Abstract
OBJECTIVES:
Evaluate safety and efficacy of filtered-sunlight phototherapy (FS-PT).
METHODS:
Term/late preterm infants ≤14 days old with clinically significant jaundice, assessed by total bilirubin (TB) levels, were recruited from a maternity hospital in Lagos, Nigeria. Sunlight was filtered with commercial window-tinting films that remove most UV and significant levels of infrared light and transmit effective levels of therapeutic blue light. After placing infants under an FS-PT canopy, hourly measurements of axillary temperatures, monitoring for sunburn, dehydration, and irradiances of filtered sunlight were performed. Treatment was deemed safe and efficacious if infants were able to stay in FS-PT for ≥5 hours and rate of rise of TB was <0.2 mg/dL/h for infants ≤72 hours of age or TB decreased for infants >72 hours of age.
RESULTS:
A total of 227 infants received 258 days of FS-PT. No infant developed sunburn or dehydration. On 85 (33%) of 258 treatment days, infants were removed briefly from FS-PT due to minor temperature-related adverse events. No infant met study exit criteria. FS-PT was efficacious in 92% (181/197) of evaluable treatment days. Mean ± SD TB change was –0.06 ± 0.19 mg/dL/h. The mean ± SD (range) irradiance of FS-PT was 38 ± 22 (2–115) µW/cm2/nm, measured by the BiliBlanket Meter II.
CONCLUSIONS:
With appropriate monitoring, filtered sunlight is a novel, practical, and inexpensive method of PT that potentially offers safe and efficacious treatment strategy for management of neonatal jaundice in tropical countries where conventional PT treatment is not available.
Keywords: newborn jaundice, hyperbilirubinemia, sunlight, phototherapy, irradiance, UV, IR, low-middle income countries
What’s Known on This Subject:
Phototherapy effectively treats unconjugated hyperbilirubinemia. However, in resource-poor settings, functional phototherapy devices are often unavailable due to financial constraints or erratic electrical power availability.
What This Study Adds:
Filtered-sunlight phototherapy could be a cost-effective option in resource-poor settings with abundant sunlight.
Severe neonatal jaundice with progression to acute bilirubin encephalopathy and kernicterus is probably the most underrecognized and underreported cause of preventable neonatal morbidity and mortality worldwide.1,2 It is a leading cause of deafness, cerebral palsy, and other neurologic damage among survivors.3 For most survivors of acute bilirubin encephalopathy/kernicterus, disabling consequences occur in locations (such as Nigeria) without the medical or technological support necessary to improve the quality of life.4,5 Survival with such distressing disabilities is indeed a tragedy, especially where prevention is possible.
Prohibitive equipment costs and the requirements for dependable electricity, monitoring, and ongoing maintenance capabilities, all of which are challenging for health care providers, frequently account for lack of functional/operational and effective phototherapy (PT) in resource-poor settings.6,7 In Nigeria, even the advent of locally fabricated PT units to increase availability and accessibility has not effectively improved treatment of neonatal jaundice, as most such units provided suboptimal irradiance levels.6,7
Historically, the use of conventional, electrically powered PT lamps had its origin from the observation by Sister Ward in England that direct sunlight bleached jaundiced premature infants. She reported this to Dr Cremer, who subsequently conducted a small study and verified a reduction in serum bilirubin levels in infants exposed to sunlight for 2 to 4 hours.8 The incidental reduction in the levels of total serum/plasma bilirubin (TB) in blood samples exposed to sunlight in the same hospital laboratory further established the therapeutic effect of sunlight toward unconjugated hyperbilirubinemia.8 Use of direct sunlight has been limited in many countries due to its high levels of infrared and UV rays with the consequent risks of sunburn, dehydration, and long-term harm to the skin. However, it is not uncommon for health officials to recommend sunlight for treating jaundice.9,10 Challenged by the continued infant morbidity and mortality from neonatal jaundice in resource-poor countries, and building on the work of Cremer et al,8 Salih,10 Olowe,11 and others, Vreman et al12 developed a novel, low-cost method to filter out virtually all UV-A, UV-B, and UV-C light, as well as significant levels of infrared radiation (heat) in natural sunlight. Unlike any other mode of phototherapy, it has the potential to provide safe therapy without the need for a source electrical power or high maintenance requirements. A pilot study was subsequently performed in Nigeria in which a small number of infants were safely treated with filtered-sunlight phototherapy (FS-PT).13
The goal of this study was to evaluate the safety and efficacy of FS-PT in a larger group of newborn infants presenting with clinical jaundice in the first 14 days of life in a resource-poor setting.
