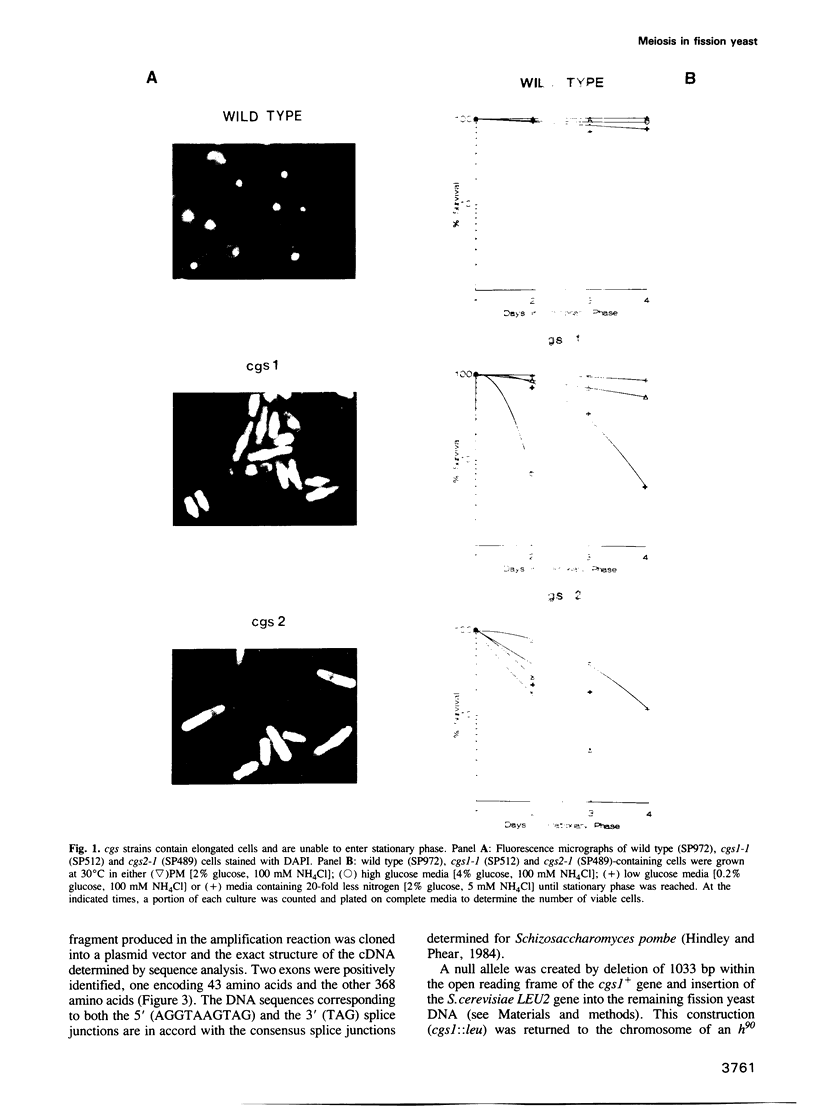
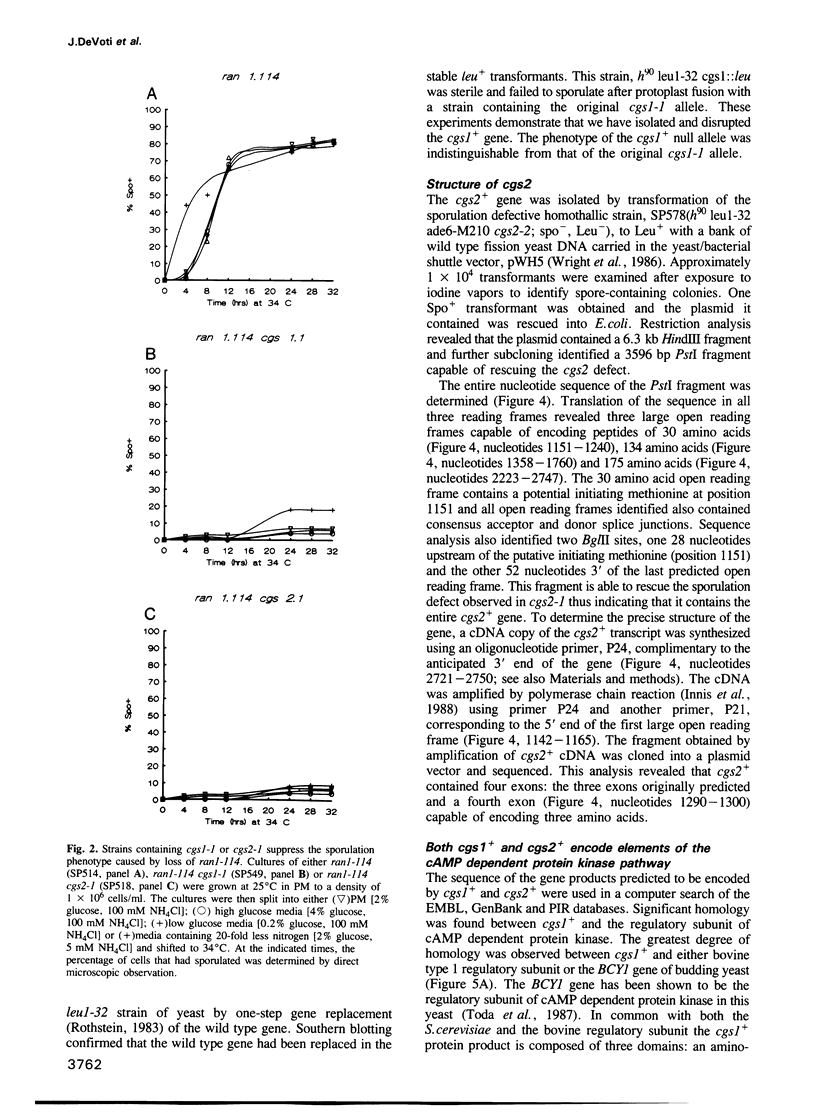
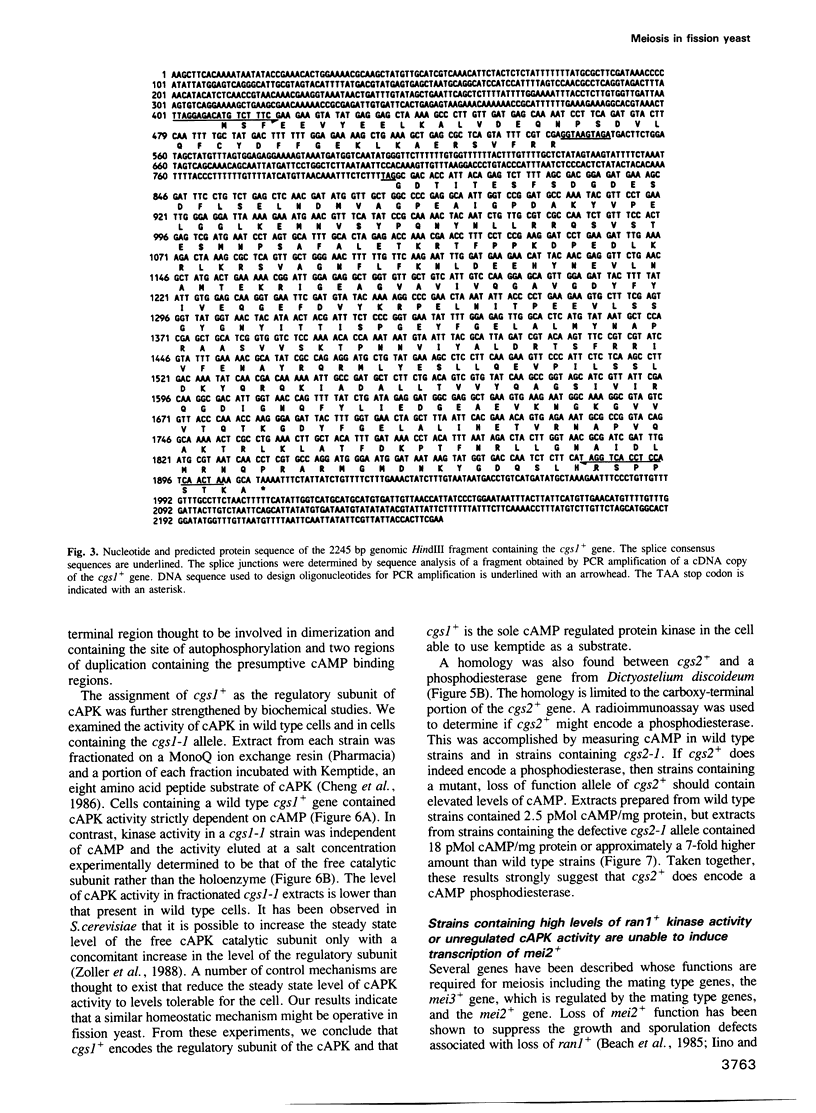
Abstract
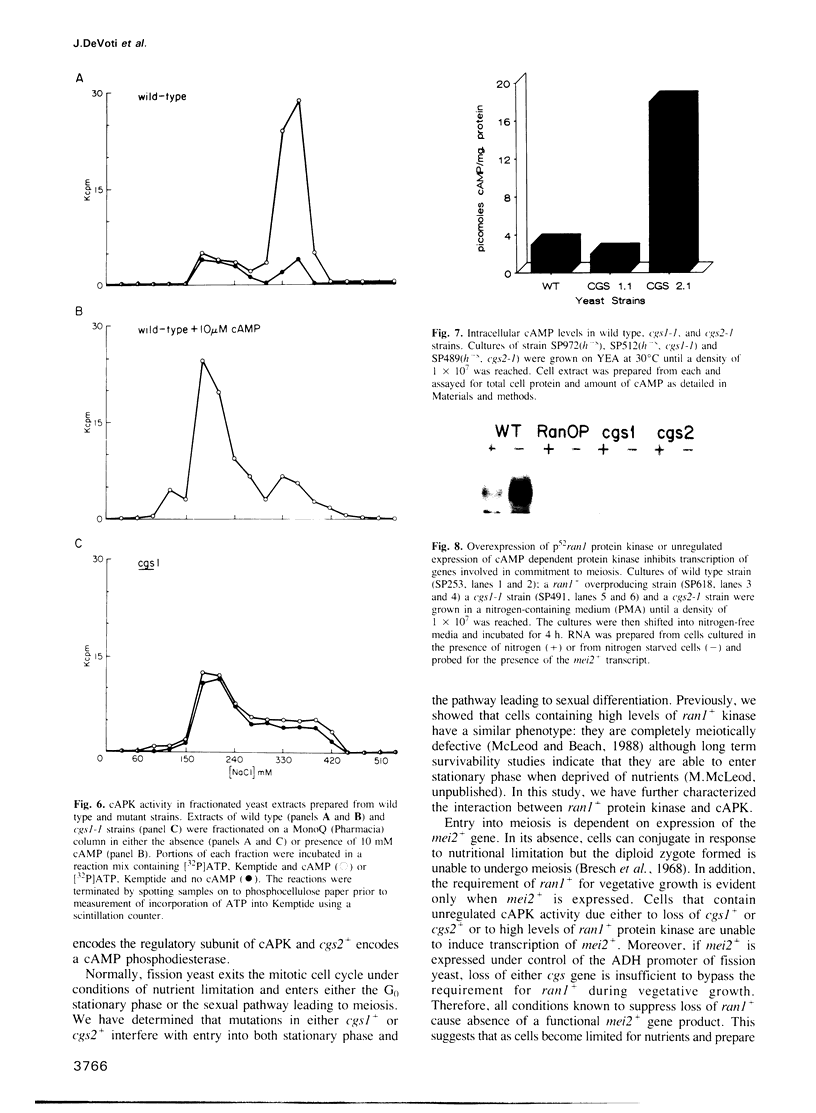
In fission yeast, meiosis is initiated by transcriptional activation of the mei3+ gene under the combined influence of the four mating type genes. The mei3+ gene product acts as a meiotic inducer by binding to and inhibiting the ran1+ protein kinase. Inactivation of ran1+ kinase is both necessary and sufficient to allow meiotic differentiation. We describe a class of mutants which are unable to undergo both normal meiosis and meiosis induced by inactivation of ran1+. In addition to these defects, the cells are sterile and unable to enter stationary phase. We have determined that the mutants define two complementation groups, designated cgs1+ and cgs2+ (continues to grow in stationary). The wild type allele of each gene has been isolated and sequence analysis of cgs1+ shows that it encodes a protein homologous to the regulatory subunit of cyclic AMP dependent protein kinase (cAPK). Biochemical studies demonstrate that in cgs1-1 containing cells, cAPK activity is unregulated by cyclic AMP (cAMP). Sequence analysis of cgs2+ shows that the predicted protein it encodes shares homology with a phosphodiesterase