Genome-wide association mapping identified four stage-specific QTLs that affect the Arabidopsis heat response and analysis of QTLs revealed candidate genes.
Abstract
For crops that are grown for their fruits or seeds, elevated temperatures that occur during flowering and seed or fruit set have a stronger effect on yield than high temperatures during the vegetative stage. Even short-term exposure to heat can have a large impact on yield. In this study, we used Arabidopsis thaliana to study the effect of short-term heat exposure on flower and seed development. The impact of a single hot day (35°C) was determined in more than 250 natural accessions by measuring the lengths of the siliques along the main inflorescence. Two sensitive developmental stages were identified, one before anthesis, during male and female meiosis, and one after anthesis, during fertilization and early embryo development. In addition, we observed a correlation between flowering time and heat tolerance. Genome-wide association mapping revealed four quantitative trait loci (QTLs) strongly associated with the heat response. These QTLs were developmental stage specific, as different QTLs were detected before and after anthesis. For a number of QTLs, T-DNA insertion knockout lines could validate assigned candidate genes. Our findings show that the regulation of complex traits can be highly dependent on the developmental timing.
INTRODUCTION
One of the major challenges in the coming decades is to ensure that global food production will increase proportionally to the increase in the global human population. High crop yields must be sustained, although climate change is expected to produce more extreme weather conditions, including longer droughts and hotter summers. Plant breeding for resistance to abiotic stresses, like drought or heat, will play a crucial role in facing this challenge. To achieve this goal, it is crucial to identify the natural range of variation for these traits within species and to gain a profound understanding of the mechanistic basis of regulatory processes. Both goals can be met by identifying key regulatory genes in large phenotypic screens of genetically diverse mapping populations, either naturally or experimentally derived.
Crops can roughly be divided into two classes: crops grown for total biomass and crops grown for seed or fruit production. For the latter, the impact of abiotic and biotic stresses on yield should be investigated during both the vegetative and the generative phases of the plant’s life cycle since both are strongly related to reproductive success. Temperature stress is one of the most detrimental abiotic stresses during the generative phase and reduced yields due to high temperatures during flowering have been reported for many species (Hedhly, 2011; Bita and Gerats, 2013). Heat stress during flowering of crops already has a large impact on the global food production, and this impact will increase in the coming years because of expected climatic changes. For example, in the year 2000, 15% of the global harvested area of maize (Zea mays) was exposed to at least five days of heat stress during the flowering period, and by 2050 this is expected to increase to 44% (Gourdji et al., 2013). Besides maize, many other cereals, such as wheat (Triticum aestivum), barley (Hordeum vulgare), and rice (Oryza sativa), are affected by high temperatures during the reproductive phase (Barnabás et al., 2008). Negative effects of heat stress have also been reported for other important crops from various plant families, such as tomato (Solanum lycopersicum) (Sato et al., 2002, 2006) and pepper (Capsicum annuum) (Marcelis et al., 2004) from the Solanaceae family, canola and mustard (Brassica juncea, Brassica rapa, and Brassica napus) (Angadi et al., 2000; Gan et al., 2004) from the Brassicaceae family, soybean (Glycine max) (Djanaguiraman et al., 2013) and chickpea (Cicer arietinum) (Clarke and Siddique, 2004) from the Fabiaceae family, and peach (Prunus persica) (Hedhly et al., 2005) and cherry (Prunus avium) (Hedhly et al., 2004) from the Rosaceae family. Thus, heat stress is an important factor that affects crop yields worldwide.
Abiotic and biotic stresses affect yield during both the vegetative and the generative phase of the plant’s life cycle, but heat stress is particularly detrimental during the reproductive stage of seed- and fruit-producing crops (Wahid et al., 2007). However, the sensitivity to temperature stress varies during flower and seed development. Two sensitive periods have been identified: during meiosis and around fertilization. Many studies, e.g., in cereals, tomato, and Arabidopsis thaliana, have shown that the development of male reproductive organs is more sensitive to elevated temperatures than the development of female reproductive organs (Kim et al., 2001; Sato et al., 2006). These studies showed that heat stress inhibits anther dehiscence, thereby reducing the release of pollen. Heat stress also leads to shortening of the anthers, due to a reduced amount of auxin (Sakata et al., 2010), which results in a mismatch between the ripening of the stigma and anthers, preventing self-fertilization in bisexual monoecious plants. Moreover, viability of pollen is reduced by disruption of male meiosis upon heat stress (Endo et al., 2009). In addition to disturbance of the development of male organs and pollen, changes in the development of the female organs have also been reported. Besides malformation of the ovule and surrounding tissues (Hedhly, 2011), changes in the receptivity of the stigma, resulting in a decrease of the number of pollen tubes reaching the ovary, are described (Saini and Aspinall, 1982). The timing and severity of the stress determine to a large extent which physiological processes are disrupted. However, responses are similar in many species, even when they are not closely related (Hedhly, 2011). This makes the study of these processes in a model plant like Arabidopsis very relevant. Surprisingly, however, many studies in this species focus on the impact of elevated temperatures on germination (Baskin and Baskin, 1983; Schmuths et al., 2006; He et al., 2014; Silva-Correia et al., 2014), flowering time (Balasubramanian et al., 2006; Y. Li et al., 2014), and plant architecture (Wahid et al., 2007; Patel and Franklin, 2009; Antoun and Ouellet, 2013), but only very few address the impact of heat on fertility (Kim et al., 2001; (Warner and Erwin, 2005; Zinn et al., 2010).
In many species, heat-tolerant and -sensitive cultivars have been identified. Also, a study in Arabidopsis reported natural variation for heat response during flowering (Warner and Erwin, 2005). Unfortunately, much less is known about the genetic factors that cause this variation in heat response. To identify the mechanisms responsible for heat tolerance, heat-tolerant and -sensitive cultivars or variable natural accessions can be compared at the physiological, molecular, and genetic levels. In tomato, metabolite and gene expression profiles have been compared in cultivars with different levels of heat tolerance. In anthers of tolerant cultivars, higher constitutive expression of heat shock proteins and heat shock transcription factors was observed (Bita et al., 2011). Moreover, higher invertase activity was reported upon heat stress in tolerant cultivars (Dorion et al., 1996; Li et al., 2012), and downregulation of a proline transporter was observed in sensitive cultivars (Sato et al., 2006). This information may be helpful for understanding tolerance mechanisms, but to be useful for breeding of temperature-tolerant crops, allelic variation in genes promoting heat tolerance needs to be identified.
Natural variation in heat tolerance between cultivars can be used to detect useful alleles in segregating mapping populations. Moreover, such studies reveal the actual genetic regulators driving a specific response, in contrast to identifying transcriptional differences upon heat stress in gene expression studies, which identifies many downstream effects of the response. Biparental mapping populations have been developed and used for quantitative trait locus (QTL) mapping of heat tolerance in various crops, such as rice (Ye et al., 2012), cowpea (Vigna unguiculata) (Lucas et al., 2013), and tomato (Grilli et al., 2007). These studies identified multiple QTLs (from 2 to 18) with an explained variance between 2 and 20%. These studies indicate that QTL analyses and subsequent fine-mapping and cloning are a promising way of identifying heat tolerance genes. In addition, the number of QTLs detected in these studies and the relatively low explained variance for most of them indicate that heat tolerance during flowering is a multigenic trait. Unfortunately, none of the studies referred to identified the underlying causal genes. Such identification in classical linkage studies is time-consuming because of the wide regions that are associated with the trait of interest. Genome-wide association (GWA) mapping in natural populations can therefore be of great use because, due to the fast linkage decay, the resolution is much higher and therefore fine-mapping is often not needed to identify candidate genes (Bergelson and Roux, 2010). Because similar heat responses are observed in many species, heat tolerance genes identified by GWA mapping in a natural population of Arabidopsis will likely have homologous equivalents in crops. Considering the variation in heat stress sensitivity observed for different developmental stages and genotypes in various species, it is timely to combine the analysis of these observations in a single study. This offers the opportunity to identify natural variation in the key regulators in the response to heat stress that exert their function in a specific phase of development. These insights might provide a deeper understanding of the regulation of developmental timing and the natural strategies and mechanisms by which plants cope with abiotic stresses.
We aimed to investigate in a systematic way the genetic basis of heat tolerance for various developmental phases in the reproduction of Arabidopsis. Not all stages of flower and seed development were equally sensitive, and we were able to distinguish between heat responses before and after anthesis. GWA mapping was performed on data obtained for different regions along the inflorescence, resulting in developmental stage-specific QTLs. Strong associations could be observed for pre- and postanthesis heat response, and several candidate genes were identified that have not previously been associated with heat stress. A number of these candidate genes could be functionally validated by T-DNA insertion knockout analyses and artificially induced mutants, providing confidence in the applied methods.
RESULTS
To investigate natural variation in the reduction of fertility caused by short-term elevated temperatures, a collection of natural accessions of Arabidopsis was subjected to heat stress and subsequently used for GWA mapping.
Quantification of Heat Stress Response by Determination of Silique Length
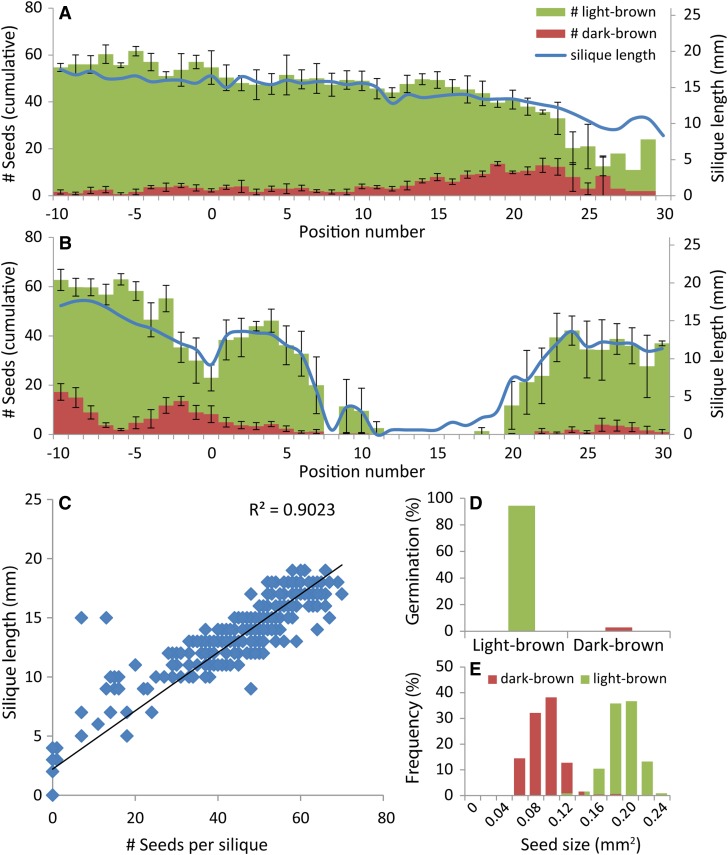
Flowering plants of a wide range of natural accessions of Arabidopsis were exposed to a 1-d treatment at 35°C. First, we tested if the effect of heat stress could be quantified by taking the length of each silique along the main inflorescence as a measure for the number of seeds. Accession Di-1 (origin Dijon, France), which showed an intermediate response to the heat treatment, was selected for detailed analysis of silique length, seed size, and seed number per silique for all siliques along the main inflorescence (Figure 1). A linear correlation between the length of the silique and the number of seeds per silique was observed (Figure 1C; r2 = 0.90). Such a high correlation has also been reported previously for accessions Landsberg erecta and Cape Verde Islands (Alonso-Blanco et al., 1999). The reduction in silique length is predominantly caused by fewer seeds per silique rather than by smaller seeds. Two types of seeds were typically observed: a majority of light-brown seeds and a lower number of dark-brown small seeds (Figure 1E). Germination tests revealed that most small dark-brown seeds were not able to germinate while almost all light-brown seeds did germinate (Figure 1D). The small dark-brown seeds were mainly present in siliques in which embryogenesis had already started at the day of the treatment. Therefore, these small dark-brown seeds are most probably aborted seeds. These putative seed abortions did not lead to shorter siliques, which indicates that the length of the silique is a measure of successful fertilization, resulting in the initiation of embryogenesis, and might not always reflect the number of viable seeds within the silique. Siliques from control plants also contained a few small dark-brown seeds (Figure 1A), which indicates that seed abortion does not only result from heat stress, but may be caused by other factors as well.