Methods
Term and near-term infants ≤14 days old were recruited between December 2011 and August 2012 from Island Maternity Hospital, Lagos, Nigeria, an inner-city maternity hospital where effective PT is not always available. Infants were eligible for screening for jaundice if their mothers consented. Infants were screened daily for jaundice using the BiliChek Transcutaneous Bilirubinometer (Philips Healthcare North America, Monroeville, PA) before newborn discharge from the hospital, for up to 7 days of life, and after discharge whenever possible up to 14 days if mothers brought their infants in to be checked for jaundice.
Infants were enrolled in the study if their TB (measured using a BR-2 Bilirubin StatAnalyzer; Advanced Instruments, Inc, Norwood, MA) exceeded 3 mg/dL below the recommended level for initiating PT as per the American Academy of Pediatrics (AAP) PT guideline for high-risk infants.14 This entry level was chosen because in most Nigerian hospitals, PT is routinely started at lower levels, because of the high incidence of glucose-6-phosphate dehydrogenase deficiency, ABO incompatibility, and limited use of Rho(D) Immune Globulin (Rhogam). Infants were excluded from the study if they were clinically unstable for FS-PT, needed treatment, such as oxygen therapy that could not be performed under FS-PT, met criteria for an exchange blood transfusion, or if consent was not obtained. Because of the high incidence of acute bilirubin encephalopathy and the generally poor quality of phototherapy, exchange blood transfusions are performed when bilirubin levels are ≥5 mg/dL in infants <24 hours of age and phototherapy is initiated when bilirubin levels are >12 mg/dL regardless of an infant’s age. Phototherapy is discontinued when bilirubin levels fall below 12 mg/dL. Additionally, it was felt that these conservative guidelines were appropriate because FS-PT was a previously untested, novel mode of therapy. FS-PT was discontinued when the infant’s TB level fell below that needed to meet the criteria for enrollment.
An educational video on diagnosis, prevention, and treatment of severe neonatal jaundice was produced and presented to parents or caretakers of the patient by one of the authors (BOO).15 This provided a nonthreatening format forum for all to ask questions and receive answers and for discussion of common harmful practices and beliefs.
