Abstract
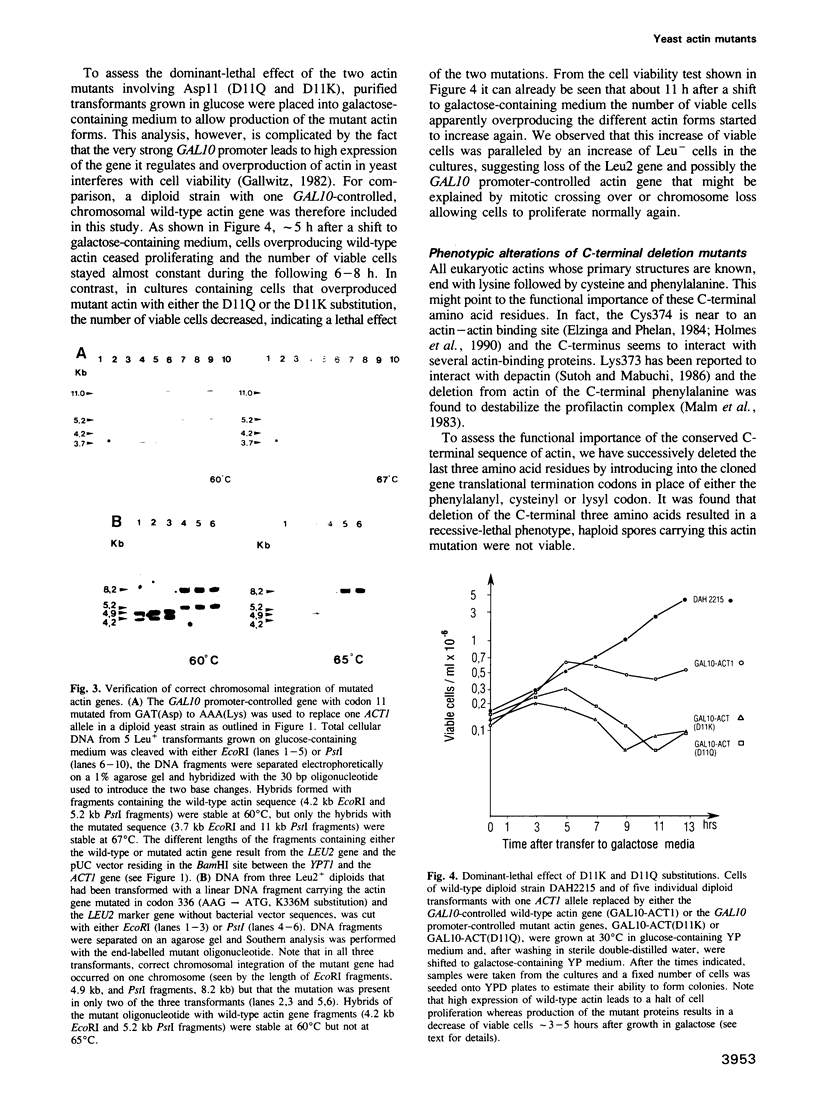
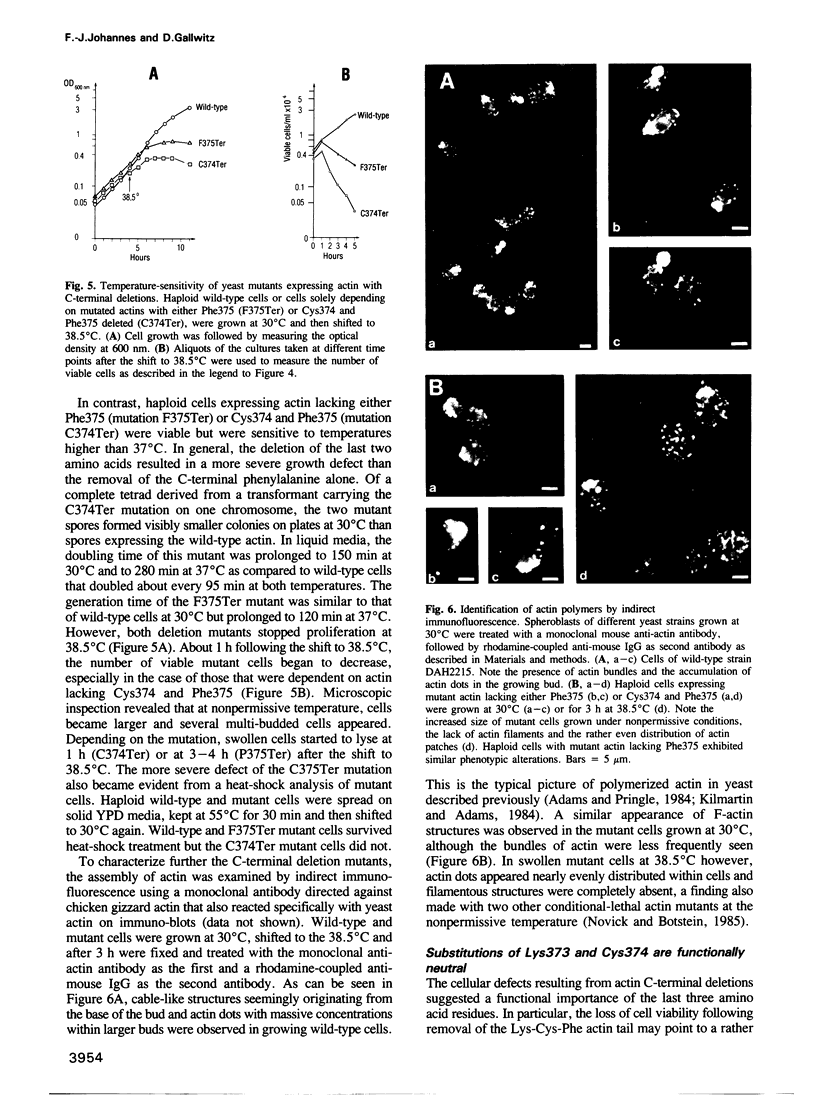
The yeast Saccharomyces cerevisiae has a single actin gene, ACT1, whose protein product is essential for cell viability. To study the structure-function relationship of this evolutionarily highly conserved protein, we have introduced into the gene several mutations leading to substitutions of amino acids that, by chemical crosslinking experiments, have previously been identified as potential sites for the interaction of actin with several actin-binding proteins and of actin monomers in filaments. The in vitro mutated actin genes were used to replace one chromosomal ACT1 allele in diploid cells. From diploid transformants, haploids that solely depended on mutant actins were isolated and their phenotypic alterations studied. The replacement of the N-terminal acidic residues (Asp2 and Glu4) with valine was functionally neutral. Substitutions of Asp11 led to dominant lethality. Substitutions of Lys191, Lys336, Trp356, Lys373 and Cys374 were without observable effect on cell growth, proliferation and morphology. Deletion of the C-terminal end, Lys-Cys-Phe-COOH, was lethal, whereas successive removal of the C-terminal Phe375 or Cys374 and Phe375 resulted in temperature sensitivity. At the nonpermissive temperature, the mutant cells were characterized by an increase in size, a tendency to lyse and significant alterations of the actin cytoskeleton.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Botstein D., Drubin D. G. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science. 1989 Jan 13;243(4888):231–233. doi: 10.1126/science.2643162. [DOI] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda J. F., Cannon J. F., Tatchell K., Hug C., Cooper J. A. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature. 1990 Mar 22;344(6264):352–354. doi: 10.1038/344352a0. [DOI] [PubMed] [Google Scholar]
- Barden J. A., Phillips L. 19F NMR study of the myosin and tropomyosin binding sites on actin. Biochemistry. 1990 Feb 6;29(5):1348–1354. doi: 10.1021/bi00457a034. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Kassab R., Boyer M., Roustan C., Benyamin Y. Cross-linking of the skeletal myosin subfragment 1 heavy chain to the N-terminal actin segment of residues 40-113. Biochemistry. 1988 Jul 26;27(15):5728–5736. doi: 10.1021/bi00415a050. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Crain W. R., Jr Complete nucleotide sequence of a sea urchin actin gene. Nucleic Acids Res. 1982 Jul 10;10(13):4081–4092. doi: 10.1093/nar/10.13.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Higashida M., Kido S. Plasma-gelsolin-binding sites on the actin sequence. Eur J Biochem. 