Abstract
Study Objectives:
Video-polysomnography (v-PSG) is the gold standard for the diagnosis of sleep disorders. Quantitative assessment of type and distribution of physiological movements during sleep for the differentiation between physiological and pathological motor activity is lacking. We performed a systematic and detailed analysis of movements during physiological sleep using v-PSG technology.
Design:
Prospective v-PSG investigation.
Setting:
Academic referral center sleep laboratory.
Participants:
One hundred healthy sleepers aged 19–77 years recruited from a representative population sample after a two-step screening.
Interventions:
N/A.
Measurements and Results:
All subjects underwent v-PSG. In all cases where electromyographic activity > 100 msec duration was visible during sleep in the mentalis, submentalis, flexor digitorum superficialis, or anterior tibialis muscles, the time-synchronized video was analyzed. Visible movements were classified according to movement type and topography, and movement rates were computed for the different sleep stages. A total of 9,790 movements (median 10.2/h, IQR 4.6–16.2) were analyzed: 99.7% were elementary, 0.3% complex. Movement indices were higher in men than women (men: median 13/h, interquartile range 7.1–29.3, women: median 7.9/h, interquartile range 3.4–14.5; P = 0.006). The majority of movements involved the extremities (87.9%) and were classified as focal (53.3%), distal (79.6%), and unilateral (71.5%); 15.3% of movements were associated with arousals. REM-related movements (median 0.8 sec, IQR 0.5–1.2) were shorter than NREM-related movements (median 1.1 sec, IQR 0.8–1.6; P = 0.001). Moreover, REM-related movements were predominantly myocloniform (86.6%), whereas NREM-related movements were more often non-myocloniform (59.1%, P < 0.001).
Conclusion:
Minor movements are frequent during physiological sleep, and are associated with low arousal rates. REM-related movements were predominantly myocloniform and shorter than NREM movements, indicating different influences on motor control during both sleep states.
Citation:
Stefani A, Gabelia D, Mitterling T, Poewe W, Högl B, Frauscher B. A prospective video-polysomnographic analysis of movements during physiological sleep in 100 healthy sleepers. SLEEP 2015;38(9):1479–1487.
Keywords: sleep laboratory, movement, motor activity, video analysis, healthy subject, myocloniform, REM sleep behavior disorder
INTRODUCTION
Video-polysomnography (v-PSG) is the gold standard for the diagnosis of sleep disorders, and is of special importance for the evaluation of movement disorders during sleep or parasomnias.1
The first attempts to study physiological movements during sleep date back to the 1970s, and movement analysis at those times relied on a variety of approaches, including direct observation, the “sensitive bed” method, photography or videotaping over the electroencephalographic (EEG) artifact method, the use of different semiconductor strain gauges, as well as registration of the electromyographic (EMG) signal.2–6 Recently, time-synchronized v-PSG has become widely available. The advantage of this technology is that movements can be recorded continuously over the complete registration period, analyzed offline in high temporal resolution, and directly correlated to PSG signals.
Movements during sleep in adults have been analyzed in various pathologies, such as NREM parasomnias, REM sleep behavior disorder (RBD), and narcolepsy, and also in other neurological disorders such as early Parkinson disease.7–13
Systematic v-PSG studies of physiological movements are lacking, but would be of interest for the differential diagnosis between normal and pathological movements, as abnormal motor activity during sleep is a hallmark of an impending neurodegenerative disease.14–16 RBD is a salient example, where qualitative and quantitative differentiation between RBD movements and non-RBD physiological movements is essential for diagnostic accuracy of RBD. Nevertheless, according to present criteria, “abnormal” REM-related motor behaviors are not further specified, and their rating may be influenced by the individual scorer's subjective impression and experience.17
Against this background, we aimed to perform a detailed and systematic video analysis of movements during both NREM and REM sleep with contemporary technology of time-synchronized high-resolution v-PSG in healthy subjects, who were carefully selected from an existing population-based sample. Special attention was paid to the type and topographical distribution of movements during both NREM and REM sleep, as well as movement-associated occurrence of arousals.
METHODS
This study was part of a larger project on motor activity during sleep in health and disease. Data on normative values of motor activity as assessed with surface EMG have been reported elsewhere.18 Results on video-analysis have not been published.