Sunlight was filtered using commercially available plastic window-tinting films.12 After extensive testing, 2 films were selected: the Air Blue 80 (CP Films, Inc, subsidiary of Eastman Chemical Co, Fieldale, VA) (Canopy I) and Gila Titanium (CP Films, Inc) (Canopy II). Both films excluded virtually all UV-B and UV-A radiation. The Air Blue 80, chosen for use during overcast sky periods, transmits 79% of the >400- to 520-nm wavelength blue light and only 0.1% of the 315- to 400-nm UV-A. The Gila Titanium film, chosen for periods of direct sunlight, transmits 33% of the 400- to 520-nm wavelength light and 0.4% of the UV-A radiation. During laboratory testing, the 2 films provided partial shade that reduced the temperature under a cloudy and cloudless sky by 6.0°C and 9.5°C, respectively.12 The films (240 × 360 cm or 8 × 12 ft) were each stretched over separate 0.750-inch white polyvinyl chloride irrigation tube frames, which were internally reinforced with 0.375-inch thick steel rebar, creating a treatment canopy large enough (240 cm [8 ft] wide × 240 cm [8 ft] long × 180 cm [6 ft] high) to accommodate 6 infants and their mothers (see Fig 1). The films’ edges were reinforced with folded-over 5-cm (2-inch) wide blue painter’s tape, fitted with peripheral 12-mm (0.5-inch) brass grommets, and secured to the frame with rubber bands. The cost of the film for a typical canopy ranged from approximately $200 for the Air Blue 80 film to $50 for the Gila Titanium. Additional costs for the frames, rebar, tape, grommets, and so forth were ∼$50 per canopy. Infants were treated under each canopy as dictated by either cloudy or clear sky. Because of weather and wear, the canopies were replaced every 6 months approximately. Typically, depending on the time of the year, the sun at zenith in a clear sky delivered an irradiance of 120 µW/cm2/nm of blue light, as measured with a Bili-Blanket Meter II (General Electric, Fairfield, CT). When filtered by Gila Titanium film canopy, the remaining treatment irradiance was approximately (0.33 × 120 µW/cm2/nm=) 40 µW/cm2/nm, an irradiance well above the level of what is defined as intensive PT by the AAP.14 In contrast, the irradiance delivered by an overcast sky was ∼25 µW/cm2/nm. Under this condition, the maximal irradiance delivered by the Air Blue 80 film was (0.79 × 25 μW/cm2/nm=) 20 µW/cm2/nm. Canopies were placed in a location suitable for daylong sun exposure, preferably one also protected from potentially violent wind currents. Irradiance measurements were recorded every 30 to 60 minutes outside of and under the canopy using a Bili-Blanket Meter II.
FIGURE 1.

Aerial photograph of canopies (note: nurses put a white sheet under the canopy they were not using for their work area and moved that as needed when infants moved, on right side in the photo).
After eligible infants were placed under the FS-PT canopy, each infant’s axillary body temperature (ABT) was measured and clinical evaluation for evidence of sunburn (new-onset pink skin) or dehydration (dry mucus membranes, sunken eyes, dry eyes, skin tenting) was performed hourly. Infants were removed from FS-PT when the ABT fell to <35.5°C or exceeded 38°C. If the ABT returned to >35.5°C within 1 hour, FS-PT was resumed. If the ABT did not return to >35.5°C within 1 hour after removal, infants exited FS-PT on that day, but were eligible to resume FS-PT on the following day. If moderate hypothermia or hyperthermia (<35°C or >39°C) occurred more than once, infants were excluded from any further FS-PT. To promote normothermia, we placed infants on wet white towels during hotter periods of the day, when an infant’s temperature began to rise above ∼37.5°C, or when an infant’s temperature was >38.0°C. Conversely, infants were wrapped up and/or brought inside to warm up if they had hypothermia.
Total bilirubin was determined daily before initiation and on termination of treatment. The goal was to place infants under FS-PT for 5 to 6 hours per day because maximum irradiance occurred at midday. Early morning and late afternoon irradiances were often subtherapeutic and staffing issues precluded longer days. Whenever possible, mothers stayed with and cared for their infants during FS-PT. To maximize the efficacy of FS-PT, only white fabrics were used under the canopies. Infants were placed on white sheets, beds were covered with white cloths, mothers wore white gowns while seated on white chairs to care for their infants, and nurses wore white aprons.16 Elastic sock tops (2–4 cm wide) were used for eye covers owing to lack of availability of commercial eye patches and the need to use economical, local resources.
Definitions
Treatment Day
A day on which an infant received any FS-PT regardless of the duration.
Safety
Treatment was deemed safe if the infant was able to stay in FS-PT for ≥5 hours per day. Furthermore, treatment days were considered safe if they were shorter than 5 hours due to late arrival of the infant for FS-PT or the onset of rain. However, treatment days <5 hours of FS-PT were not evaluated for efficacy.