from Dictyostelium discoideum and biochemical studies demonstrate that cells containing a mutant allele of cgs2+ have elevated levels of cAMP. Thus, both genes encode proteins that regulate the activity of cAPK. We have previously shown that cells overproducing ran1+ kinase are meiotically defective. Here, we provide direct evidence that the meiotic defect caused by either unregulated cAPK activity or unregulated ran1+ kinase activity is due to inability to induce transcription of the mei2+ gene, which is required for meiotic initiation. We propose that the switch from vegetative growth to meiosis in fission yeast requires inactivation of ran1+ kinase and is prevented by unregulated levels of cAPK.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Beach D., Rodgers L., Gould J. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet. 1985;10(4):297–311. doi: 10.1007/BF00365626. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nairn A. C., Kuo J. F. Protein kinases 1988: a current perspective. FASEB J. 1988 Nov;2(14):2957–2969. doi: 10.1096/fasebj.2.14.2972578. [DOI] [PubMed] [Google Scholar]
- Bresch C., Müller G., Egel R. Genes involved in meiosis and sporulation of a yeast. Mol Gen Genet. 1968;102(4):301–306. doi: 10.1007/BF00433721. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Fantes P., Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977 Jul;107(2):377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Field J., Vojtek A., Ballester R., Bolger G., Colicelli J., Ferguson K., Gerst J., Kataoka T., Michaeli T., Powers S. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990 Apr 20;61(2):319–327. doi: 10.1016/0092-8674(90)90812-s. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Iino Y., Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Beach D. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature. 1988 Apr 7;332(6164):509–514. doi: 10.1038/332509a0. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J. 1986 Dec 20;5(13):3665–3671. doi: 10.1002/j.1460-2075.1986.tb04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M. Regulation of meiosis: from DNA binding protein to protein kinase. Bioessays. 1989 Jul;11(1):9–14. doi: 10.1002/bies.950110104. [DOI] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen O., Egel R. The pat1 protein kinase controls transcription of the mating-type genes in fission yeast. EMBO J. 1990 May;9(5):1401–1406. doi: 10.1002/j.1460-2075.1990.tb08255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda C., Uehira M., Kishida M., Fujioka H., Iino Y., Watanabe Y., Yamamoto M. Cloning and analysis of transcription of the mei2 gene responsible for initiation of meiosis in the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1987 Jan;169(1):93–96. doi: 10.1128/jb.169.1.93-96.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Amino acid sequence of the regulatory subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4200–4206. doi: 10.1021/bi00313a029. [DOI] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Scott J. D., McMullen B., Hurwitz M., Krebs E. G., Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Apr;7(4):1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Lino Y., Furuhata K., Shimoda C., Yamamoto M. The S.pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 1988 Mar;7(3):761–767. doi: 10.1002/j.1460-2075.1988.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Maundrell K., Heyer W. D., Beach D., Nurse P. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid. 1986 Mar;15(2):156–158. doi: 10.1016/0147-619x(86)90051-x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Kuret J., Cameron S., Levin L., Johnson K. E. Purification and characterization of C1, the catalytic subunit of Saccharomyces cerevisiae cAMP-dependent protein kinase encoded by TPK1. J Biol Chem. 1988 Jul 5;263(19):9142–9148. [PubMed] [Google Scholar]