Abstract
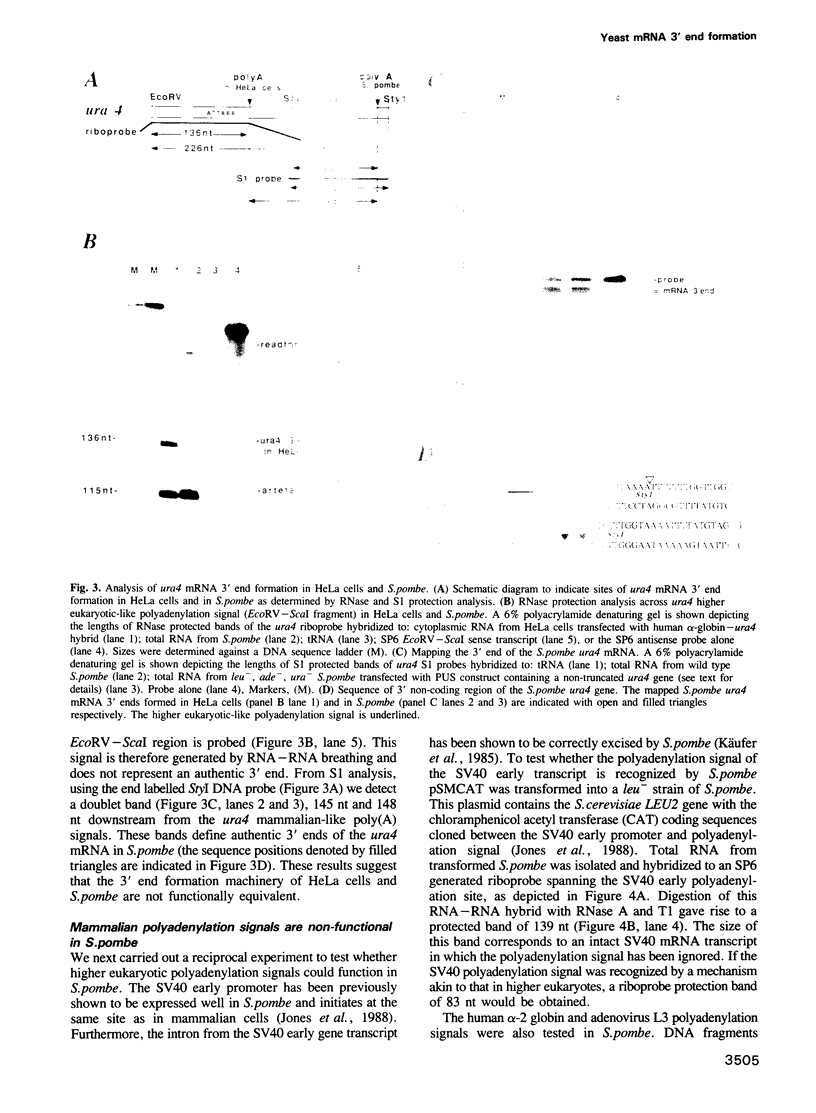
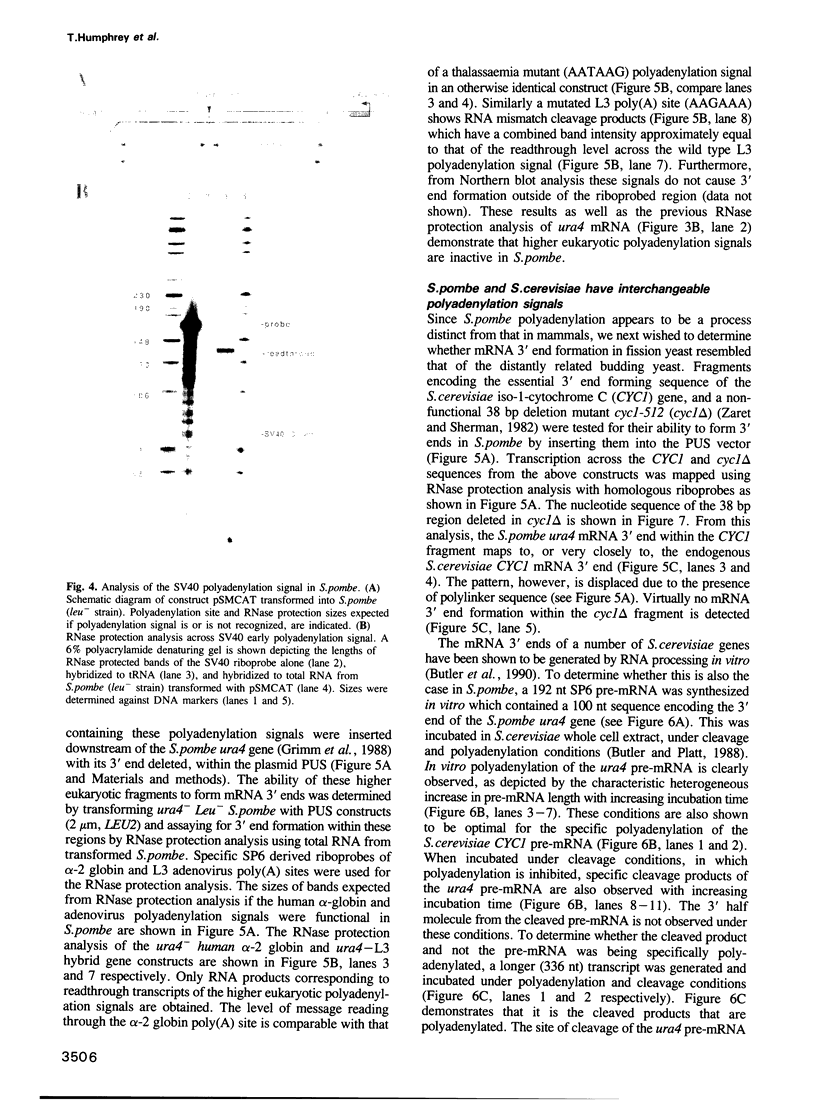
Sequences resembling polyadenylation signals of higher eukaryotes are present downstream of the Schizosaccharomyces pombe ura4+ and cdc10+ coding regions and function in HeLa cells. However, these and other mammalian polyadenylation signals are inactive in S. pombe. Instead, we find that polyadenylation signals of the CYC1 gene of budding yeast Saccharomyces cerevisiae function accurately and efficiently in fission yeast. Furthermore, a 38 bp deletion which renders this RNA processing signal non-functional in S. cerevisiae has the equivalent effect in S. pombe. We demonstrate that synthetic pre-mRNAs encoding polyadenylation sites of S. pombe genes are accurately cleaved and polyadenylated in whole cell extracts of S. cerevisiae. Finally, as is the case in S. cerevisiae, DNA sequences encoding regions proximal to the S. pombe mRNA 3' ends are found to be extremely AT rich; however, no general sequence motif can be found. We conclude that although fission yeast has many genetic features in common with higher eukaryotes, mRNA 3' end formation is significantly different and appears to be formed by an RNA processing mechanism homologous to that of budding yeast. Since fission and budding yeast are evolutionarily divergent, this lower eukaryotic mechanism of mRNA 3' end formation may be generally conserved.
Full text
PDF

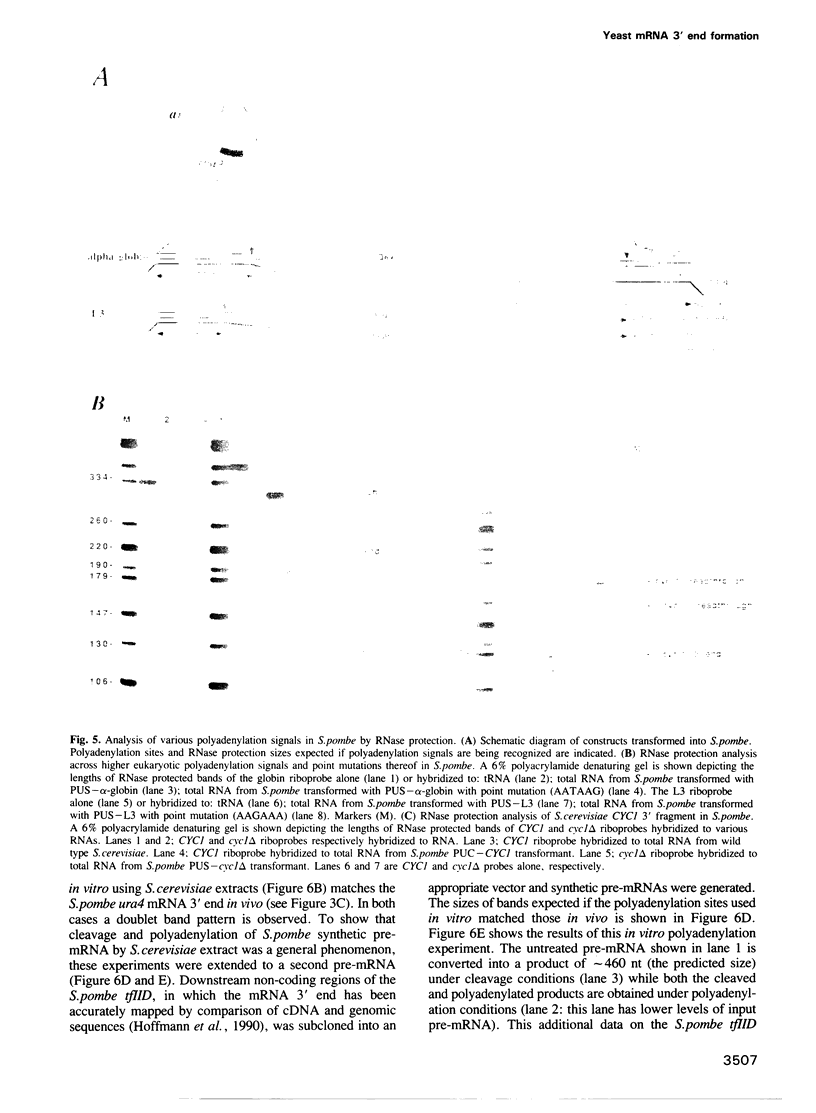




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Hiraoka Y., Fukasawa T. Signal sequence for generation of mRNA 3' end in the Saccharomyces cerevisiae GAL7 gene. EMBO J. 1990 Nov;9(11):3691–3697. doi: 10.1002/j.1460-2075.1990.tb07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aves S. J., Durkacz B. W., Carr A., Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 'start' gene. EMBO J. 1985 Feb;4(2):457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. Molecular characterisation of the DNA ligase gene, CDC17, from the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1987 Feb 2;162(3):659–667. doi: 10.1111/j.1432-1033.1987.tb10688.x. [DOI] [PubMed] [Google Scholar]