Efficacy
Treatment was considered efficacious if the rate of rise of TB was less than 0.2 mg/dL/h for an infant ≤72 hours of age or if TB decreased for an infant >72 hours of age. These criteria were chosen based on data for infants admitted <5 days of age with kernicterus that estimated a rate of TB rise of >0.25 mg/dL/h.17 These estimates are consistent with the recommendations that a TB rise of >0.5 mg/dL/h in Rh-associated hemolytic disease of the newborn is an absolute indication for a blood exchange transfusion.18
Ethical Approval
This study was approved by the Institutional Review Boards/Ethical Committees of the University of Minnesota, Minnesota Medical Research Foundation (Hennepin County Medical Center), and the Lagos State Government. Written informed consent was obtained by trained research assistants.
Statistical Analysis
Study data were initially recorded on paper forms and then entered into an Excel spreadsheet. Selected study data from the treated infants were also entered into a REDCap database.19 Demographic and blood test data were summarized for the treated infants and their mothers. Irradiance data were summarized for inside and outside of the canopies. Treatment outcomes were tabulated and exact binomial 95% confidence intervals were reported. Data cleaning and statistical analyses were carried out using the statistical software package R, version 2.14.1 (open source statistical software package, R Project, http://www.r-project.org/).
Data are presented as mean ± SD unless otherwise indicated.
Results
A total of 826 neonates were screened for neonatal jaundice; 227 (27.5%) infants (99 girls and 126 boys, 2 not recorded) met treatment criteria and were placed under FS-PT. Recorded characteristics of the treated infants are shown in Table 1. The mean age and TB levels at first FS-PT were 36 ± 23 hours and 7.9 ± 2.9 mg/dL, respectively. Of the 227 infants placed under FS-PT, 25 infants (11.0%) required a second day of treatment, and 6 infants (2.6%) required a third day, giving a total of 257 treatment days. On average, each treated infant received 1.13 days of PT. The total number of hours of phototherapy that an infant received, summed over all treatment days, ranged from 0.2 hours to 19.9 hours, with the median being 6.2 hours and a mean of 6.6 hours.
TABLE 1.
Infant Characteristics (n = 227)
| Characteristic | Mean ± SD (Range) |
|---|---|
| Birth weight, kg, n = 224a | 3.1 ± 0.5 (2.0–4.5) |
| Gestational age, wk, n = 226a | 38 ± 1.5 (27–43) |
| Age at first FS-PT, h, n = 215a | 36 ± 23 (10–166) |
| Entry TB on first FS-PT day, mg/dL, n = 220a | 7.6 ± 2.9 mg/dL (2.3–19.1) |
| Exit TB on first FS-PT day, mg/dL, n = 176a | 7.1 ± 2.5 mg/dL (3.0-14.3) |
Totals are less than 227 due to missing data.
Safety
Minor adverse events related to temperature occurred on 85 occasions, 24 with hypothermia and 61 with hyperthermia (Fig 2). Most (61 occasions, 71%) were due to mild hyperthermia with ABT >38.0°C but <39.1°C. One infant had a temperature of 39.4°C on 1 occasion, which decreased to 37.2°C after placing the infant in the shade for 19 minutes. No infant developed hyperthermia before 11:00 am. The peak period for hyperthermia was between 12:00 and 1:00 pm when almost half (46%) of the episodes occurred. Mild hypothermia (35.0–35.4°C) occurred in 21 (8.1%) of the treated infants (Fig 2). Three infants developed moderate hypothermia with ABT of <35.0°C on a single occasion (34.9°C, 33.7°C, 34.5°C). Hypothermic episodes where fewer in number but occurred throughout the day without any specific pattern. No infant incurred sunburn or significant dehydration as documented by a physician. No infant met study exit criteria.
FIGURE 2.