Study Participants
For this study, 100 healthy subjects between 19 and 77 years of age were recruited in a 2-step screening process from an existing random population sample representative for the population of Tyrol/Austria. The first step was a structured telephone interview; the second step consisted of an interview by a physician experienced in sleep medicine. The major inclusion criterion was that participating subjects perceived their sleep as healthy. Exclusion criteria included the presence of neurological, psychiatric, cardiac, pulmonary, renal, hepatic, metabolic or oncological diseases, pregnancy, regular alcohol consumption, and the presence of a history suggestive of any sleep disorder according to the International Classification of Sleep Disorders (ICSD) criteria.19 Another exclusion criterion was use of any psychiatric or neurological medication. Further details have been published elsewhere.18
This study was approved by the local ethical committee of the Medical University of Innsbruck. All participants granted written informed consent prior to study participation.
Polysomnography
All participating subjects underwent one night of v-PSG according to current standards,20,21 and sleep stages were scored according to the 2007 American Academy of Sleep Medicine (AASM) criteria.21 V-PSG consisted of vertical and horizontal electrooculography (EOG), EEG (F3, F4, C3, C4, O1, O2, M1, and M2 electrodes), cardiorespiratory recording (single channel electrocardiography [ECG], recording of nasal air flow [thermocouple], nasal pressure cannula, tracheal microphone, thoracic and abdominal respiratory movements [piezo], transcutaneous oxygen saturation), EMG of the mental, sub-mental, both flexor digitorum superficialis muscles (FDS), both anterior tibialis muscles (AT), and digital videography. The video was recorded by an infrared camera (Elbex Inc. EX series, Regensburg, Germany) and processed by a data rate of 1,500.000 bit/second. The screen resolution for video analysis was 1,280 × 960 pixels.
Video-Polysomnographic Analysis
Definition of Movements
The selection of video segments to be analyzed in detail was based on EMG recording. If any EMG activity with a duration > 100 msec was present in the mentalis, submentalis, FDS, or AT muscles during sleep (from lights off to lights on), the time-synchronized video was carefully assessed for the presence of potential video correlates.
All visible movements regardless of type, amplitude, and duration were analyzed by one of the authors (A.S.). All ambiguous cases were discussed with 2 board-certificated neurologists with a specialization in sleep medicine (B.F., B.H.).
The exact onset and offset of movements were observed in the video, and the duration determined with the chronograph of the iPhone 4s. Two movements were considered separate when they were clearly seen as separate in the video and there was an interval of 0.5 sec between the 2 corresponding EMG activities. Voluntary movements associated with arousals were not considered.
Classification of Movements
The video classification system used for this analysis was developed by the senior authors of our sleep laboratory (B.F., B.H.).8,11 Every movement was classified according to type of movement, topographical involvement, and presence of an associated arousal. For illustration, see Figure S1 (supplemental material). In addition, we classified all movements according to the presence or absence of REM behavioral events (RBEs).
Type of Movement
Regarding type, movements were dichotomized into elementary and complex. Elementary movements were defined as small involuntary movements or stereotyped movements. Complex movements were defined as movements showing complexity of action and involving more muscle groups simultaneously or violent movements.
Elementary movements were subdivided into myocloniform, non-myocloniform, and stereotyped movements. Myocloniform movements were defined as sudden, brief, jerky, and involuntary movements. We decided to use the term myocloniform rather than myoclonic because the duration of observed myocloniform movements did not always correspond to current duration criteria for myoclonic movements. Non-myocloniform movements were defined as small non-jerky excursions that usually would not be noticed by a sleeping bed partner. Stereotypes refer to automatism-like events (e.g., smacking or fumbling).
Complex movements were sub-classified into complex/ scenic or violent. Complex/scenic movements are motor events that involve multiple muscle groups at the same time or acting out movements (gesturing, reaching, grabbing). Violent movements are forceful and vehement movements in which the patient can potentially hurt or injure himself and/or the bed partner (e.g., kicking or punching).
Vocalizations (talking, crying, laughing, yelling, swearing) were also analyzed when associated with a movement. Furthermore, the apparent emotional state (positive, e.g., when the patient is laughing; negative, e.g., when the patient is screaming or crying; neutral) was assessed for complex/scenic behaviors and vocalizations.
Topographical Distribution
The involved body parts were categorized into 5 regions (head, neck, trunk, upper extremities, and lower extremities) and the number of involved body parts (maximum 5) was provided for every movement.
