Abstract
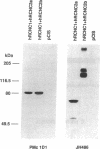
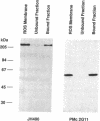
The cGMP-gated cation channel mediating visual transduction in retinal rods was recently found to comprise at least two subunits, 1 and 2 (or alpha and beta). SDS gels of the purified channel show, in addition to a 63-kDa protein band (subunit 1), a 240-kDa protein band that binds Ca(2+)-calmodulin, a modulator of the channel. To examine any connection between subunit 2 and the 240-kDa protein, cGMP-gated channels formed from the expressed cloned subunits in human embryonic kidney (HEK) 293 cells were tested for Ca(2+)-calmodulin effect. Homooligomeric channels formed by subunit 1 alone showed no sensitivity to Ca(2+)-calmodulin, and neither did heterooligomeric channels formed by subunit 1 and the short alternatively spliced form of subunit 2 (2a). By contrast, the cGMP half-activation constant (K1/2) for heterooligomeric channels formed from subunit 1 and the long form of subunit 2 (2b) was increased 1.5- to 2-fold by Ca(2+)-calmodulin, similar to the increase observed for the native channel. In Western blots of rod outer segment membranes, a subunit 2-specific antibody also recognized the 240-kDa protein. Finally, amino acid sequences derived from peptide fragments of the bovine 240-kDa protein showed approximately 80% identity to regions of subunit 2b of the human channel. These results together suggest that subunit 2b of the rod channel is a component of the 240-kDa protein and that it mediates the Ca(2+)-calmodulin modulation of the channel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. L., Gerber W. V., Karpen J. W. Specific labeling and permanent activation of the retinal rod cGMP-activated channel by the photoaffinity analog 8-p-azidophenacylthio-cGMP. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5369–5373. doi: 10.1073/pnas.90.11.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Yau K. W. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994 Apr 7;368(6471):545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Cook N. J., Hanke W., Kaupp U. B. Identification, purification, and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc Natl Acad Sci U S A. 1987 Jan;84(2):585–589. doi: 10.1073/pnas.84.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. J., Molday L. L., Reid D., Kaupp U. B., Molday R. S. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989 Apr 25;264(12):6996–6999. [PubMed] [Google Scholar]
- Cook N. J., Zeilinger C., Koch K. W., Kaupp U. B. Solubilization and functional reconstitution of the cGMP-dependent cation channel from bovine rod outer segments. J Biol Chem. 1986 Dec 25;261(36):17033–17039. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dhallan R. S., Macke J. P., Eddy R. L., Shows T. B., Reed R. R., Yau K. W., Nathans J. Human rod photoreceptor cGMP-gated channel: amino acid sequence, gene structure, and functional expression. J Neurosci. 1992 Aug;12(8):3248–3256. doi: 10.1523/JNEUROSCI.12-08-03248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Hanke W., Cook N. J., Kaupp U. B. cGMP-dependent channel protein from photoreceptor membranes: single-channel activity of the purified and reconstituted protein. Proc Natl Acad Sci U S A. 1988 Jan;85(1):94–98. doi: 10.1073/pnas.85.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Molday L. L., Cook N. J., Kaupp U. B., Molday R. S. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990 Oct 25;265(30):18690–18695. [PubMed] [Google Scholar]
- Molday R. S., Molday L. L. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol. 1987 Dec;105(6 Pt 1):2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R. S., Molday L. L., Dosé A., Clark-Lewis I., Illing M., Cook N. J., Eismann E., Kaupp U. B. The cGMP-gated channel of the rod photoreceptor cell characterization and orientation of the amino terminus. J Biol Chem. 1991 Nov 15;266(32):21917–21922. [PubMed] [Google Scholar]
- Shinozawa T., Sokabe M., Terada S., Matsusaka H., Yoshizawa T. Detection of cyclic GMP binding protein and ion channel activity in frog rod outer segments. J Biochem. 1987 Aug;102(2):281–290. doi: 10.1093/oxfordjournals.jbchem.a122052. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Wong S., Molday R. S. A spectrin-like protein in retinal rod outer segments. Biochemistry. 1986 Oct 7;25(20):6294–6300. doi: 10.1021/bi00368a069. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):9–32. [PubMed] [Google Scholar]