Figure 1.
Silique Length, Seed Number, and Seed Size per Silique along the Main Inflorescence of Di-1 Plants and Germination Rates of the Seeds.
(A) and (B) Comparison of silique length and number of seeds (light and dark brown) in control (n = 3 for seed size; n = 5 for silique length) and heat-treated (n = 5) conditions ([A] and [B], respectively). Error bars represent sd. Position numbers are relative to the flower that opened first at the day of the treatment, which received position number 0.
(C) Linear correlation between silique length and number of seeds per silique based on data represented in (A) and (B).
(D) Germination rate of light-brown (n = 213) and dark-brown (n = 244) seeds of heat-treated plants.
(E) Size distribution of light-brown (n = 575) and dark-brown (n = 526) seeds of heat-treated plants.
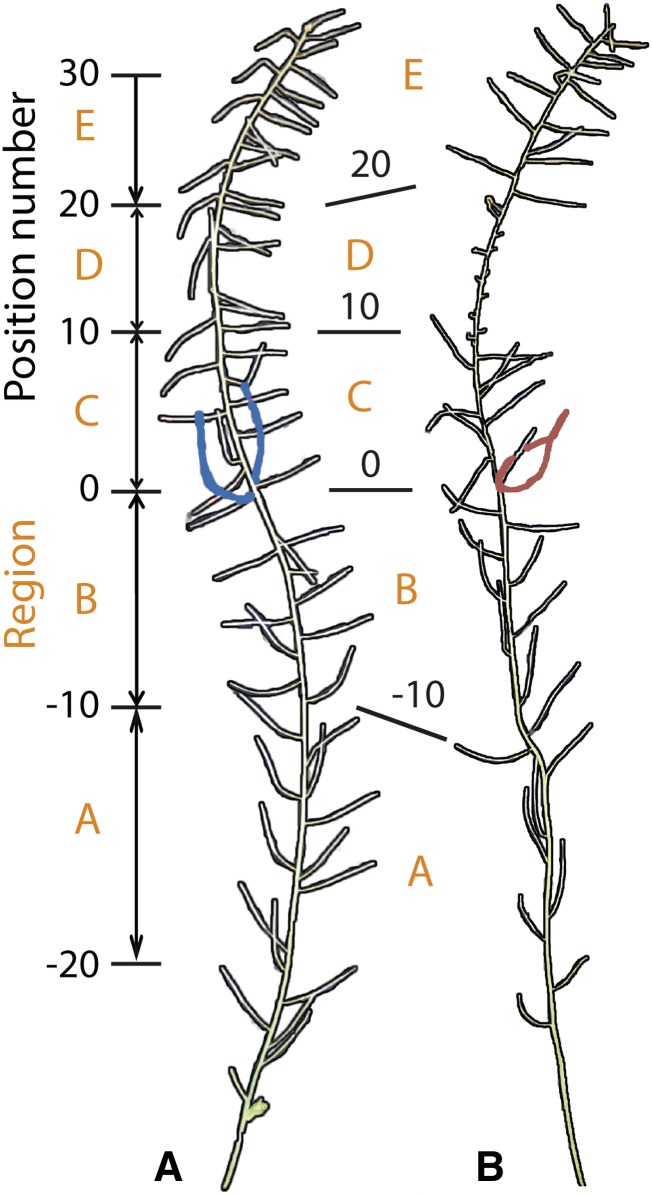
The flower that opened first on the day of the treatment was tagged for each replicate of 285 accessions used in the experiment. This tag enabled us to relate the effect of heat stress to the developmental stage of the flowers and siliques along the inflorescence (Figure 2). In reference to the tag, all siliques received a relative position number and we subsequently defined five developmental regions, A to E. To link the observed reduction in silique length with the disruption of flower development and embryogenesis, we matched the regions as defined in Figure 2 with data regarding timing and order of events taking place during these developmental processes in Arabidopsis (Table 1; Smyth et al., 1990; Mansfield and Briarty, 1991; Schneitz et al., 1995; Sanders et al., 1999). Region A (silique position numbers −20 to −10) is linked to late embryogenesis. Region B (silique position numbers −10 to 0) is related to processes that take place shortly after anthesis, like fertilization and early embryogenesis. Region C (silique position numbers 0 to 10) is related to processes just before anthesis, like anther elongation and female meiosis, and region D (silique position numbers 10 to 20) is linked to processes that take place some days before anthesis, like male meiosis. Finally, region E (silique position numbers 20 to 30) corresponds to early flower development. Although we defined each region by a fixed number of siliques without differentiating between accessions, we note that natural variation was observed for the number of flowers that opened per day. Even though such phase shifts in developmental timing might introduce noise, this did not negatively affect later analyses, probably because the size of the assigned regions is relatively large in comparison with the observed differences between accessions. Therefore, we assume that the developmental processes assigned to the different regions are correct for most accessions and analyses described hereafter.
Figure 2.
Outline of Main Inflorescence.
Outline of photographed inflorescences of a representative susceptible accession grown in control (A) and heat-treated (B) conditions. Definition of position number and developmental regions; the silique bearing the tag originated from the flower that opened first at the day of the treatment. Position numbers are relative to the tag. Five regions (A to E) were defined each containing ten siliques and representing different developmental stages as indicated in Table 1.
Table 1. Matching of Landmark Events in Flower and Seed Development with the Developmental Regions along the Inflorescence as Defined in Figure 2.
| Stagea | Landmark Event at Beginning of Stagea | Duration (h)a | Landmark Event in Reproduction and Seed Developmentb | Regionc | ||
|---|---|---|---|---|---|---|
| 1 | Flower buttress arises | 24 | ||||
| 2 | Flower primordium forms | 30 | ||||
| 3 | Sepal primordia arise | 18 | ||||
| 4 | Sepals overlie flower meristem | 18 | ||||
| 5 | Petal and stamen primordia arise | 6 | ||||
| 6 | Sepals enclose bud | 30 | E | |||
| 7 | Long stamen primordia stalked at base | 24 | E | |||
| 8 | Locules appear in long stamens | 24 | E | |||
| 9 | Petal primordia stalked at base | 60 | Male meiosis | E | D | |
| 10 | Petals level with short stamens | 12 | D | |||
| 11 | Stigmatic papillae appear | 30 | Anther elongation | Female meiosis | C | D |
| 12 | Petals level with long stamens | 42 | Anther elongation | C | D | |
| 13 | Bud opens, petals visible, anthesis | 6 | Anther dehiscence, pollen tube growth, fertilization | C | B | |
| 14 | Long anthers extend above stigma | 18 | Early embryogenesis until heart stage | Anther dehiscence, pollen tube growth, fertilization | B | |
| 15 | Stigma extends above long anthers | 24 | Early embryogenesis until heart stage | B | ||
| 16 | Petals and sepals withering | 12 | Early embryogenesis until heart stage | B | ||
| 17 | All organs fall from green siliques | 192 | Early embryogenesis until heart stage | Late embryogenesis from torpedo stage to mature embryo, seed coat formation | A | B |
| 18 | Siliques turn yellow | 36 | Late embryogenesis from torpedo stage to mature embryo, seed coat formation | A | ||
| 19 | Valves separate from dry silique | 24 | Late embryogenesis from torpedo stage to mature embryo, seed coat formation | |||
| 20 | Seeds fall | Late embryogenesis from torpedo stage to mature embryo, seed coat formation | ||||
Data are taken from Smyth et al. (1990).
Data are taken from Mansfield and Briarty (1991), Schneitz et al. (1995), and Sanders et al. (1999).
Regions along the inflorescence as defined in Figure 2. Because of natural variation in number of flowers that open per day, matching with other data in the table relies on estimates.
Natural Variation of Heat Response Is Dependent on Developmental Stage
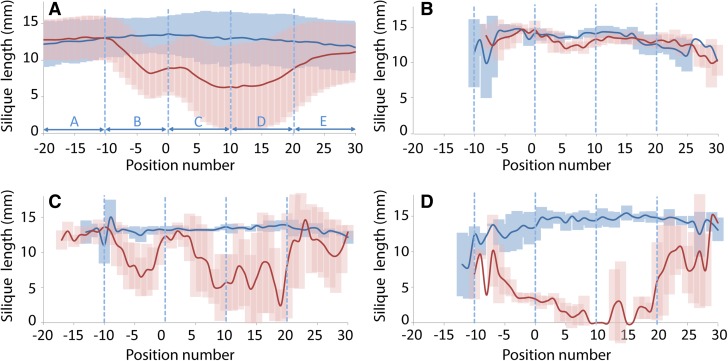
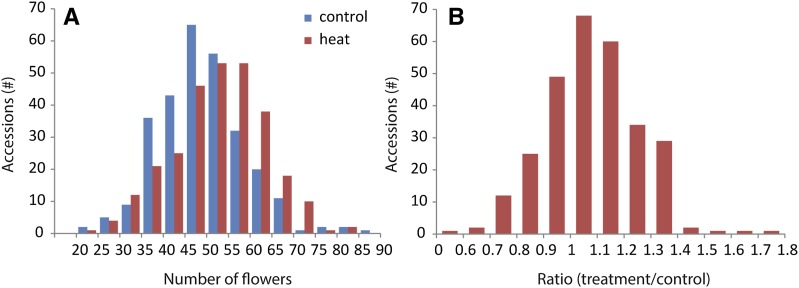
Natural variation in seed set upon heat stress was recorded by measuring the length of all siliques along the main inflorescence of each individual plant of all accessions. To determine the general response pattern, the average of the silique length of all plants in the experiment was calculated for all silique positions along the main inflorescence (Figure 3A). The effect of the treatment varied along the inflorescence. For instance, region A (late embryogenesis) and region E (early flower development) were least affected by the heat treatment in all accessions (Figure 3A), while the siliques in other regions were at least affected in some accessions. After heat treatment, two minima were observed in the overall average silique length. The first minimum was found for siliques in the phase of early embryogenesis (region B) with the absolute minimum at position minus three. On average silique length at that position decreased from 13.4 to 8.2 mm, corresponding to a decrease of ∼21 seeds per silique on average (Figure 1C). The second minimum spanned a large region in which male and female meiosis takes place. The lowest value was reached at position ten with an average decrease in silique length from 13.0 to 6.3 mm, corresponding to a decrease of ∼27 seeds per silique on average (Figure 1C). Overall, large differences were observed in the response to the treatment, ranging from accessions without a clear response, to complete absence of siliques; consequently, the two minima were not always detected in all accessions. For instance, accession C24 (origin Portugal) displayed no reduction in silique length at any position, whereas accession Rmx-A180 (origin Michigan) was clearly affected in both regions. In addition, accession Arby-1 (origin Sweden) was severely affected in silique length along most parts of the inflorescence (Figures 3B to 3D). The variation in silique length in control and heat stress conditions was high between accessions, whereas the variation within accessions was low. This resulted in high broad-sense heritabilities (H2 between 0.58 and 0.73; Table 2), indicating that the observed variation in silique length between accessions is for the most part determined by genetic factors. In addition, substantial variation between the different accessions was observed for the total number of flowers formed along the main inflorescence during the reproductive period (Figure 4A). In the majority of accessions, the number of flowers formed in heat-treated plants was higher than in control conditions (Figure 4B). The average number of flowers formed in all heat-treated plants was also significantly larger than the average number in control plants (P < 0.001). On average, heat-treated plants developed 12% more flowers than the plants in control conditions, suggesting a compensation mechanism for the loss of viable seeds.