Spatial distribution of movements was subdivided into categories (focal, segmental, multifocal, or generalized), as has been previously described.22 Focal movements are localized to one body part. Segmental movements involve one or more contiguous body parts. Multifocal indicates involvement of non-contiguous body parts. Generalized motor events involve multiple (≥ 3 non-contiguous with trunk involvement) or all body parts.
In addition, every movement was classified as proximal, distal, or axial. Laterality was described as unilateral if movements involved only one side of the body or bilateral if both sides were involved.
Association with Arousal
An arousal was defined according to the AASM 2007 guidelines,21 and scored as associated if the arousal preceded or followed the movement by ≤ 0.5 s. The time interval ≤ 0.5 s was chosen according to scoring rules for periodic leg movement in sleep-related arousals.23
REM Sleep Behavioral Events
We analyzed all movements during REM sleep for the presence of RBEs.13 An RBE is defined as a motor behavior and/or vocalization with a purposeful component, seemingly expressive of a subject's mentation. Comfort moves, neck myoclonus, respiratory noises, and events related to arousals are not taken into account. At least 2 separate behavioral events and/or vocalizations during REM sleep are needed to classify a subject as “RBE positive.”13 All potential RBEs were independently discussed with 2 board-certified neurologists with specialization in sleep medicine (B.F., B.H.).
Derived Measures
All identified movements were tabulated in an Excel spreadsheet, and associated to sleep stage and arousal. The exact onset and offset of every movement was observed in the video and linked to the corresponding 3-sec mini-epochs.
For each patient, we summed the total number of movements and calculated an index (movement index, MI) of each movement per hour of total sleep, NREM sleep and its respective sleep stages, and REM sleep. Movement-related arousal indices represent movement-related arousal counts per hour.
SINBAR (Sleep Innsbruck Barcelona) Index
As described in detail elsewhere,18,24 “any” EMG activity in the mentalis muscle and phasic EMG activity in right and left FDS were quantified according to SINBAR criteria. The SINBAR EMG activity index represents the percentages of 3-sec mini-epochs containing “any” or phasic EMG activity divided by all evaluated mini-epochs.
Statistics
IBM SPSS 21 (SPSS, Inc., Chicago, IL) was used for all statistical analysis. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Descriptive statistics are given as means ± standard deviations (SD) for normally distributed data, and medians ± interquartile ranges (IQR) for not normally distributed data, as well as frequencies, as applicable. In case of normal distribution, t-tests (independent and dependent as applicable) were calculated. In case of non-normal distribution, nonparametric statistics were applied (Mann-Whitney U-test for 2 unrelated variables, Wilcoxon test for 2 related variables). In addition, we calculated percentiles to establish the quantitative range of respective movement indices that was reached by 10%, 25%, 50%, 75%, and 90% of investigated subjects. Correlations were calculated using Spearman correlation coefficients. To account for sexand age-dependent changes of various rates of movements, a linear regression analysis was calculated. For the linear regression analysis, variables were naturally log-transformed, as rates of movements were not normally distributed. In case of performing multiple comparisons, Bonferroni correction was applied, and the significance level (normally P ≤ 0.05) was set accordingly to 0.004.
RESULTS
Study Sample and General Sleep Parameters
A total of 100 healthy subjects (60 women, 40 men) participated in this study. The median age at time of PSG was 43 (range 19–77) years. Participants were pooled in 5 age groups: up to 30 years (total 20; 9 women, 11 men); between 31 and 40 years (total 21; 10 women, 11 men); between 41 and 50 years (total 20; 15 women, 5 men); between 51 and 60 years (total 15; 9 women, 6 men); and older than 60 years (total 24; 17 women, 7 men).
Median total sleep time was 403 (IQR 361.9–438) min. Median sleep latency was 16.1 (IQR 9.2–30.9) min, and median REM latency was 119 (IQR 88.5–163.9) min. Median sleep efficiency was 84% (IQR 76.1% to 90.4%) of sleep period time (median 462, IQR 446.5–471.7 min). The median percentages of the respective sleep stages were 9.6% for N1 (IQR 7.4% to 12.7%), 45.4% for N2 (IQR 37.9% to 50.7%), 17.4% for N3 (IQR 13.3% to 21%), and 13.6% for stage REM (IQR 9.8% to 16.5%). Forty-three percent of all participants snored occasionally during v-PSG. The median apnea-hypopnea index was 0.7 (IQR 0.1–3.5) per hour.
