]
- 21.Mazeau AP, Scott R, Tuffuor B. Kampala, Uganda: 2012. Sanitation—a neglected essential service in the unregulated urban expansion of Ashaiman, Ghana. Paper presented at Sustainable Futures: Architecture and Urbanism in the Global South, June 27–30, 2012. [Google Scholar]
- 22.Biran A, Jenkins MW, Dabrase P, Bhagwat I. Patterns and determinants of communal latrine usage in urban poverty pockets in Bhopal, India. Trop Med Int Health. 2011;16:854–862. doi: 10.1111/j.1365-3156.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins M, Freeman M, Routray P. Measuring the safety of excreta disposal behavior in India with the new safe San Index: reliability, validity and utility. Int J Environ Res Public Health. 2014;11:8319–8346. doi: 10.3390/ijerph110808319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luby SP, Halder AK, Tronchet C, Akhter S, Bhuiya A, Johnston RB. Household characteristics associated with handwashing with soap in rural Bangladesh. Am J Trop Med Hyg. 2009;81:882–887. doi: 10.4269/ajtmh.2009.09-0031. [DOI] [PubMed] [Google Scholar]
- 25.Muang U K, Khin M, Wai NN, Hman W, Myint TT, Butler T. Risk factors for the development of persistent diarrhoea and malnutrition in Burmese children. Int J Epidemiol. 1992;21:1021–1029. doi: 10.1093/ije/21.5.1021. [DOI] [PubMed] [Google Scholar]
- 26.Tiimub BM, Forson MA, Obiri-Danso K, Rahaman IA. Addis Ababa, Ethiopia: 2009. Pointed gaps in the provision, quality, patronage and management of toilet facilities in Bawku East District. Paper presented at 34th WEDC International Conference, May 18–22, 2009. [Google Scholar]
- 27.Mattioli MC, Boehm AB, Davis J, Harris AR, Mrisho M, Pickering AJ. Enteric pathogens in stored drinking water and on caregiver's hands in Tanzanian households with and without reported cases of child diarrhea. PLoS One. 2014;9:e84939. doi: 10.1371/journal.pone.0084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram PK, Jahid I, Halder AK, Nygren B, Islam MS, Granger SP, Molyneaux JW, Luby Sp. Variability in hand contamination based on serial measurements: implications for assessment of hand-cleansing behavior and disease risk. Am J Trop Med Hyg. 2011;84:510–516. doi: 10.4269/ajtmh.2011.10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashbolt N, Grabow W, Snozzi M. Indicators of microbial water quality. In: Fewtrell L, Bartram J, editors. Water Quality: Guidelines, Standards and Health. London, United Kingdom: IWA Publishing; 2001. [Google Scholar]
- 30.Boisson S, Stevenson M, Shapiro L, Kumar V, Singh LP, Ward D, Clasen T. Effect of household-based drinking water chlorination of diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med. 2014;10 doi: 10.1371/journal.pmed.1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, Ensink J, Freeman M, Jenkins M, Odagiri M, Ray S, Sinha A, Suar M, Schmidt W-P. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. The Lancet Global Health. 2014;2:e645–e653. doi: 10.1016/S2214-109X(14)70307-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.