- Beltrame M., Bianchi M. E. Sequence of the cDNA for one acidic ribosomal protein of Schizosaccharomyces pombe. Nucleic Acids Res. 1987 Nov 11;15(21):9089–9089. doi: 10.1093/nar/15.21.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. S., Platt T. RNA processing generates the mature 3' end of yeast CYC1 messenger RNA in vitro. Science. 1988 Dec 2;242(4883):1270–1274. doi: 10.1126/science.2848317. [DOI] [PubMed] [Google Scholar]
- Butler J. S., Sadhale P. P., Platt T. RNA processing in vitro produces mature 3' ends of a variety of Saccharomyces cerevisiae mRNAs. Mol Cell Biol. 1990 Jun;10(6):2599–2605. doi: 10.1128/mcb.10.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Cheroutre H., Degrave W., Fiers W. Simple, efficient in vitro synthesis of capped RNA useful for direct expression of cloned eukaryotic genes. Nucleic Acids Res. 1982 Oct 25;10(20):6353–6362. doi: 10.1093/nar/10.20.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S., Chang C. W., Schweingruber M. E., Schaller J., Rickli E. E., Carbon J. Isolation and characterization of the structural gene for secreted acid phosphatase from Schizosaccharomyces pombe. J Biol Chem. 1986 Feb 25;261(6):2936–2941. [PubMed] [Google Scholar]
- Gil A., Proudfoot N. J. A sequence downstream of AAUAAA is required for rabbit beta-globin mRNA 3'-end formation. 1984 Nov 29-Dec 5Nature. 312(5993):473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Kelly J. D., Cohen E. H. Transcription terminates in yeast distal to a control sequence. Cell. 1983 Jun;33(2):607–614. doi: 10.1016/0092-8674(83)90441-5. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G., Stein M., Beach D. Sucl+ encodes a predicted 13-kilodalton protein that is essential for cell viability and is directly involved in the division cycle of Schizosaccharomyces pombe. Mol Cell Biol. 1987 Jan;7(1):504–511. doi: 10.1128/mcb.7.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A., Horikoshi M., Wang C. K., Schroeder S., Weil P. A., Roeder R. G. Cloning of the Schizosaccharomyces pombe TFIID gene reveals a strong conservation of functional domains present in Saccharomyces cerevisiae TFIID. Genes Dev. 1990 Jul;4(7):1141–1148. doi: 10.1101/gad.4.7.1141. [DOI] [PubMed] [Google Scholar]