Analysis of Movements during Sleep
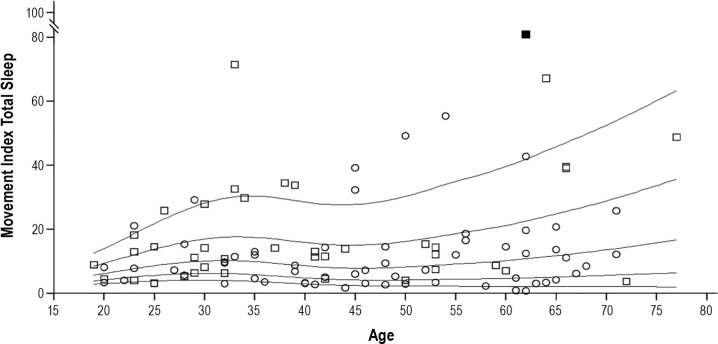
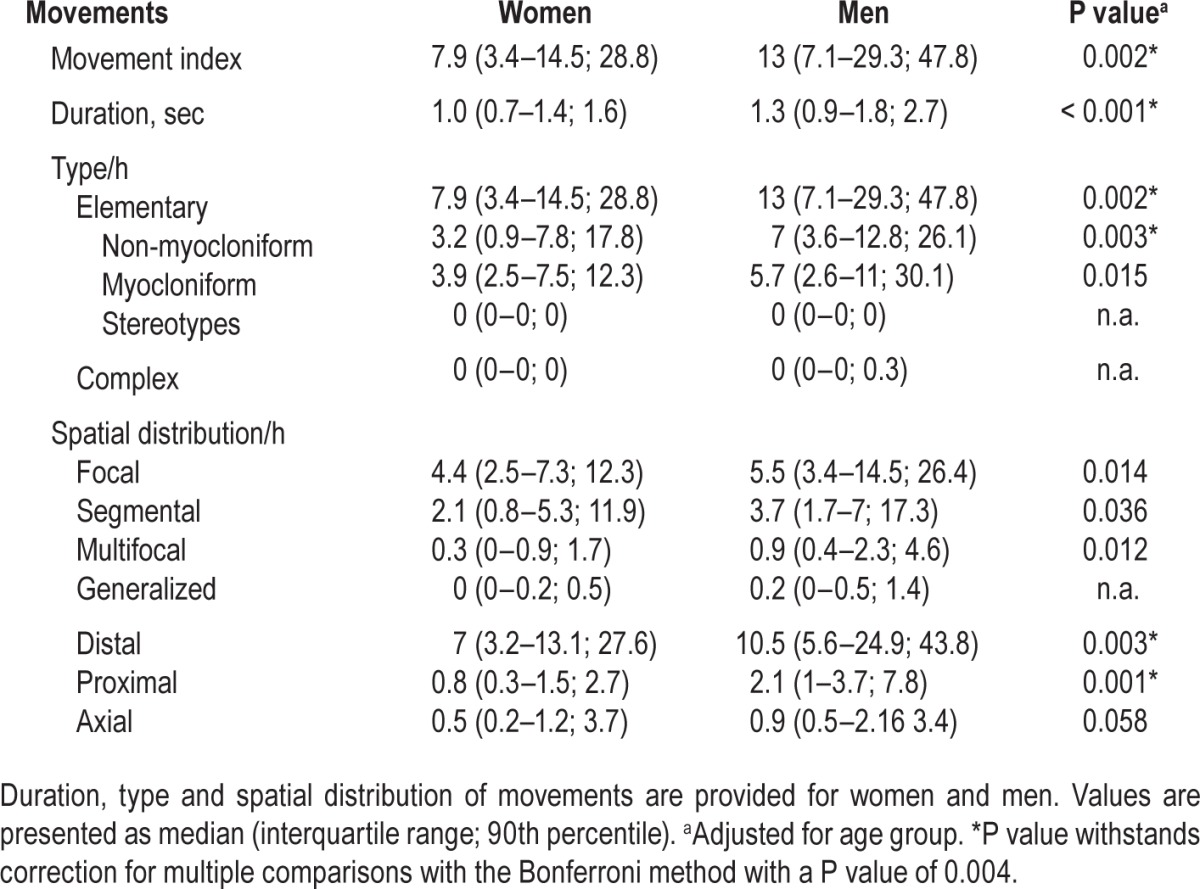
A total of 9,790 movements were analyzed—8,057 during NREM sleep and 1,733 during REM sleep. Median number of movements was 10.2/h in total sleep (IQR 4.6–16.2; 90th percentile 38.6), 9.2/h in NREM sleep (IQR 3.3–17; 90th percentile 39.6), and 14.1/h in REM sleep (IQR 6.3–22; 90th percentile 34.7). The distribution of movements per hour across the different sleep stages is given in Figure 1. Figure 2 provides the percentile curves for movement rates in total sleep across men and women and the different age groups. Of note, men had higher indices than women (P = 0.006). This difference remained significant after adjusting for age (standardized coefficient = −0.316, P = 0.002) and Bonferroni correction for multiple comparisons. Age, in contrast, was not associated with changes in movement rates (P = 0.319).