Figure 3.
Silique Length along the Main Inflorescences of Control (Blue) and Heat-Treated (Red) Plants (Mean ± sd).
(A) Overall average of all plants in the experiment.
(B) Representative tolerant accession, C24.
(C) Representative moderately sensitive accession, Rmx-A180.
(D) Representative extremely sensitive accession, Arby-1.
(B) to (D) Control, n = 3; treatment, n = 5. Bars indicate the sd of silique lengths among replicates, and solid lines indicate mean values.
Table 2. Broad-Sense Heritability of Silique Length in Control and Heat Stress Conditions per Developmental Region (B, C, and D, as Defined in Figure 2) and of Flowering Time.
| Trait | Developmental Region | H2 |
|---|---|---|
| Control silique length | B | 0.58 |
| C | 0.57 | |
| D | 0.66 | |
| Heat silique length | B | 0.70 |
| C | 0.84 | |
| D | 0.73 | |
| Flowering time | 0.96 |
Figure 4.
Comparison of Total Number and Ratio of Flowers along the Main Inflorescence in Control and Heat-Treated Plants for All Accessions.
(A) Frequency distribution of the total number of flowers per main inflorescence averaged per accession.
(B) Frequency distribution of the ratio between the numbers of flowers along the main inflorescence in heat treated and control conditions per accession.
To quantify the impact of heat on the different developmental stages, the average silique length per developmental region was calculated for control and heat-treated plants. Regions A and E were not taken into account because for some accessions with a short main inflorescence not all positions in these regions developed a flower. In addition, we observed that after determination of the developmental sensitivity window, for most accessions, these regions were outside this window. To quantify the impact of the heat independently of the size of the siliques in control conditions, simple residuals of the silique length of control and heat-treated plants were calculated using linear regression. Correlation analysis was performed for all these fertility traits to determine whether they are interconnected (Table 3). Flowering time was also included in this correlation analysis. Significant positive correlations were found between the lengths of the siliques of heat-treated plants in regions B, C, and D. This indicates that plants that are sensitive in region B in general are also sensitive in regions C and D and vice versa. Although the heat responses in the different regions can be interdependent, a correlation does not always reflect a direct causal relationship and might result from downstream effects of early events. Furthermore, a strong negative correlation was observed between flowering time and silique length in heat-treated plants (Table 3). To synchronize flowering, the plants were vernalized for 10 weeks, but the variation in flowering time still spanned 4 weeks between the earliest and latest flowering accessions (Supplemental Data Set 1). Therefore, the heat treatment was given in batches to keep the time interval between the start of flowering and the treatment for both early and late flowering accessions similar. The correlation between flowering time and heat response also suggests a role for developmental timing in the sensitivity to heat stress. As for the heat response, we also observed high heritability for flowering time (H2 = 0.96; Table 2).
Table 3. Spearman's Correlation between Average Silique Length of Control and Heat-Treated Plants in Three Developmental Regions (B, C, and D, as Defined in Figure 2), the Corresponding Residuals for Those Regions, and Flowering Time.
| Average Silique Length |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Heat |
Residuals |
||||||||
| B | C | D | B | C | D | B | C | D | ||
| Control | B | 1.00 | ||||||||
| C | 0.70 | 1.00 | ||||||||
| D | 0.44 | 0.82 | 1.00 | |||||||
| Heat | B | 0.51 | 0.43 | 0.37 | 1.00 | |||||
| C | 0.22 | 0.42 | 0.46 | 0.55 | 1.00 | |||||
| D | 0.13 | 0.37 | 0.48 | 0.40 | 0.76 | 1.00 | ||||
| Residuals | B | 0.02 | 0.11 | 0.19 | 0.83 | 0.50 | 0.41 | 1.00 | ||
| C | −0.04 | 0.07 | 0.19 | 0.42 | 0.91 | 0.68 | 0.53 | 1.00 | ||
| D | −0.02 | 0.07 | 0.11 | 0.31 | 0.65 | 0.91 | 0.40 | 0.69 | 1.00 | |
| Flowering time | 0.06 | −0.10 | −0.25 | −0.31 | −0.65 | −0.58 | −0.41 | −0.67 | −0.53 | |
QTLs Associated with Stage-Specific Heat Responses
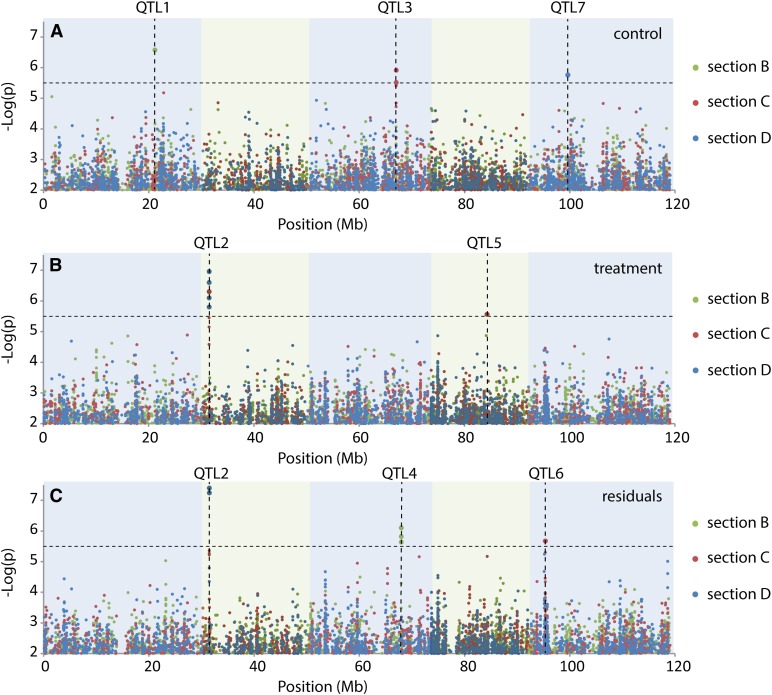
To search for the genetic variation that causes the phenotypic variation observed in the fertility traits, we performed GWA mapping on data from control and heat-treated plants and for the residuals. Traits analyzed were average silique length in regions B, C, and D, and flowering time. GWA mapping detected 15 strongly associated single nucleotide polymorphisms (SNPs) with a –log(P value) above the arbitrarily set significance threshold of 5.5 (Figure 5). In addition, 297 moderately associated SNPs with a –log(P value) between 4 and 5.5 were identified (Supplemental Table 2). The arbitrary threshold of –log(P value) = 5.5 was set to focus on the SNPs with the largest explained variance and to minimize the chance of detecting false positives. Some of these significant SNPs are in linkage disequilibrium with each other and therefore are very likely to represent a single locus. This resulted in seven unique major QTLs: three related to silique length in control conditions and four related to the heat response (Table 4). Variation in silique length as a result of heat stress could partly be explained by QTL2, QTL4, QTL5, and QTL6. Total explained phenotypic variance of these four QTLs reached 35.7%, which is relatively high for GWA studies, especially considering that this may be an underestimation due to the possible presence of multiple alleles per locus. However, the large fraction of missing heritability indicates that other genetic factors, possibly with large effects, remained undetected, e.g., due to low allele frequencies or overcorrection for population structure. None of the significant SNPs were identified in data from control conditions, suggesting that the observed variation in silique length explained by these SNPs is solely due to the heat treatment. The QTL with the strongest association and the largest effect size (QTL2) consists of four significant SNPs located at the top of chromosome 2 within a region of 0.7 kb (Table 4). All four SNPs were strongly associated with regions C and D, but not with region B. This indicates that this QTL is specific for the observed variation for average silique length at the position of the second minimum and can therefore be related to preanthesis processes, such as male and female meiosis (Table 1). QTL5 and QTL6 were specific for developmental region C. Besides female meiosis, anther elongation also takes place in that developmental stage at the time of the heat treatment. QTL4 is the only QTL found to be associated with the first minimum in average silique length and is therefore related to postanthesis processes like early embryogenesis. Although the main aim of this research was to identify genes involved in heat tolerance, the collected data also give insight into the regulation of silique development under control conditions. Variation observed between the accessions in the silique length in control conditions could be partly explained by three QTLs: QTL1, QTL3, and QTL7. Although each control QTL was identified in a distinct developmental region, moderate associations were identified for at least one other region, indicating that genes that determine silique length in one developmental region may also play a role in another region (Table 4). For QTL1, a weak association was also found for variation in silique length in region B of heat-treated plants in addition to a strong association in control conditions, suggesting that the causal gene has an effect on silique length independent of the environmental conditions. Although a strong negative correlation was observed between flowering time and silique length in heat-treated plants, no significant associations (−log(P value)>5.5) were detected for flowering time. That said, flowering time was moderately associated with QTL6 (−log(P value) = 4.96). This QTL maps to the position of the well known flowering time gene FLOWERING LOCUS C (FLC), which might explain the observed variation in flowering time and suggests a role for the regulation of flowering time in the heat stress response. To complete our analyses, data were also collected for silique length averaged over three regions (B, C, and D together) and for the total number of siliques smaller than 5 mm, which probably do not contain viable seeds. These traits are independent of a possible bias introduced by developmental phase shifts between accessions and might therefore reveal additional regulatory loci. However, GWA mapping did not reveal significant associations other than the ones described above (Supplemental Data Set 2), further strengthening our assumption that the developmental processes assigned to the different regions are correct for most accessions and that possible deviations do not affect our analyses drastically.
Figure 5.
Manhattan Plots Representing the Associations between SNP Markers and Silique Length in the Regions B, C, and D.
(A) GWA mapping of silique length in control plants.
(B) GWA mapping of silique length in heat-treated plants.
(C) GWA mapping of residuals, representing heat response independent of silique length in control conditions.
Dotted line represents the significance threshold of –log(P value) = 5.5.