- Humphrey T., Christofori G., Lucijanic V., Keller W. Cleavage and polyadenylation of messenger RNA precursors in vitro occurs within large and specific 3' processing complexes. EMBO J. 1987 Dec 20;6(13):4159–4168. doi: 10.1002/j.1460-2075.1987.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysmans E., Dams E., Vandenberghe A., De Wachter R. The nucleotide sequences of the 5S rRNAs of four mushrooms and their use in studying the phylogenetic position of basidiomycetes among the eukaryotes. Nucleic Acids Res. 1983 May 11;11(9):2871–2880. doi: 10.1093/nar/11.9.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L. E., Seiler S. H., Whoriskey J., Moore C. L. Point mutations upstream of the yeast ADH2 poly(A) site significantly reduce the efficiency of 3'-end formation. Mol Cell Biol. 1991 Apr;11(4):2004–2012. doi: 10.1128/mcb.11.4.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelbrecht I. L., Herman L. M., Dekeyser R. A., Van Montagu M. C., Depicker A. G. Different 3' end regions strongly influence the level of gene expression in plant cells. Plant Cell. 1989 Jul;1(7):671–680. doi: 10.1105/tpc.1.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Moreno S., Nurse P., Jones N. C. Expression of the SV40 promoter in fission yeast: identification and characterization of an AP-1-like factor. Cell. 1988 May 20;53(4):659–667. doi: 10.1016/0092-8674(88)90581-8. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res. 1987 Dec 10;15(23):9627–9640. doi: 10.1093/nar/15.23.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käufer N. F., Simanis V., Nurse P. Fission yeast Schizosaccharomyces pombe correctly excises a mammalian RNA transcript intervening sequence. Nature. 1985 Nov 7;318(6041):78–80. doi: 10.1038/318078a0. [DOI] [PubMed] [Google Scholar]
- Levitt N., Briggs D., Gil A., Proudfoot N. J. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989 Jul;3(7):1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J. 1986 Dec 20;5(13):3665–3671. doi: 10.1002/j.1460-2075.1986.tb04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins P., Gallwitz D. A single intronless action gene in the fission yeast Schizosaccharomyces pombe: nucleotide sequence and transcripts formed in homologous and heterologous yeast. Nucleic Acids Res. 1987 Sep 25;15(18):7369–7379. doi: 10.1093/nar/15.18.