Figure 1.
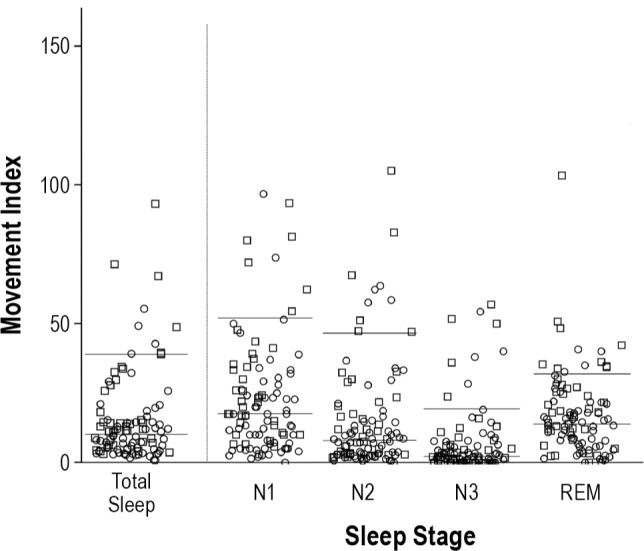
Distribution of the movement indices across the different sleep stages. The figure shows the distribution of the movement indices across total sleep and the different sleep stages (N1, N2, N3 and REM sleep). Individuals are presented as circles (women) or squares (men). The horizontal lines represent the 50th and 90th percentiles. Note that one extreme outlier in N1 sleep stage (movement index 167.5/h) is not illustrated.
Figure 2.
Sex- and age-related distribution of the movement index during total sleep. The panel shows the percentiles (10th, 25th, 50th, 75th and 90th) for the movement index during total sleep in relation to age. Individuals are presented as circles (women) or squares (men). Note that one extreme outlier (movement index in total sleep 93.2/h) is illustrated as a filled square.
The median duration of movements was 1.1 sec (IQR 0.8–1.5; 90th percentile 1.9) in total sleep time, 1.1 sec (IQR 0.8–1.6; 90th percentile 2.4) in NREM sleep and 0.8 sec (IQR 0.5–1.2; 90th percentile 1.7) in REM sleep (P = 0.001). We found a sex difference, with a shorter duration of movements in women than men (P = 0.001). This difference remained significant after adjustment for age (standardized coefficient = −0.346, P < 0.001) and Bonferroni correction for multiple comparisons. There was no statistically significant difference between age groups after adjusting for sex (P = 0.204).
The majority of movements (84.7%) were not associated with arousals; 15.3% of all motor events were associated with arousals (median 1.3/h IQR 0.5–3.7; 90th percentile 6.6). This was more pronounced in NREM (median 1.3 movements/h, IQR 0.5–4.2; 90th percentile 7.1), than in REM sleep (median 0 movements/h, IQR 0–1.7; 90th percentile 4.3), P < 0.001 (see Figure 3).
Figure 3.