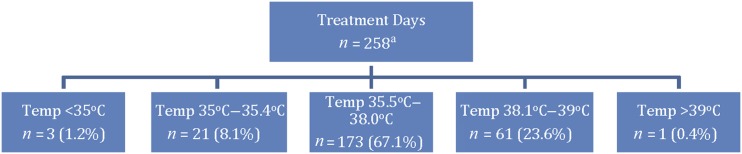
Treatment days with 1 or more axillary temperature excursions in the designated ranges. On 174 treatment days, no temperature excursions occurred. aOne infant was too hot and too cold in 1 day, accounting for 259 events.
Efficacy
There were 258 total treatment days; 91% of days were ≥5 hours; 77.5% had both pre and post TB reported. FSPT was efficacious on 92% of evaluable treatment days (Table 2). The average TB change rate was –0.06 ± 00.19 mg/dL/h for all evaluable treatment days. The TB change rate varied between groups based on age and initial TB level (Table 2).
TABLE 2.
Efficacy of FSPT as a Function of Both Age and Initial Total Serum/plasma Bilirubin (TB) Level for 197 Evaluable Treatment Days
| Age at Morning TB | Initial TB Level | Efficacious Days/Total Days | % Efficacious (95% CI) | TB Change Rate in mg/dL/h, Mean ± SD (Range) |
|---|---|---|---|---|
| All | All | 181/197 | 91.9% (87.1%–95.3%) | −0.060 ± 0.188 (−0.837 to +0.409) |
| ≤72 h old | Low, <12 mg/dL | 157/168 | 93.5% (88.6%–96.7%) | −0.042 ± 0.171 (−0.789 to +0.385) |
| ≤72 h old | High, ≥12 mg/dL | 12/13 | 92.3% (64.0%–99.8%)a | −0.201 ± 0.302 (−0.837 to +0.205) |
| >72 h old or unknown age | Low, <12 mg/dL | 4/6 | 66.7% (22.3%–95.7%)a | −0.017 ± 0.224 (−0.221 to +0.409) |
| >72 h old or unknown age | High, ≥12 mg/dL | 8/10 | 80.0% (44.4%–97.5%)a | −0.194 ± 0.148 (−0.379 to +0.032) |
The wide confidence interval (CI) is due to the small sample size in this category.
Irradiance
Irradiance data were recorded for 215 of the 227 treated infants in the study, on 243 of the 257 treatment days, spanning 109 unique calendar days. Of the 2643 (99.4%) irradiance measurements with known times, 2441 (92.4%) were taken under Canopy I (Air Blue 80), 46 (1.7%) were taken under Canopy II (Titanium), and 156 (5.9%) were not identified. Because most treatment days were under Canopy I, measurements for canopies were combined.
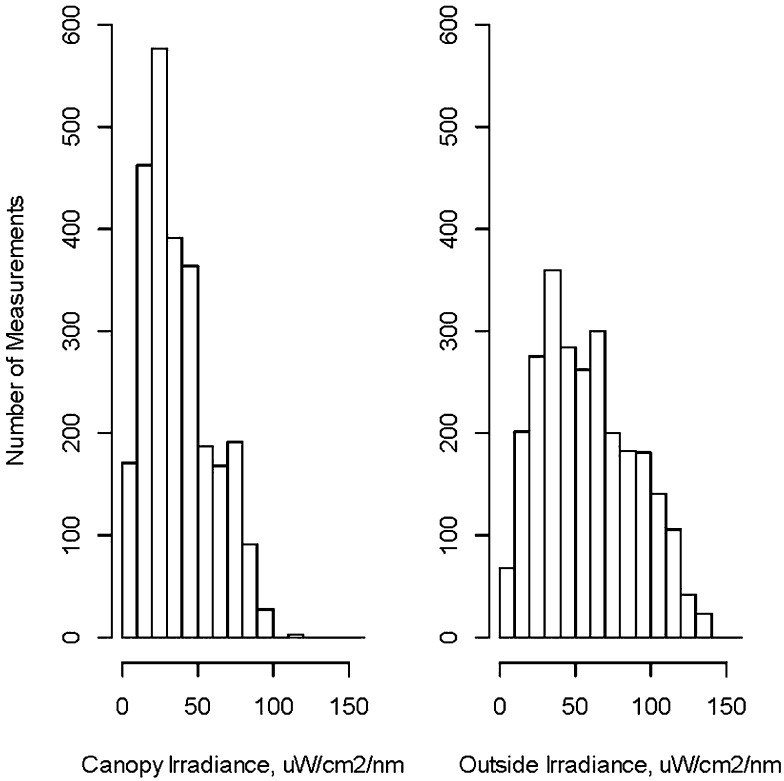
The average hourly or semihourly irradiance measurements (both canopies combined) was 38 ± 22 (range 2–115) µW/cm2/nm inside the canopy and 59 ± 31 µW/cm2/nm (range 4–157) outside the canopy (Fig 3). The irradiance inside the canopy exceeded 8 µW/cm2/nm (AAP guideline for conventional PT14) for 97.5% and exceeded 30 µW/cm2/nm (defined by the AAP as intensive PT14) for 56% of all measurements. As expected, the maximum irradiances were