Table 4. Major QTLs Identified by GWA Mapping for Flowering Time and Average Silique Length in Developmental Regions B, C, and D (as Defined in Figure 2) in Control and Heat-Treated Conditions and Their Corresponding Residuals for Those Regions.
| QTL | Chromosome | Position (Mb) | Control |
Heat |
Residuals |
Effect Size | Explained Genotypic Variance | Explained Phenotypic Variance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT | B | C | D | B | C | D | B | C | D | ||||||
| 1 | 1 | 21.17 | 6.6 | 3.5 | 3.7 | −2.16 | 9.88 | 8.43 | |||||||
| 2 | 2 | 1.06 | 2.2 | 6.3 | 6.1 | 5.3 | 4.8 | −2.63 | 9.72 | 7.93 | |||||
| 2 | 1.06 | 5.5 | 5.8 | 3.8 | 4.3 | −3.14 | 9.45 | 6.94 | |||||||
| 2 | 1.06 | 5.1 | 7 | 5.4 | 7.4 | −3.40 | 25.10 | 11.54 | |||||||
| 2 | 1.07 | 4.6 | 6.6 | 5.2 | 7.2 | −3.32 | 24.41 | 11.22 | |||||||
| 3 | 3 | 16.89 | 4.1 | 5.9 | 3.5 | 2.1 | −1.46 | 9.99 | 7.18 | ||||||
| 3 | 16.89 | 3.5 | 5.5 | 2.2 | −1.92 | 9.28 | 6.67 | ||||||||
| 4 | 3 | 17.85 | 2.8 | 5.6 | 1.53 | 14.22 | 8.09 | ||||||||
| 3 | 17.85 | 2.8 | 5.6 | 1.53 | 14.22 | 8.09 | |||||||||
| 3 | 17.85 | 2.7 | 6.1 | 1.62 | 15.40 | 8.76 | |||||||||
| 3 | 17.85 | 2.8 | 5.6 | 1.53 | 14.22 | 8.09 | |||||||||
| 3 | 17.85 | 2.9 | 5.8 | 1.58 | 14.73 | 8.38 | |||||||||
| 5 | 4 | 10.72 | 5.6 | 5.2 | −2.09 | 8.04 | 6.55 | ||||||||
| 6 | 5 | 3.19 | 4.96 | 4.5 | 5.7 | −2.45 | 15.66 | 8.88 | |||||||
| 7 | 5 | 7.48 | 3.1 | 5.8 | 1.44 | 7.02 | 5.37 | ||||||||
Columns represent the exact position of SNPs and their association with above-mentioned traits and the effect size (Col-0 allele is positive; non-Col-0 allele is negative) and explained genetic and phenotypic variance for the highest association detected. Only SNPs that are strongly associated with at least one trait (−log(P value) > 5.5) are shown. Bold indicates significantly associated SNPs; no value indicates no association (−log(P value) < 2).
Assignment of Candidate Genes Based on Linkage Disequilibrium, Gene Annotation, and Expression
Resequencing data (1001genomes.org) of 502 accessions were used to assign possible causal candidates to each of the seven observed QTL. For 124 of those 502 accessions, the heat response was investigated in this study. Genes in linkage disequilibrium (LD) with the significant SNPs (threshold LD > 0.4) were considered as candidates (Supplemental Figure 1). In addition, gene annotation and gene expression patterns in different plant tissues were obtained from TAIR and the Arabidopsis eFP browser (Winter et al., 2007), respectively. For a number of candidate genes, selected for likelihood and detection confidence, the heat response of knockout mutants was investigated in two independent experiments (Supplemental Data Set 3).
Preanthesis Sensitivity to Heat Stress
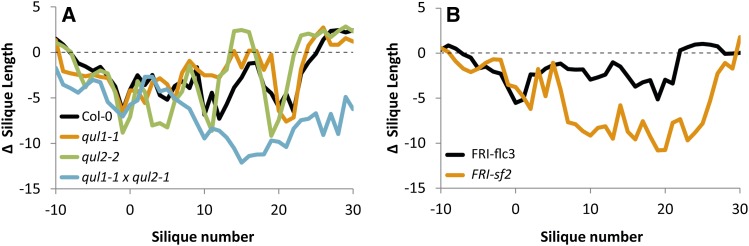
The four strongly associated SNPs of QTL2, explaining variation in silique length upon preanthesis heat treatment, are in linkage with nine other SNPs, as identified in the resequencing data. All 13 SNPs are located in the introns of the AT2G03505 locus, which is therefore the only candidate gene in LD with the significant SNPs. AT2G03505 encodes an unknown protein of the carbohydrate binding X8 domain superfamily. This gene is relatively high expressed in flowers and especially carpels (Laubinger et al., 2008). As a start of the validation process, a Gabi-Kat knockout line was tested for its heat response in two independent experiments. No differences were observed between the heat response of this line and the Col-0 wild type (Supplemental Data Set 3). This suggests that this gene might not be the causal gene, although the wild-type Col-0 allele in itself can already be weak or nonfunctional and additional experiments of knockout and overexpression lines of non-Col-0 alleles of this gene are needed for final determination. Because causal genes are sometimes also detected outside the LD interval of significantly associated SNPs (Chao et al., 2014), due to, among others, low linkage between causal and associated SNPs (Freedman et al., 2011), other genes in the 20-kb region around the significant SNPs (10 kb upstream and 10 kb downstream) were considered as candidates, in accordance to the average linkage distance in Arabidopsis of 10 kb (Kim et al., 2007). This resulted in the identification of a candidate gene, QUASIMODO-LIKE2 (QUL2; AT2G03480) that is highly expressed during the development of the male gametophyte (Honys and Twell, 2004). This process takes place in region D, in which the highest association was observed. A triple mutant of QUL2 and its two paralogs, QUA2 (AT1G78240) and QUL1 (AT1G13860), was previously found to have temperature-dependent changes in stem diameter and stem lignification (Fuentes et al., 2010). Therefore, the heat response of the T-DNA insertion lines of QUL2, its closest paralog QUL1, and the corresponding double mutant were investigated (Figure 6; Supplemental Data Set 3). Expression analysis confirmed that the single and double mutants of QUL1 and QUL2 were true knockdowns (Supplemental Figure 2). The double mutant was more heat sensitive in regions C, D, and E (Figure 6) but not in region B, whereas both single mutants resembled the wild type (Supplemental Data Set 3). This indicates that QUL1 and QUL2 have probably very similar complementary functions with direct or indirect positive effects on silique formation upon heat. This result also emphasizes that different genes might be responsible for the heat response before and after anthesis.
Figure 6.
Mutant Analyses.
Difference between silique length of heat-stressed and control plants (average silique length of heat stress plants minus average silique length of control plants) of Col-0 wild type and the single and double knockdown mutant lines of QUL1 and QUL2 (A), and of isogenic lines with FRI strong allele and FLC null allele (early flowering, FRI-flc3) or FLC functional allele (late flowering, FRI-sf2) (B). Results of one experiment are shown. Results of a second independent experiment can be found in Supplemental Data Set 3.
A second significant association (QTL5) assigned to the preanthesis stage is located in a chromosomal region with very fast linkage decay (Supplemental Figure 1). The significant SNP is not in LD with resequence SNPs that are located upstream and only with a limited number of SNPs located 4 kb downstream. This led to the identification of one candidate gene, AT4G19710, coding for an aspartate kinase-homoserine dehydrogenase. This gene is expressed in many tissues in control conditions (Schmid et al., 2005).
Postanthesis Sensitivity to Heat Stress
QTL4, at the bottom of chromosome three, is specific for the heat response in developmental region B and assumed to be related to fertilization or embryo formation. QTL4 is determined by five tightly linked SNPs, which are in high linkage disequilibrium (LD > 0.9; Supplemental Figure 1) with resequenced SNPs in a gene encoding a kinetochore protein (AT3G48210). This protein is involved in cell division, a process that is crucial for the growing embryo. Functional characterization of this gene has not been performed, but it is expressed in many tissues, including the embryo (Schmid et al., 2005). High linkage (LD > 0.7; Supplemental Figure 1) was also observed between the strongly associated SNPs, and resequence SNPs located in AT3G48190 and its promoter region. AT3G48190 is suggested to play a role in DNA repair upon stress (Huefner et al., 2011) and might be important in heat-stressed embryos. However, the slow LD decay in this region results in a linkage block of 140 kb (Supplemental Figure 1) in which 37 genes are located, and further analyses are needed to confirm the causal gene.
A Possible Genetic Link between Developmental Timing of Flowering and Response to Heat Stress
The strongly associated SNP of QTL6, at the top of chromosome five, is in LD with 17 SNPs identified in the resequence data (Supplemental Figure 1). Four of those SNPs were included in the GWA mapping and were moderately associated (−log(P value)>4) with heat stress response in region C. The strongest associated SNP is responsible for a nonsynonymous change in the protein sequence of AT5G10170, which codes for Myo-Inositol 1-Phosphate Synthase3 (MIPS3). MIPS3 expression was most prominent in vascular tissue (Donahue et al., 2010), and heat-induced expression was reported during the seedling stage (Khurana et al., 2012). The Arabidopsis genome has three MIPS genes. Analysis of the close relative MIPS2 showed that the expression of this gene is highly induced upon heat stress treatment in seedlings, flowers, and mature siliques (Khurana et al., 2012). Investigation of heat response of the T-DNA knockout mutant of MIPS3 did not reveal differences between the heat response of this line and wild-type Col-0 (Supplemental Data Set 3), suggesting again that this gene might not be causal for the observed natural variation. In addition, mutants of two other genes in the LD region of QTL6 were analyzed. AT5G10200 codes for an ARM-repeat/Tetratricopeptide repeat-like protein. Like most ARM-repeat proteins in Arabidopsis, the function of AT5G10200 is unknown. In rice, a genome-wide study of ARM-repeat gene expression showed that several genes of this family are regulated by abiotic stresses (Sharma et al., 2014). However, investigation of a T-DNA knockout mutant did not reveal an immediate role for this gene in the heat response of Arabidopsis (Supplemental Data Set 3). This is in agreement with the observation that another Arabidopsis ARM-repeat protein, ARO1, is required for pollen tube germination and penetration into the stigma (Gebert et al., 2008). These processes take place postanthesis, whereas QTL6 is associated with preanthesis heat responses.
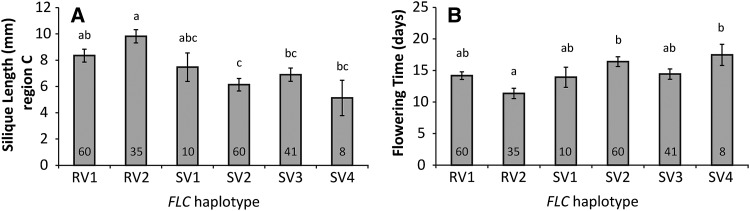
Interestingly, the other gene in LD with QTL6, AT5G10140, encodes FLC, which is involved in the timing of flowering. Two isogenic lines, containing different FLC alleles and a strong functional allele of FRIGIDA (FRI; AT4G00650) in a Columbia background, were tested for their heat response. A strong FRI allele is needed because FRI is an upstream regulator of FLC and the presence of a nonfunctional allele (like in the Columbia wild-type accession) will result in early flowering, independent of the FLC allele. Line FRI-sf2, containing the functional Columbia allele of FLC, requires vernalization to flower, whereas line FRI-flc3, containing a null allele of FLC, flowers early. Both lines were vernalized for 10 weeks, after which FRI-sf2 still flowered a week later than FRI-flc3. Heat treatment was given approximately 1 week after the start of flowering. The late flowering line, FRI-sf2, was much more sensitive to the heat stress in regions C, D, and E than the early flowering line FRI-flc3 (Figure 6). Both lines had similar heat responses for region B, suggesting that FLC is responsible for part of the preanthesis heat response. This experiment is in line with the observation in the association panel that heat response and flowering time are negatively correlated, and it suggests that the two processes are, directly or indirectly, genetically linked. To investigate whether this result is specific for the Columbia allele, the six most common FLC alleles (P. Li et al., 2014) were compared for flowering time after 10 weeks of vernalization and for their heat response. This comparison confirmed the negative correlation observed between the two traits (Figure 7). Accessions with the Col-0 allele (haplotype RV2), which flowered early, had significantly larger siliques in region C (Figure 7) and region D (Supplemental Table 4) than accessions belonging to haplotypes SV2, SV3, and SV4, which flowered later. Haplotype RV1 was, like RV2, identified because of its strong response to vernalization (P. Li et al., 2014), but no significant difference was found for silique length upon heat between accessions of this haplotype and the SV haplotypes with slow response to vernalization, indicating that RV2 represents the FLC allele associated with heat resistance. However, large variation in flowering time and heat response was observed between accessions belonging to the same haplotype, which might be a consequence of sequence variation at other flowering time loci (Shindo et al., 2005) or heat response genes. Differences between the alleles were not significant anymore when corrected for population structure (Supplemental Data Set 4), which corresponds with the observation of P. Li et al. (2014) that haplotypes with different geographical distribution were also functionally different.