1987 Apr 1;164(1):89–94. doi: 10.1111/j.1432-1033.1987.tb10997.x. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Miller K. G., Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988 Dec;107(6 Pt 2):2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Mulholland J., Zhu Z. M., Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990 Jan 18;343(6255):288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., Peckham M., Sparrow J. C., White D. C. Alteration in crossbridge kinetics caused by mutations in actin. Nature. 1990 Nov 29;348(6300):440–442. doi: 10.1038/348440a0. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Phelan J. J. F-actin is intermolecularly crosslinked by N,N'-p-phenylenedimaleimide through lysine-191 and cysteine-374. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6599–6602. doi: 10.1073/pnas.81.21.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C., Schekman R. Actin from Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1270–1278. doi: 10.1128/mcb.2.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi G., Szilagyi L., Elzinga M. Photoaffinity labeling of the nucleotide binding site of actin. Biochemistry. 1986 Sep 23;25(19):5793–5798. doi: 10.1021/bi00367a067. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford C., Nellen W., Niessing J., Gallwitz D. Yeast is unable to excise foreign intervening sequences from hybrid gene transcripts. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1496–1500. doi: 10.1073/pnas.80.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. P., Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989 Apr 21;57(2):233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- Malm B., Larsson H., Lindberg U. The profilin--actin complex: further characterization of profilin and studies on the stability of the complex. J Muscle Res Cell Motil. 1983 Oct;4(5):569–588. doi: 10.1007/BF00712116. [DOI] [PubMed] [Google Scholar]
- Mertins P., Gallwitz D. A single intronless action gene in the fission yeast Schizosaccharomyces pombe: nucleotide sequence and transcripts formed in homologous and heterologous yeast. Nucleic Acids Res. 1987 Sep 25;15(18):7369–7379. doi: 10.1093/nar/15.18.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Karr T. L., Kellogg D. R., Mohr I. J., Walter M., Alberts B. M. Studies on the cytoplasmic organization of early Drosophila embryos. Cold Spring Harb Symp Quant Biol. 1985;50:79–90. doi: 10.1101/sqb.1985.050.01.012. [DOI] [PubMed] [Google Scholar]