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Yamamoto M. Identification of ras-related, YPT family genes in Schizosaccharomyces pombe. EMBO J. 1990 May;9(5):1417–1422. doi: 10.1002/j.1460-2075.1990.tb08257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne B. I., Guarente L. Mutational analysis of a yeast transcriptional terminator. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4097–4101. doi: 10.1073/pnas.86.11.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Rutherford T. R., Partington G. A. Transcriptional analysis of human zeta globin genes. EMBO J. 1984 Jul;3(7):1533–1540. doi: 10.1002/j.1460-2075.1984.tb02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola H., Baker S. M., Parker R., Platt T. Orientation-dependent function of a short CYC1 DNA fragment in directing mRNA 3' end formation in yeast. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5041–5045. doi: 10.1073/pnas.85.14.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. R. Evolutionary divergence of the mRNA transcription initiation mechanism in yeast. Nature. 1983 Jan 13;301(5896):167–169. doi: 10.1038/301167a0. [DOI] [PubMed] [Google Scholar]
- Russo P., Sherman F. Transcription terminates near the poly(A) site in the CYC1 gene of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8348–8352. doi: 10.1073/pnas.86.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfaçon H., Brodmann P., Hohn T. A dissection of the cauliflower mosaic virus polyadenylation signal. Genes Dev. 1991 Jan;5(1):141–149. doi: 10.1101/gad.5.1.141. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3' end processing in the human alpha 2 globin gene. EMBO J. 1986 Nov;5(11):2915–2922. doi: 10.1002/j.1460-2075.1986.tb04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. Mutationally altered 3' ends of yeast CYC1 mRNA affect transcript stability and translational efficiency. J Mol Biol. 1984 Jul 25;177(1):107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]