Figure 7.
Comparison of Silique Length and Flowering Time of Different FLC Haplotypes.
(A) Length of siliques (average ± se) in region C upon heat stress.
(B) Flowering time (average ± se ) after 10 weeks of vernalization for accessions of the six most common FLC haplotypes (P. Li et al., 2014). Numbers in the bars indicate the number of accessions belonging to the haplotype indicated below the bar; Col-0 belongs to the RV2 haplotype. Significant differences (P < 0.05), as indicated by letters above the bars, are determined by one-way ANOVA with Bonferroni correction for multiple testing.
Natural Variation in Silique Length in Control Conditions Suggests a Role for Pollen Allergens
Genes in linkage with the strongly associated SNPs detected in control conditions were also analyzed to identify candidates that could be involved in the determination of silique length and consequently seed yield. For QTL1, the control QTL with the strongest associated SNP and the largest effect size, a second weaker associated SNP (−log(P value) = 4.2) in moderate LD (LD = 0.424) was detected 30 kb downstream. The significant SNP is located in an intron of a white rust resistance gene (AT1G56510), which is highly expressed in leaves. The weaker associated SNP is located between AT1G56580, a gene associated with trichome architecture, and AT1G56590, a gene involved in vesicle trafficking between the trans-Golgi network and vacuoles. None of these three genes are obvious candidates. However, the LD decay in this region was very slow, resulting in a linkage block of almost 176 kb, 8 kb upstream, and 168 kb downstream of the significant SNP (threshold: LD = 0.4), in which 127 genes are located (Supplemental Figure 1). Most of these genes are pre-tRNAs (81 genes) or genes with unknown biological function (22 genes). None of the genes with an annotated function is an obvious candidate, and further analyses are needed to identify the causal gene. For QTL3 and QTL7, candidate genes could be identified based on functional annotation. QTL3 is determined by two significant SNPs that are located 0.2 kb from each other. Three other moderately associated SNPs were identified in close proximity (within 0.4 kb). These five SNPs are located in the promoter of AT3G45970, 1.2 kb upstream of the start codon, and are in low LD (0.2 < LD < 0.4) with SNPs in the gene AT3G45960 (Supplemental Figure 1). Both genes are annotated as expansin-like A and are suggested to be involved in cell wall loosening (Cosgrove et al., 2002). The significant SNP of QTL7 is located in a large linkage block starting 43 kb upstream and ending 76 kb downstream of the significant SNP. Two candidate genes were identified within this linkage block: AT5G22430, a gene of a conserved allergen family, which is suggested to play a role in pollen-pistil interactions (Jiménez-López et al., 2011), and AT5G22640, a gene essential for proper embryo development (Liang et al., 2010). Remarkably, both identified expansin-like proteins of QTL3 and AT5G22430 of QTL7 contain a pollen allergen domain. Proteins with such a domain are known to be present in pollen and cause allergic reactions in humans. It is suggested that these proteins loosen maternal cell walls to aid penetration of the stigma by the pollen tube (Cosgrove, 2000; Sharova, 2007). Inconsistencies in the interaction between pollen and pistil can lead to reduced fertilization and can therefore result in shorter siliques. In addition, proper embryogenesis is essential for the development of seeds. If this process is disrupted, fewer seeds will be present per silique, resulting in shorter siliques.
Our results indicate that the observed heat response is dependent on both the genotype and the developmental stage of the flowers and embryos during the heat treatment. GWA mapping resulted in the identification of developmental stage-specific QTLs, and covariance between the heat response and flowering time suggests a direct or indirect link between the two regulatory pathways. In addition, QTLs for silique length in control conditions were identified. Candidate genes were suggested for most QTLs, of which some could be provisionally validated by analyzing knockout mutants.
DISCUSSION
Over the past decade much effort has been put in collecting large numbers of natural variants for many species, including the model species Arabidopsis. Technological advancements have made it possible to characterize the genetic diversity in such collections in great detail. A number of studies have shown the use of such densely genotyped natural populations in GWA mapping approaches (Cockram et al., 2010; Chan et al., 2011; Chao et al., 2014; Verslues et al., 2014). Such studies not only disclose the large amount of natural variation present in species for many traits but also hint at candidate genes causal for the observed variation. The latter is an important argument in favor of GWA mapping over, e.g., gene expression studies because causal genes often represent key regulators of, or essential components in biological processes, whereas differentially expressed genes might merely represent transcriptional program changes in response to genetic or environmental perturbations. As such, GWA mapping can add directionality in genetic regulatory networks and identify important anchor points in biological processes. In addition to genetic perturbations, many complex traits might depend on interaction with the environment and the developmental timing of the subject of study, a complication that is often neglected. Including genotypic diversity and developmental dependence in a single study of environmental perturbation can therefore provide a deeper understanding of the developmental processes involved in the regulation of abiotic stress responses.
Identification of a Developmental Sensitivity Window
In this study, the impact of short-term heat on fertility was measured in a developmental stage specific way. Large variation was observed in the sensitivity of different developmental stages of the flowers and embryos to the stress. Early flower development and late embryogenesis, assumed to take place in regions A and E, respectively, were not sensitive to heat stress in most of the accessions (Figure 3, Table 1). Two minima in the average silique length, corresponding to reduced seed set, were identified indicating two sensitive periods in development. One of the minima observed in our experiment corresponds to floral stages 9, 10, 11, and 12 (Smyth et al., 1990), representing the developmental stages in which male and female meiosis occur and anther elongation takes place (Table 1). The other minimum corresponds to flowers in floral stages 14 and 15 that were heat treated after anthesis, during these stages early embryo formation takes place. Male meiosis, which takes place during floral stage 9, is a process that is sensitive to heat in many crops (Hedhly, 2011). An earlier study in Arabidopsis investigated the effect of heat on flower development (but not on embryo development) in the accession Columbia by counting fertile siliques along the main inflorescence. Although the heat treatment in this study was more severe (42°C/4 h), a sensitive period at floral stage 9 was observed, similar to our study. In addition, a sensitive period at floral stage 12 was identified (Kim et al., 2001). A more extensive study in Arabidopsis, investigating floral bud abortion upon heat stress in 13 natural accessions, demonstrated that in the Columbia accession floral stage 12, but not floral stage 9, was most sensitive (Warner and Erwin, 2005). That floral stage 9 was not identified could be caused by the longer period of heat that was applied (36°C for 72 h). However, other accessions were shown to be more sensitive than Columbia, and floral bud abortion was also observed in earlier stages than floral stage 12. Floral stage 12 corresponds to anther elongation. Reduction in anther elongation was observed upon heat stress as a result of decreased auxin levels in the developing anthers. Reduced anther elongation upon heat stress has also been found in other species, such as barley (Sakata et al., 2010). The observations of Kim et al. (2001) and Warner and Erwin (2005) are in line with our observation that natural variation exists both in terms of duration of the sensitivity window and the degree of reduction in seed set within the sensitivity window. However, comparison of floral stages between different studies should be done carefully since the matching between the developmental regions and the landmark events in flower and seed development are based on generalization and natural variation was observed for the number of flowers that opened per day.
Also in other Brassicaceae species, heat response patterns similar to our observations have been reported. Studies of canola and mustard species (B. napus and B. juncea) showed that heat stress during flowering or pod development led to more reduction in yield than heat stress during bud formation (Gan et al., 2004). In addition, it was observed that pods that passed a critical developmental threshold were tolerant toward heat stress (Angadi et al., 2000). This threshold corresponds with late embryogenesis, the developmental stage that was also not sensitive to heat in any accession tested in our experiment. Even though the study of Angadi et al. (2000) confirms the existence of a developmental sensitivity window in which heat stress affects yield, a comparison between these results and the observations in our experiment also reveals an interesting difference. We observed a higher impact of stress before flowering, while in other Brassicaceae, the impact seems to be more dominant after the start of flowering. This may be related to embryo abortion during early silique development, which was observed in our experiments (Figure 1). Moreover, the findings of Gan et al. (2004) are based on total seed yield and should be interpreted carefully. If compensation mechanisms are active (Figure 4; Angadi et al., 2003), total seed yield might not be the best measure to determine a developmental sensitivity window.
In species other than Brassicaceae, heat responses similar to our observations have been reported. In green bean (Phaseolus vulgaris), the most sensitive period was 10 d before anthesis and could be related to a reduction in pollen viability (Suzuki et al., 2001). This indicates a relationship with male meiosis, which corresponds to the sensitive floral stage 9 in Arabidopsis. In tomato, flower buds of 9 to 5 d before anthesis and flowers of 1 to 3 d after anthesis are highly susceptible to high temperatures (Sugiyama et al., 1965). These two periods correspond with the two minima identified in our experiment.
The finding that our observations in Arabidopsis are in line with data collected for heat responses in various crops increases the potential of translating our results about molecular regulation of the heat response to crop species. Interestingly, besides heat stress, drought also has an impact on developing flowers and embryos. In cereals, two sensitive periods are found upon drought stress, the first one centers around the period of male and female meiosis and the second one during anthesis and initial grain development (Saini, 1997). This suggests that similar regulatory mechanisms may be involved, increasing the relevance of this work.
GWA Mapping Reveals Stage-Specific QTLs
Although a few studies have reported on the developmental sensitivity window upon heat stress (Kim et al., 2001; Warner and Erwin, 2005), this study is unique as it investigates this sensitivity window using a large natural population. Therefore, the data could be subjected to GWA mapping, which allowed the identification of stage-specific QTLs. Two choices in our experimental setup were crucial in identifying stage specific QTLs. First, the 1-d exposure time ensured that developing flowers received stress in only one developmental stage. In experiments in which the stress is applied for longer periods, the identification of stage-specific QTLs is less accurate because a single flower may receive stress in multiple developmental stages. Second, the quantification of the impact of the heat stress by measuring the length of individual siliques along the main inflorescence provided high resolution of development-specific heat effects. Studies that quantify the heat stress response by total seed or fruit yield will have less power to identify effects that are only present in a small developmental window. Compensation mechanisms of plants (Figure 4; Angadi et al., 2003; Young et al., 2004) make total seed or fruit yield as a measure even less reliable. Our findings should increase awareness of the fact that the regulation of a trait of interest can change during development. Experimental design is a crucial factor here, which, in most cases, determines whether developmental stage specificity of QTLs or regulatory networks can be detected or not. Accurate measurements or large population sizes will not contribute to a higher power to detect stage specific QTLs when a nonoptimal design is chosen.