generally higher during the middle of the day both inside and outside the canopy but the irradiances varied widely depending on cloud cover. During midday when ambient temperatures were higher, infants had to be monitored more closely for elevated temperatures and, as noted previously, were often placed prophylactically on wet white towels during these periods of the day. See supplemental information regarding change in TB levels after a day of FSPT and TB level at age of first treatment.
FIGURE 3.
Distribution of the hourly or semi-hourly irradiance measurements under the canopy (left) and outside the canopy (right).
Discussion
The current AAP-recommended treatment of neonatal jaundice using intensive PT is exposure to blue light (spectral range of 400–520 nm and peak emission of 450 ± 10 nm) with an irradiance (intensity) of 30 µW/cm2/nm.14 Blue light of this quality and intensity effectively alters the bilirubin molecule by photoisomerization/degradation, so that the bilirubin can be readily excreted into bile and cleared via the intestinal tract and kidneys. Our results demonstrate that FS-PT is safe and efficacious in our population. Moreover, the daily average irradiance inside the canopies used for this study (range 8–65 µW/cm2/nm) met or exceeded the AAP guideline14 for conventional PT (8–10 µW/cm2/nm) 100% of the time (243/243 days measured) and exceeded that needed for intensive PT (≥30 µW/cm2/nm) 69% of the time. Currently available commercial PT devices using fluorescent, halogen, fiberoptic, or light-emitting diode lamps deliver irradiances between 10 and 60 μW/cm2/nm, depending on the device.20 Although the devices with high irradiance appear to be most effective, maximally effective irradiance levels have not yet been established.
FS-PT was well accepted by both mothers and hospital staff. Using large canopies kept mothers and their infants together, facilitating bonding, care, and feeding. It also promoted socialization between mothers and enabled simultaneous treatment of multiple mother/infant pairs. Low cost and sustainable measures, such as placing the infant on a wet towel when the ambient temperature was high or when the infant’s temperature began to rise improved the safety profile. Other low-cost measures that improved safety and/or efficacy included lining the bed with white cloths,16 and providing white aprons for the mothers and low-cost eye patches made from sock tops.