- Miller L., Kalnoski M., Yunossi Z., Bulinski J. C., Reisler E. Antibodies directed against N-terminal residues on actin do not block acto-myosin binding. Biochemistry. 1987 Sep 22;26(19):6064–6070. doi: 10.1021/bi00393a018. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen W., Gallwitz D. Actin genes and actin messenger RNA in Acanthamoeba castellanii. Nucleotide sequence of the split actin gene I. J Mol Biol. 1982 Jul 25;159(1):1–18. doi: 10.1016/0022-2836(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Hiromi Y., Ishikawa E., Yamada T., Isoda K., Maekawa H., Hotta Y. Molecular characterization of mutant actin genes which induce heat-shock proteins in Drosophila flight muscles. EMBO J. 1986 Mar;5(3):589–596. doi: 10.1002/j.1460-2075.1986.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Complete nucleotide sequence of a soybean actin gene. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1022–1026. doi: 10.1073/pnas.79.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L. R., Rubenstein P. A. Studies on the role of actin's N tau-methylhistidine using oligodeoxynucleotide-directed site-specific mutagenesis. J Biol Chem. 1987 Aug 15;262(23):11382–11388. [PubMed] [Google Scholar]
- Solomon T. L., Solomon L. R., Gay L. S., Rubenstein P. A. Studies on the role of actin's aspartic acid 3 and aspartic acid 11 using oligodeoxynucleotide-directed site-specific mutagenesis. J Biol Chem. 1988 Dec 25;263(36):19662–19669. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Actin-actin and actin-deoxyribonuclease I contact sites in the actin sequence. Biochemistry. 1984 Apr 24;23(9):1942–1946. doi: 10.1021/bi00304a009. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Mabuchi I. Improved method for mapping the binding site of an actin-binding protein in the actin sequence. Use of a site-directed antibody against the N-terminal region of actin as a probe of its N-terminus. Biochemistry. 1986 Oct 7;25(20):6186–6192. doi: 10.1021/bi00368a053. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Yin H. L. End-label fingerprintings show that the N- and C-termini of actin are in the contact site with gelsolin. Biochemistry. 1989 Jun 13;28(12):5269–5275. doi: 10.1021/bi00438a052. [DOI] [PubMed] [Google Scholar]
- Szilagyi L., Lu R. C. Changes of lysine reactivities of actin in complex with myosin subfragment-1, tropomyosin and troponin. Biochim Biophys Acta. 1982 Dec 20;709(2):204–211. doi: 10.1016/0167-4838(82)90462-9. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Watts F. Z., Shiels G., Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987 Nov;6(11):3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G. A putative ancestral actin gene present in a thermophilic eukaryote: novel combination of intron positions. Nucleic Acids Res. 1988 Mar 25;16(6):2553–2564. doi: 10.1093/nar/16.6.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]