In our study, the main focus was on the identification of stage-specific QTLs. Two minima were identified in the silique length after heat stress corresponding to two distinct processes in flower and seed development (Figure 3, Table 1). Stage-specific information can be helpful to prioritize candidate genes for future functional analyses. Genes that are not expressed in the developmental stage in which the QTL is identified or that are not expressed in flowers or embryos can be given lower priority. In this study, four QTLs were identified to be highly associated with heat response: one specific for the response after anthesis and three specific for the response before anthesis. Based on LD, annotated gene function, expression profiles, and mutant analysis, one or two genes per QTL could be prioritized as candidate genes. The knockout analyses of several candidate genes indicated the involvement of these genes in heat stress responses of reproductive processes. However, for a number of genes, their involvement could not be confirmed. This might be due to the genetic background of the mutant line (Col-0), whose wild type is used for comparison. On the other hand, knockouts that do show a significant effect on the trait under study do not conclusively rule out the involvement of other genes explaining the observed variation in the support window of the QTL. Further evidence should be obtained from functional complementation studies in different genetic backgrounds.
In many studies describing heat stress during flowering, a prominent role is given to heat shock proteins (Hsps) and heat shock transcription factors (Hsfs) (Giorno et al., 2013). For instance, constitutive and heat stress induced accumulation of Hsps in reproductive tissue (especially anthers) is associated with heat tolerance (Bita et al., 2011). Hsps stabilize proteins that are unfolding due to heat stress (Al-Whaibi, 2011). Hsps, as chaperones, prevent the irreversible aggregation of other proteins and participate in refolding proteins during heat stress conditions (Waters, 2013). Surprisingly, in our study, no Hsp or Hsf was found in close proximity of a QTL (threshold –log(P) = 5.5), and only two Hsps were identified within 20 kb of moderately associated SNPs (threshold –log(P) = 4), suggesting that allelic variation in Hsps or Hsfs is not the main cause of natural variation in heat tolerance during flowering. The lack of these obvious candidates in our results shows that an untargeted analysis, like GWA mapping, will, in many cases, result in the identification of novel gene functions, while hypothesis-driven research, in most cases, results in the proof or disproof of an already suggested function.
Genetic Coregulation of Heat Response and Flowering
A high negative correlation and coincidence of strongly (Table 4) and moderately associated SNPs (Supplemental Data Set 2) were observed for heat stress tolerance and flowering time. QTL6 was detected for heat stress response but was also moderately associated with flowering time. The significant SNP of QTL6 was in linkage with SNPs in FLC, a flowering time repressor, and its promoter. The impact of heat stress was evaluated in two isogenic lines, one with the FLC functional allele, requiring vernalization to flower, and one containing a FLC null allele resulting in early flowering. Heat stress led to significantly more reduction in silique length in the late flowering line compared with the early flowering line. Analysis of the six most common FLC haplotypes further confirmed that heat sensitivity is associated with late flowering and might be based on crosstalk between regulatory networks. Even though variation in heat stress response mapped to FLC, we cannot rule out an indirect effect of flowering time differences. Especially because FLC expression diminishes during the 10 weeks of vernalization, severely reducing the variation in FLC expression during the treatment (Michaels and Amasino, 1999; Shindo et al., 2006). Although other known genetic factors affecting flowering time might also indirectly contribute to the variation in heat stress response, the analysis of this relationship was outside the scope of this study. A positive correlation was also found for chilling tolerance and flowering time in soybean, where high tolerance was associated with late flowering (Kurosaki et al., 2004). Differences in tolerance were shown to be due to natural variation in a well known maturation locus. While the response to nonoptimal temperatures can be linked to natural variation in flowering time or maturation genes, we cannot exclude that the correlation found in our experiment was caused by cold priming. The time between the cold period, needed to synchronize the flowering, and the treatment is not equal for all accessions: Accessions that flowered earlier inherently received the treatment sooner after the cold treatment than later flowering ones. It may be that recently cold-primed plants are less sensitive to the heat stress. This priming might be released over time, resulting in increased sensitivity to heat stress over time. However, natural variation was observed for accessions that received the heat treatment on the same day after the cold treatment, indicating that a major part of the natural variation cannot be explained by differences in the period between cold and heat treatment. Therefore, we propose that the variation in heat tolerance, observed between early and late flowering accessions, is a result of local adaptation. Early flowering accessions are summer annuals that germinate in spring and flower in the summer, whereas late flowering accessions are winter annuals that germinate in autumn, survive winter in the vegetative stage, and flower in spring. This suggests that early flowering accessions will benefit from a heat tolerance mechanism in their flowers, whereas late flowering accessions will not because flowers will rarely be exposed to high temperatures during spring.
Homology of Heat Stress Responses between Species
As mentioned, similar stage-specific heat responses are observed in several crop species, suggesting common regulatory mechanisms of the response of plants to elevated temperature during flowering, and fruit and seed set. Such a common response mechanism is promising for the translation of findings from model species to crops. Unfortunately, comparison of the QTLs observed in this study with earlier identified QTLs in other mapping studies in crops aiming to identify heat tolerance genes during flowering is difficult because most QTLs have not been cloned yet (Grilli et al., 2007; Ye et al., 2012; Lucas et al., 2013). All earlier studies were done in low-resolution biparental mapping populations; therefore, the associated regions still contain many genes. Because none of the tested crops (rice, tomato, and cowpea) are closely related to Arabidopsis, comparison of genome regions is difficult due to the lack of synteny. However, because of the high resolution of GWA mapping and the enormous amount of prior information available in Arabidopsis, it is promising to follow up the prioritized candidate genes identified in this study with further functional analyses to identify their functions and subsequently to be able to identify the heat response network. Translation of a response network from one species to the other can be more fruitful than single gene comparisons (Ferrier et al., 2011). Homologs of proteins in the pathway can be identified, and knowledge about protein function and expression, protein-protein interactions, and protein-metabolite interactions can be used to identify a similar response pathway in another species (Assmann, 2013).
Although the focus of this article is on silique length in heat-stressed plants, results obtained from plants grown in control conditions are also relevant for breeding purposes. To improve yield and consequently produce more oil, breeding is intensively done in B. napus and B. juncea varieties. Total yield is determined by several plant traits, such as total fruits per plant, seed weight, seeds per fruit, and fruit length. The last three are correlated and many coinciding QTLs have been reported (Alonso-Blanco et al., 1999; Dechaine et al., 2014; N. Li et al., 2014). Fruit length is considered to be a suitable trait to select for in B. napus (Samizadeh et al., 2010) because high heritability is observed for this trait, which is less sensitive to GxE than, for example, the total number of fruits per plant (Chay and Thurling, 1989; Dechaine et al., 2014). In this experiment, high heritability was also observed for silique length (Table 3). Because Arabidopsis is a member of the Brassicaceae family, similar mechanisms are likely to determine silique length and translation of mapping results from this model species to crops should be relatively easy.
Three candidate genes were identified for silique length in control conditions, which all contained a pollen allergen domain. Pollen allergens are known to cause allergic reactions in humans and are therefore studied intensively. Although their biological function in plants is not fully elucidated yet (Jiménez-López et al., 2011), it is suggested that these proteins may weaken the cell wall of the maternal tissue to ease the penetration of the pollen tube into the stigma (Cosgrove et al., 1997; Mollet et al., 2013). In tomato, competition experiments have been performed between wild-type pollen and pollen in which one of these pollen allergens has been mutated. It revealed that pollen with the mutated protein was outcompeted by the wild-type pollen because of slower pollen tube growth, indicating an important role of pollen allergens for the access of pollen to the ovule (Valdivia et al., 2007). Silique length is correlated with the number of seeds per silique (Figure 1). Slower penetration of the pollen tube may result in less efficient fertilization; as a consequence, less seeds will develop and siliques will be shorter. For clinical immunological reasons, variation of these allergens is studied between species and within species (Radauer and Breiteneder, 2006; Songnuan, 2013), but the impact of natural variation on fertilization success has not been studied yet.
In conclusion, the chosen experimental design was very appropriate to determine developmental stage-specific genetic regulators of the heat response of reproductive processes. Provisional validation by knockout analyses was provided for two preanthesis QTLs, strengthening the candidacy of two genes in close proximity of the significantly associated SNPs. Furthermore, the high resolution of GWA mapping, the similarities between flower and seed development in different species, and the suggested common regulatory mechanisms of heat response make the translation of findings in Arabidopsis to crops promising. To reach that goal, further functional analyses of candidate genes in Arabidopsis and their homologs in crops are needed.
METHODS
Plant Material
A collection consisting of 285 natural accessions of Arabidopsis thaliana (Supplemental Data Set 1) selected to capture most of the genetic variation present within the species (Platt et al., 2010) and genotyped with ∼215k SNP markers (Affymetrix SNP-tilling array Atsnptile1; Atwell et al., 2010; Li et al., 2010) was obtained from the ABRC Stock Center (Baxter et al., 2010).
For functional gene analyses, two lines, FRI-sf2 and FRI-flc3, containing two different alleles of FLC in a Columbia background (Michaels and Amasino, 1999), were kindly provided by M. Koornneef (MPI Köln). FRI-sf2 (CS6209) can also be obtained from the ABRC Stock Center. In addition, the following SALK T-DNA insertion mutants (Alonso et al., 2003) and GABI-Kat T-DNA insertion mutants (Kleinboelting et al., 2012) were obtained from NASC: qul2-2 (SALK_065604c), AT2G03505 (GABI_745A07), AT2G03490 (SALK_125910), mips3-2 (SALK_120131c; Donahue et al., 2010), and AT5G10200 (SALK_040912c). Seeds of the mutants qul1-1 and qul1-1 qul2-1 were kindly provided by L. Østergaard (Fuentes et al., 2010). Plants carrying a homozygote T-DNA insert were genotyped by PCR using the primers listed in Supplemental Table 1.
Experimental Setup
For each accession, eight plants were grown in controlled conditions. Five replicates received a heat treatment and three replicates served as controls. Seeds were sown in Petri dishes on wet filter paper. After 4 d of cold treatment, they were placed at room temperature in the light for one and a half day to germinate. Germinated seeds were placed on rockwool blocks saturated with nutrient solution (Hyponex, 1mM N, 1.1 mM P, and 5.9 mM K) in a climate room (16 h light, 125 umol/s/m2, 70% humidity, 20/18°C). Three times a week the rockwool blocks were saturated with nutrient solution using an automated flooding system. After 3 weeks, the plants were vernalized for 10 weeks to synchronize flowering. After vernalization, the plants were returned to the climate room (same conditions as above). One to two weeks after the first replicate of an accession started to flower, the heat treatment was applied to all replicates of that accession. A small number of accessions received the treatment outside this window because of large variation in flowering time between the replicates (Supplemental Data Set 1). The first flower that opened on the day of the treatment was tagged with a thread. Three replicates per accession were kept in the climate room as controls. Five replicates per accession were transferred to a climate cabinet where they received heat treatment. At the start of the day, the temperature was raised from 20 to 35°C in 2 h. The temperature was kept at 35°C for 13.5 h. At the end of the light period, the temperature was decreased again to 20°C for 2 h. The day after the treatment the plants were returned to the climate room.
The same procedure was followed to test the heat response in FRI-sf2 and FRI-flc3. For the other T-DNA insertion mutants used in this study, the same procedure was used but without the vernalization period. All lines were tested in two independent experiments (Experiments A and B).
Harvest and Analysis of Main Inflorescence Siliques
When the plant stopped producing new flowers or when at least 40 flowers above the thread tag had developed into siliques, the main inflorescence was cut off and stored in a paper bag for later analysis. Control and treated plants of the same accession were always harvested on the same day. Stored material was used to determine the length of all siliques present at the main inflorescence. The silique tagged with the thread was labeled as position number 0. Siliques below the tag received negative position numbers and siliques above the tag received positive position numbers (Figure 2).