The utility of FS-PT depends on the amount of daily sunlight available and is therefore most likely to be applicable in geographic areas where the proportion of daily direct sunlight is high and where seasonal variation is reduced. Additionally, this study would need to be replicated in other locations and other climates to develop local adaptations to make it both safe and effective in low-resources settings. However, because sunlight appears to be more effective than conventional PT, a shorter period of treatment may be required.10 Solar-powered or battery backups for conventional PT devices would be required to provide PT around the clock or during times when sunlight was inadequate. In colder climates, a room with a glass/plexiglass roof would be required to maintain normothermia. Furthermore, a permanent or semipermanent structure to protect the films from rain and storms would significantly improve the longevity of the films and would be helpful toward making FS-PT sustainable in low-resource settings. No subject progressed to an exchange blood transfusion level. However, due to our study design, infants were screened early and even in the absence of any clinical jaundice. Therefore, when FS-PT is used in other more standard settings after the onset of clinical neonatal jaundice, it would be expected that some infants might progress to exchange levels.
Strengths of FS-PT include the ability to provide effective low-cost PT without electricity and relatively expensive conventional PT devices; to provide a forum to educate both mothers and health care providers on the prevention, recognition, and treatment of neonatal jaundice; and to allow mothers to interact with each other as well as with the health care providers while caring for their infants without interrupting therapy.
The limitations of FS-PT in this study include the need to import the film material, locally manufacture the canopies, as well as difficulties of protecting the films during stormy weather, lack of ability to provide FS-PT at night or on rainy days, and inability to use open-ended canopies in colder climates. However these limitations can be addressed by encouraging local production of films and/or keeping import costs low by working with companies investing in resource-poor settings, building glass- or plexiglass-roofed buildings for larger health care centers or hospitals using this technology, and heating these structures in colder climates. Additionally, because FS-PT was a new therapy, this trial was not designed to test maximum clinical efficacy.
Further study is needed to enable pragmatic and safe scaling-up of this novel potentially life-saving therapy to other urban and more remote health centers. Expected challenges, which are not insurmountable, include maintenance of normothermia where temperature monitoring is not routinely available, monitoring TB levels without standard laboratory analysis, and timely referral to a higher level of care. It will be important to evaluate this treatment of infants with higher TB levels in more urgent treatment scenarios. Until this information is available, infants placed under FS-PT need to continue to be closely monitored, as was done in this study. A randomized controlled noninferiority trial comparing FS-PT with conventional PT in Nigeria is currently ongoing.21
Conclusions
With appropriate infant temperature monitoring, the use of FS-PT ≥5 hours per day, offers a novel, yet practical, inexpensive, safe, and efficacious strategy for the management of neonatal jaundice in areas of the world where no other treatment is readily or only sporadically available.
Supplementary Material
Acknowledgments
The authors thank the Staff and Mothers at Island Maternity Hospital, Vinod K. Bhutani, MD, FAAP, professor of pediatrics, Stanford University, for his contribution to the analysis of efficacy of the FS-PT and consultation regarding the study, and Tu Quan, MPH, research manager, and Bonnie Crissman, grants administrator, for their extra support in administrating this grant.
Glossary
- AAP
American Academy of Pediatrics
- ABT
axillary body temperature
- FS-PT
filtered-sunlight phototherapy
- PT
phototherapy
- TB
total serum/plasma bilirubin
Footnotes