Seed Properties
To determine the number of seeds per silique and their seed size, seeds were spread on white paper and pictures were taken in constant light conditions to enable easy image processing. The pictures were processed with the software package ImageJ (Schneider et al., 2012). Based on the size distribution of dark-brown and light-brown seeds, a threshold of 0.12 mm2 was used to define small and normal sized seeds, indicative for nonviable and viable seeds, respectively. To determine germination rates, seeds were placed on wet filter paper in Petri dishes sealed with Parafilm. The Petri dishes were placed in the dark at 4°C for 4 d. Thereafter the Petri-dishes were placed in the light at room temperature. The numbers of germinated and nongerminated seeds were counted after 48 h in the light.
Expression Analysis QUL1 and QUL2
Expression of QUL1 and QUL2 was measured in the qul1-1, qul2-2, and qul1-1 qul2-1 mutants as well as in Col-0 in 3-week-old plants grown at 22°C in 16 h light. RNA was isolated from 20 to 100 mg of plant rosette with the Invitrap Spin Plant RNA Mini Kit (Strateco) according to the manufacturer’s instructions. For the cDNA synthesis, 1 µg of RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. The cDNA was subsequently diluted to 150 μL end volume for use in RT-qPCR. Absence of genomic DNA was confirmed by comparing cDNA samples with RNA samples that were not reverse transcribed (minus RT control). The RT-qPCR was performed on the CFX-Connect (Bio-Rad) in 96-well plates (HSP-9645; Bio-Rad) using per well, 10 μL total volume consisting of 2.5 μL of cDNA, 0.5 μL of 10 µM primer mix, 5 μL iQ SYBR Green (Bio-Rad), and 2 μL of water. The following program was used for every reaction: 95°C for 3min, followed by 40 cycles of 95°C for 15s, and 60°C for 30s, followed by a melt curve analysis from 65 to 95°C. Each of the four biological replicates was measured in duplicate. Expression of QUL1 and QUL2 was measured using the primers described by Fuentes et al. (2010). For the reference gene MONENSIN SENSITIVITY1 (MON1; AT2G28390), the primer set described by Czechowski et al. (2005) was used. Expression data was normalized with the 2−ΔΔCT method (Schmittgen and Livak, 2008).
Statistical Analysis
GWA mapping was performed on fertility and architectural traits as well as flowering time. GENSTAT (VSN International, 2013) was used to calculate the simple residuals, which characterize the impact of the heat, corrected for difference between accessions in the size of the siliques in control conditions. Simple residuals were calculated for the average silique length in the different regions along the inflorescence, representing different developmental stages. To calculate the simple residuals, the mean of data collected under control and heat stress conditions was compared by a linear regression (least sum of squares) including all accessions. GWA mapping was performed with the EmmaX software package (Kang et al., 2010). A mixed model was used correcting for population structure based on a kinship matrix of all SNPs. SNPs with a minor allele frequency below 0.05 were excluded from the analysis. For each SNP, it was calculated how much of the total phenotypic and genotypic variance could be explained by the alleles of that SNP. Spearman’s rank correlation coefficient (ρ) (two-tailed, α = 0.05) was used to determine correlations between data series using SPSS (version 19.0; IBM Corp.). A one-sided, two-sample t test, assuming unequal variances, was used to compare the mean number of flowers formed on the main inflorescence in control and heat-treated plants. ANOVA and analysis of covariance were used to compare the phenotypic means for each of the FLC haplotypes (P. Li et al., 2014) (Supplemental Data Set 4). The first 10 principal components of the kinship matrix were used as covariates. Subsequent pairwise comparisons were done using Bonferroni correction.
All sequences from the resequenced Arabidopsis accessions were obtained from 1001genomes.org for 525 accessions, and 2012 nucleotide variation files compared with Col-0 (TAIR10) were downloaded. Custom Perl scripts were developed to determine positions with an allele frequency >2% (SNPs must be shared by more than 11 accessions). Another Perl script parsed these positions per accession and outputs either a 1 or 0 for compliance or no compliance with Col-0. The resulting data are stored as data frames (.csv) on disk. In order to calculate the LD, required data were extracted from the .scv files with the gnu program “cut” in order to slice out the region of interest. The sliced data frame is read into R (R Development Core Team, 2012) and column-wise the LD (r2 or correlation coefficient) can be determined by invoking the R function “cor()” followed by a quadratic operation. LD scores are made available to the user via a Web interface (in-house access only). The user can calculate the LD in any region on the genome.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT1G13860, AT1G56510, AT1G56580, AT1G56590, AT1G78240, AT2G03480, AT2G03490, AT2G03505, AT2G28390, AT3G45960, AT3G45970, AT3G48190, AT3G48210, AT4G00650, AT4G19710, AT5G10140, AT5G10170, AT5G10200, AT5G10200, AT5G22430, and AT5G22640.
Supplemental Data
Supplemental Figure 1. LD between significant SNPs detected by GWA mapping and surrounding SNPs obtained from resequence data.
Supplemental Figure 2. Expression of QUL1 and QUL2 in single and double mutants and Col-0 wild type.
Supplemental Table 1. Left and right border primers used to determine homozygosity of T-DNA inserts.
Supplemental Data Set 1. Dates on which the first replicate of an accession started to flower and dates on which the accessions received the heat treatment.
Supplemental Data Set 2. SNPs identified by GWA mapping for flowering time and average silique length in developmental regions B, C, and D (as defined in Figure 2) in control and heat-treated conditions and their corresponding residuals for those regions.
Supplemental Data Set 3. Silique length along the inflorescence of heat-stressed and control plants of several T-DNA insertion lines and two lines with functional (FRI-sf2) and nonfunctional (FRI-flc3) FLC alleles.
Supplemental Data Set 4. Average silique length in regions B, C, and D and flowering time for accessions of the six most common FLC alleles (P. Li et al., 2014).
Supplementary Material
Acknowledgments
We thank Matthijs van Houwen, Rik Kooke, Laurens Deurhof, and Jiaming Yang for their assistance during sowing and harvesting and for their work to determine silique lengths. We also thank Henri van der Geest and Rik Kooke for the development of the LD-SNP tool. Finally, we thank Caroline Dean and Peijin Li for generous sharing of the FLC haplotype information, Maarten Koornneef for the transgenic lines FRI-sf2 and FRI-flc3, and Lars Østergaard for the qul1-1 and qul1-1 qul2-1 seeds. This research is part of the “Learning from Nature” program, supported by the Dutch Technology Foundation (STW Grant 1099), which is part of the Netherlands Organization for Scientific Research.
AUTHOR CONTRIBUTIONS
J.A.B.-M. designed and performed experiments, analyzed data, discussed results, and wrote the article. E.F.F. performed experiments, analyzed data, discussed results, and wrote the article. F.F.M.B. performed experiments, discussed results, and wrote the article. J.A.R. performed experiments and discussed results. J.v.d.S. performed experiments. D.V. and J.J.B.K. designed experiments, discussed results, and wrote the article.
Glossary
- QTL
quantitative trait locus
- GWA
genome-wide association
- SNP
single nucleotide polymorphism
- LD
linkage disequilibrium
References
- Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C., Blankestijn-de Vries H., Hanhart C.J., Koornneef M. (1999). Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96: 4710–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Whaibi, M.H. (2011). Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 23: 139–150.
- Angadi S.V., Cutforth H.W., McConkey B.G., Gan Y. (2003). Yield adjustment by canola grown at different plant populations under semiarid conditions. Crop Sci. 43: 1358–1366. [Google Scholar]
- Angadi S.V., Cutforth H.W., Miller P.R., McConkey B.G., Entz M.H., Brandt S.A., Volkmar K.M. (2000). Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 80: 693–701. [Google Scholar]
- Antoun M., Ouellet F. (2013). Growth temperature affects inflorescence architecture in Arabidopsis thaliana. Botany 91: 642–651. [Google Scholar]
- Assmann S.M. (2013). Natural variation in abiotic stress and climate change responses in Arabidopsis: implications for twenty-first-century agriculture. Int. J. Plant Sci. 174: 3–26. [Google Scholar]
- Atwell S., et al. (2010). Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S., Sureshkumar S., Lempe J., Weigel D. (2006). Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabás B., Jäger K., Fehér A. (2008). The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 31: 11–38. [DOI] [PubMed] [Google Scholar]
- Baskin J.M., Baskin C.C. (1983). Seasonal changes in the germination responses of buried seeds of Arabidopsis thaliana and ecological interpretation. Bot. Gaz. 144: 540–543. [Google Scholar]
- Baxter I., Brazelton J.N., Yu D., Huang Y.S., Lahner B., Yakubova E., Li Y., Bergelson J., Borevitz J.O., Nordborg M., Vitek O., Salt D.E. (2010). A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet. 6: e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson J., Roux F. (2010). Towards identifying genes underlying ecologically relevant traits in Arabidopsis thaliana. Nat. Rev. Genet. 11: 867–879. [DOI] [PubMed] [Google Scholar]
- Bita C.E., Gerats T. (2013). Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 4: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bita C.E., Zenoni S., Vriezen W.H., Mariani C., Pezzotti M., Gerats T. (2011). Temperature stress differentially modulates transcription in meiotic anthers of heat-tolerant and heat-sensitive tomato plants. BMC Genomics 12: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E.K.F., Rowe H.C., Corwin J.A., Joseph B., Kliebenstein D.J. (2011). Combining genome-wide association mapping and transcriptional networks to identify novel genes controlling glucosinolates in Arabidopsis thaliana. PLoS Biol. 9: e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D.-Y., Chen Y., Chen J., Shi S., Chen Z., Wang C., Danku J.M., Zhao F.-J., Salt D.E. (2014). Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol. 12: e1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay P., Thurling N. (1989). Identification of genes controlling pod length in spring rapeseed, Brassica napus L., and their utilization for yield improvement. Plant Breed. 103: 54–62. [Google Scholar]
- Clarke H.J., Siddique K.H.M. (2004). Response of chickpea genotypes to low temperature stress during reproductive development. Field Crops Res. 90: 323–334. [Google Scholar]
- Cockram J., et al. ; AGOUEB Consortium (2010). Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. Proc. Natl. Acad. Sci. USA 107: 21611–21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. (2000). Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J., Bedinger P., Durachko D.M. (1997). Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. USA 94: 6559–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J., Li L.C., Cho H.-T., Hoffmann-Benning S., Moore R.C., Blecker D. (2002). The growing world of expansins. Plant Cell Physiol. 43: 1436–1444. [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.-R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechaine J.M., Brock M.T., Weinig C. (2014). QTL architecture of reproductive fitness characters in Brassica rapa. BMC Plant Biol. 14: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djanaguiraman M., Prasad P.V.V., Boyle D.L., Schapaugh W.T. (2013). Soybean pollen anatomy, viability and pod set under high temperature stress. J. Agron. Crop Sci. 199: 171–177. [Google Scholar]
- Donahue J.L., Alford S.R., Torabinejad J., Kerwin R.E., Nourbakhsh A., Ray W.K., Hernick M., Huang X., Lyons B.M., Hein P.P., Gillaspy G.E. (2010). The Arabidopsis thaliana Myo-inositol 1-phosphate synthase1 gene is required for Myo-inositol synthesis and suppression of cell death. Plant Cell 22: 888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion S., Lalonde S., Saini H.S. (1996). Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol. 111: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tsuchiya T., Hamada K., Kawamura S., Yano K., Ohshima M., Higashitani A., Watanabe M., Kawagishi-Kobayashi M. (2009). High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 50: 1911–1922. [DOI] [PubMed] [Google Scholar]
- Ferrier T., Matus J.T., Jin J., Riechmann J.L. (2011). Arabidopsis paves the way: genomic and network analyses in crops. Curr. Opin. Biotechnol. 22: 260–270. [DOI] [PubMed] [Google Scholar]
- Freedman M.L., et al. (2011). Principles for the post-GWAS functional characterization of cancer risk loci. Nat. Genet. 43: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S., Pires N., Østergaard L. (2010). A clade in the QUASIMODO2 family evolved with vascular plants and supports a role for cell wall composition in adaptation to environmental changes. Plant Mol. Biol. 73: 605–615. [DOI] [PubMed] [Google Scholar]
- Gan Y., Angadi S.V., Cutforth H., Potts D., Angadi V.V., McDonald C.L. (2004). Canola and mustard response to short periods of temperature and water stress at different developmental stages. Can. J. Plant Sci. 84: 697–704. [Google Scholar]
- Gebert M., Dresselhaus T., Sprunck S. (2008). F-actin organization and pollen tube tip growth in Arabidopsis are dependent on the gametophyte-specific Armadillo repeat protein ARO1. Plant Cell 20: 2798–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno F., Wolters-Arts M., Mariani C., Rieu I. (2013). Ensuring reproduction at high temperatures: the heat stress response during anther and pollen development. Plants 2: 489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdji S.M., Sibley A.M., Lobell D.B. (2013). Global crop exposure to critical high temperatures in the reproductive period: historical trends and future projections. Environ. Res. Lett. 8: 024041. [Google Scholar]
- Grilli G.V.G., Braz L.T., Lemos E.G.M. (2007). QTL identification for tolerance to fruit set in tomato by fAFLP markers. Crop Breed. Appl. Biotechnol. 7: 234–241. [Google Scholar]
- He H., de Souza Vidigal D., Snoek L.B., Schnabel S., Nijveen H., Hilhorst H., Bentsink L. (2014). Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot. 65: 6603–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhly A. (2011). Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 74: 9–16. [Google Scholar]
- Hedhly A., Hormaza J.I., Herrero M. (2004). Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 91: 558–564. [DOI] [PubMed] [Google Scholar]
- Hedhly, A., Hormaza, J.I., and Herrero, M. (2005). The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biol. (Stuttg.) 7: 476–483. [DOI] [PubMed]
- Honys D., Twell D. (2004). Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huefner N.D., Mizuno Y., Weil C.F., Korf I., Britt A.B. (2011). Breadth by depth: expanding our understanding of the repair of transposon-induced DNA double strand breaks via deep-sequencing. DNA Repair (Amst.) 10: 1023–1033. [DOI] [PubMed] [Google Scholar]
- Jiménez-López, J.C., Rodríguez-García, M.I., and Alché, J. (2011). Systematic and phylogenetic analysis of the Ole e 1 pollen protein family members in plants. In Systems and Computational Biology - Bioinformatics and Computational Modeling, N.-S. Yang, ed (InTech), doi/10.5772/19175. [Google Scholar]
- Kang H.M., Sul J.H., Service S.K., Zaitlen N.A., Kong S.Y., Freimer N.B., Sabatti C., Eskin E. (2010). Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana N., Chauhan H., Khurana P. (2012). Expression analysis of a heat-inducible, Myo-inositol-1-phosphate synthase (MIPS) gene from wheat and the alternatively spliced variants of rice and Arabidopsis. Plant Cell Rep. 31: 237–251. [DOI] [PubMed] [Google Scholar]
- Kim S., Plagnol V., Hu T.T., Toomajian C., Clark R.M., Ossowski S., Ecker J.R., Weigel D., Nordborg M. (2007). Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat. Genet. 39: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Hong C.B., Lee I. (2001). Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana. J. Plant Res. 114: 301–307. [Google Scholar]
- Kleinboelting N., Huep G., Kloetgen A., Viehoever P., Weisshaar B. (2012). GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40: D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki H., Yumoto S., Matsukawa I. (2004). Correlation of cold-weather tolerance with pubescence color and flowering time in yellow hilum soybeans in Hokkaido. Breed. Sci. 54: 303–311. [Google Scholar]
- Laubinger S., Zeller G., Henz S.R., Sachsenberg T., Widmer C.K., Naouar N., Vuylsteke M., Schölkopf B., Rätsch G., Weigel D. (2008). At-TAX: a whole genome tiling array resource for developmental expression analysis and transcript identification in Arabidopsis thaliana. Genome Biol. 9: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Shi J., Wang X., Liu G., Wang H. (2014). A combined linkage and regional association mapping validation and fine mapping of two major pleiotropic QTLs for seed weight and silique length in rapeseed (Brassica napus L.). BMC Plant Biol. 14: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Filiault D., Box M.S., Kerdaffrec E., van Oosterhout C., Wilczek A.M., Schmitt J., McMullan M., Bergelson J., Nordborg M., Dean C. (2014). Multiple FLC haplotypes defined by independent cis-regulatory variation underpin life history diversity in Arabidopsis thaliana. Genes Dev. 28: 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang Y., Bergelson J., Nordborg M., Borevitz J.O. (2010). Association mapping of local climate-sensitive quantitative trait loci in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107: 21199–21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cheng R., Spokas K.A., Palmer A.A., Borevitz J.O. (2014). Genetic variation for life history sensitivity to seasonal warming in Arabidopsis thaliana. Genetics 196: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Palmer W.M., Martin A.P., Wang R., Rainsford F., Jin Y., Patrick J.W., Yang Y., Ruan Y.-L. (2012). High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J. Exp. Bot. 63: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Lu X., Jiang L., Wang C., Fan Y., Zhang C. (2010). EMB1211 is required for normal embryo development and influences chloroplast biogenesis in Arabidopsis. Physiol. Plant. 140: 380–394. [DOI] [PubMed] [Google Scholar]
- Lucas M., Ehlers J., Huynh B.-L., Diop N.-N., Roberts P., Close T. (2013). Markers for breeding heat-tolerant cowpea. Mol. Breed. 31: 529–536. [Google Scholar]
- Mansfield S.G., Briarty L.G. (1991). Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can. J. Bot. 69: 461–476. [Google Scholar]
- Marcelis L.F.M., Heuvelink E., Hofman-Eijer L.R., Bakker J.D., Xue L.B. (2004). Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 55: 2261–2268. [DOI] [PubMed] [Google Scholar]
- Michaels S.D., Amasino R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet J.-C., Leroux C., Dardelle F., Lehner A. (2013). Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2: 107–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D., Franklin K.A. (2009). Temperature-regulation of plant architecture. Plant Signal. Behav. 4: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A., et al. (2010). The scale of population structure in Arabidopsis thaliana. PLoS Genet. 6: e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radauer C., Breiteneder H. (2006). Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J. Allergy Clin. Immunol. 117: 141–147. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2012). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing). [Google Scholar]
- Saini H.S. (1997). Effects of water stress on male gametophyte development in plants. Sex. Plant Reprod. 10: 67–73. [Google Scholar]
- Saini H.S., Aspinall D. (1982). Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Ann. Bot. (Lond.) 49: 835–846. [Google Scholar]
- Sakata T., Oshino T., Miura S., Tomabechi M., Tsunaga Y., Higashitani N., Miyazawa Y., Takahashi H., Watanabe M., Higashitani A. (2010). Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. USA 107: 8569–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samizadeh H., Yazdi-Samadi B., Bihamta M., Taleii A., Stringam G. (2010). Study of pod length trait in doubled haploid brassica napus population by molecular markers. J. Agric. Sci. Technol. 9: 129–136. [Google Scholar]
- Sanders P.M., Bui A.Q., Weterings K., McIntire K.N., Hsu Y.C., Lee P.Y., Truong M.T., Beals T.P., Goldberg R.B. (1999). Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 11: 297–322. [Google Scholar]
- Sato S., Peet M.M., Thomas J.F. (2002). Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. J. Exp. Bot. 53: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Sato S., Kamiyama M., Iwata T., Makita N., Furukawa H., Ikeda H. (2006). Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. (Lond.) 97: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M., Davison T.S., Henz S.R., Pape U.J., Demar M., Vingron M., Schölkopf B., Weigel D., Lohmann J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37: 501–506. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schmuths H., Bachmann K., Weber W.E., Horres R., Hoffmann M.H. (2006). Effects of preconditioning and temperature during germination of 73 natural accessions of Arabidopsis thaliana. Ann. Bot. (Lond.) 97: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz K., Hülskamp M., Pruitt R.E. (1995). Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J. 7: 731–749. [Google Scholar]
- Sharma M., Singh A., Shankar A., Pandey A., Baranwal V., Kapoor S., Tyagi A.K., Pandey G.K. (2014). Comprehensive expression analysis of rice Armadillo gene family during abiotic stress and development. DNA Res. 21: 267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova E.I. (2007). Expansins: Proteins involved in cell wall softening during plant growth and morphogenesis. Russ. J. Plant Physiol. 54: 713–727. [Google Scholar]
- Shindo C., Lister C., Crevillen P., Nordborg M., Dean C. (2006). Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes Dev. 20: 3079–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C., Aranzana M.J., Lister C., Baxter C., Nicholls C., Nordborg M., Dean C. (2005). Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 138: 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Correia J., Freitas S., Tavares R.M., Lino-Neto T., Azevedo H. (2014). Phenotypic analysis of the Arabidopsis heat stress response during germination and early seedling development. Plant Methods 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.R., Bowman J.L., Meyerowitz E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songnuan W. (2013). Wind-pollination and the roles of pollen allergenic proteins. Asian Pac. J. Allergy Immunol. 31: 261–270. [PubMed] [Google Scholar]
- Sugiyama T., Iwahori S., Takahashi K. (1965). Effect of high temperature on fruit setting of tomato under cover. Acta Hortic. 4: 63–69. [Google Scholar]
- Suzuki K., Tsukaguchi T., Takeda H., Egawa Y. (2001). Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. J. Am. Soc. Hortic. Sci. 126: 571–574. [Google Scholar]
- Valdivia E.R., Wu Y., Li L.-C., Cosgrove D.J., Stephenson A.G. (2007). A group-1 grass pollen allergen influences the outcome of pollen competition in maize. PLoS ONE 2: e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., Lasky J.R., Juenger T.E., Liu T.-W., Kumar M.N. (2014). Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. 164: 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VSN International (2013). GenStat for Windows, 16th ed. (Hemel Hempstead, UK: VSN International). [Google Scholar]
- Wahid A., Gelani S., Ashraf M., Foolad M.R. (2007). Heat tolerance in plants: An overview. Environ. Exp. Bot. 61: 199–223. [Google Scholar]
- Warner R.M., Erwin J.E. (2005). Naturally occurring variation in high temperature induced floral bud abortion across Arabidopsis thaliana accessions. Plant Cell Environ. 28: 1255–1266. [Google Scholar]
- Waters E.R. (2013). The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 64: 391–403. [DOI] [PubMed] [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G.V., Provart N.J. (2007). An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C., Argayoso M.A., Redoña E.D., Sierra S.N., Laza M.A., Dilla C.J., Mo Y., Thomson M.J., Chin J., Delaviña C.B., Diaz G.Q., Hernandez J.E. (2012). Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 131: 33–41. [Google Scholar]
- Young L.W., Wilen R.W., Bonham-Smith P.C. (2004). High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 55: 485–495. [DOI] [PubMed] [Google Scholar]
- Zinn K.E., Tunc-Ozdemir M., Harper J.F. (2010). Temperature stress and plant sexual reproduction: uncovering the weakest links. J. Exp. Bot. 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.