Dr Slusher conceptualized and designed the study, approved the design and implementation of the study, coordinated and supervised data collection, drafted the initial manuscript, and critically reviewed and revised the manuscript; Dr Vreman contributed to the design of the study and took responsibility for the selection of the films, design, procurement and building of the treatment canopies, and also critically reviewed and revised the draft manuscript; Dr Olusanya approved the design and implementation of the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript; Mr Wong and Dr Stevenson contributed to the design of the study, participated in the film-selection studies, and critically reviewed and revised the draft manuscript; Dr Brearley served as the statistician for the study, and critically reviewed and revised the manuscript; Dr Vaucher contributed to study design, data analysis, and manuscript preparation, and also critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Thrasher Research Fund, Salt Lake City, UT. Philips Healthcare (Monroeville, PA) donated the BiliChek Transcutaneous Bilirubinometer and a portion of the probes; Advanced Instruments (Norwood, MA) donated the Advanced BR2 Bilirubin Stat-Analyzer. Clinical and Translational Science Institute grant support (UL1TR000114 from the National Center for Research Resources).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Young Infants Clinical Signs Study Group . Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;371(9607):135–142 [DOI] [PubMed] [Google Scholar]
- 2.Slusher TM, Olusanya BO. Neonatal jaundice in low and middle income countries. In: Stevenson DK, Maisels MJ, JF Watchko, eds. Care of the Jaundiced Neonate. New York, NY: McGraw-Hill; 2012:263–273 [Google Scholar]
- 3.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379(9814):445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunlesi T, Ogundeji M, Ogunfowora O, Olowu A. Socioclinical issues in cerebral palsy in Sagamu, Nigeria. SAJCH. 2008;2:12024 [Google Scholar]
- 5.Olusanya BO, Somefun AO. Sensorineural hearing loss in infants with neonatal jaundice in Lagos: a community-based study. Ann Trop Paediatr. 2009;29(2):119–128 [DOI] [PubMed] [Google Scholar]
- 6.Owa JA, Adebami OJ, Fadero FF, Slusher TM. Irradiance readings of phototherapy equipment: Nigeria. Indian J Pediatr. 2011;78(8):996–998 [DOI] [PubMed] [Google Scholar]
- 7.Cline BK, Vreman HJ, Faber K, et al. Phototherapy device effectiveness in Nigeria: irradiance assessment and potential for improvement. J Trop Pediatr. 2013;59(4):321–325 [DOI] [PubMed] [Google Scholar]
- 8.Cremer RJ, Perryman PW, Richards DH. Influence of light on the hyperbilirubinaemia of infants. Lancet. 1958;1(7030):1094–1097 [DOI] [PubMed] [Google Scholar]
- 9.Harrison SL, Buettner PG, MacLennan R. Why do mothers still sun their infants? J Paediatr Child Health. 1999;35(3):296–299 [DOI] [PubMed] [Google Scholar]
- 10.Salih FM. Can sunlight replace phototherapy units in the treatment of neonatal jaundice? An in vitro study. Photodermatol Photoimmunol Photomed. 2001;17(6):272–277 [DOI] [PubMed] [Google Scholar]
- 11.Olowe SA. Sunshine phototherapy cot: utilization of sunlight for phototherapy. Niger J Paediatr. 1985;12:6970 [Google Scholar]
- 12.Vreman HJ, Slusher TM, Wong RJ, Schulz S, Olusanya BO, Stevenson DK. Evaluation of window-tinting films for sunlight phototherapy. J Trop Pediatr. 2013;59(6):496–501 [DOI] [PubMed] [Google Scholar]
- 13.Slusher T, Cline B, Amubunosi E, Ofovwe G, Wong R, Stevenson D, Vreman H. Selectively filtered sunlight phototherapy for treatment of neonatal jaundice in Nigeria. E-PAS2011 2011;2918.254.
- 14.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia . Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316 [DOI] [PubMed] [Google Scholar]
- 15.Olusanya BO. Educational video for training mothers about neonatal jaundice. 2012.
- 16.Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358(9):920–928 [DOI] [PubMed] [Google Scholar]
- 17.Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 2009;29(suppl 1):S25–S45 [DOI] [PubMed] [Google Scholar]
- 18.Moller J, Ebbesen F. Phototherapy in newborn infants with severe rhesus hemolytic disease. J Pediatr. 1975;86(1):135–137 [DOI] [PubMed] [Google Scholar]
- 19.Harris PATR, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vreman HJ, Wong RJ, Murdock JR, Stevenson DK. Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr. 2008;97(3):308–316 [DOI] [PubMed] [Google Scholar]
- 21.Slusher TM, Olusanya BO, Vreman HJ, et al. Treatment of neonatal jaundice with filtered sunlight in Nigerian neonates: study protocol of a non-inferiority, randomized controlled trial. Trials. 2013;14(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.