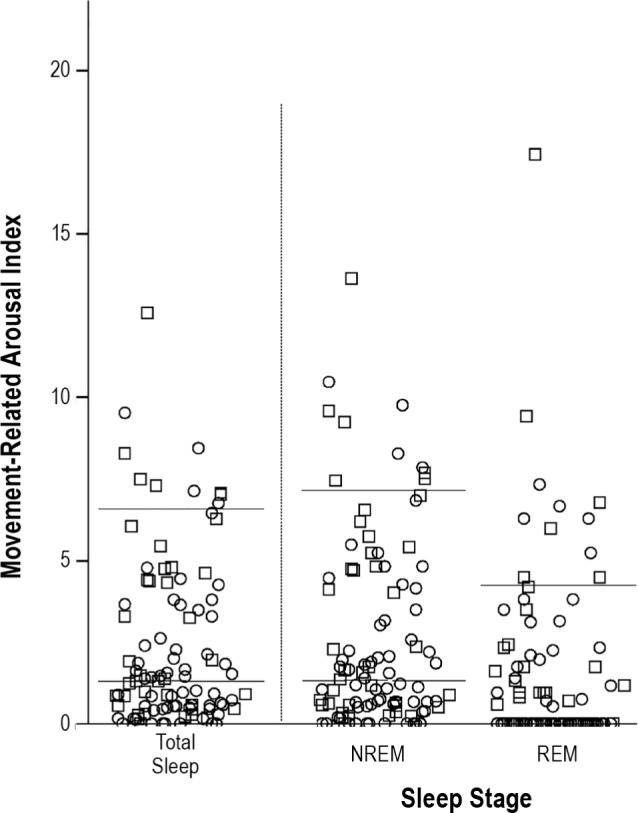
Movement-related arousal indices across the different sleep stages. The figure shows movement-related arousal indices in total sleep, NREM sleep and REM sleep. Individuals are presented as circles (women) or squares (men). The horizontal lines represent the 50th and 90th percentile for the different sleep stages. In REM sleep, only the 90th percentile is shown, as the 50th percentile corresponds to 0.
Type of Movement
The vast majority of movements (99.7%, median 10.1/h, IQR 4.6–15.3; 90th percentile 38.6) were classified as elementary; only 0.3% (median 0/h, IQR 0–0; 90th percentile 0.2) of the events were classified as complex. None of these complex movements was classified as violent or scenic. For differences between NREM and REM sleep see Table 1.
Table 1.
Movement indices across sleep stages.
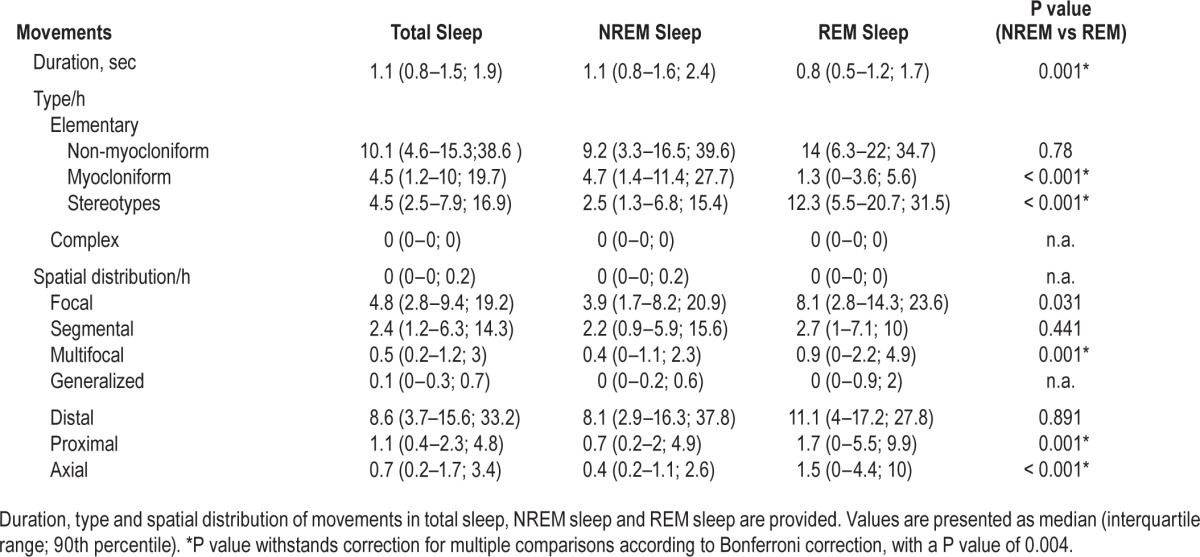
The distribution of elementary movements for total sleep, NREM, and REM sleep is given in Figure 4. Of note, elementary movements during REM sleep were predominantly myocloniform (86.6%, median 12.3, IQR 5.5–20.7; 90th percentile 31.5), whereas NREM sleep exhibited more often non-myocloniform movements (59.1%, median 4.7/h, IQR 1.4–11.4, 90th percentile 27.7, P < 0.001). Men had a higher rate of elementary movements than women (P = 0.006), and this withstood adjustment for age (standardized coefficient = −0.315, P = 0.002) as well as Bonferroni correction. We found no statistically significant difference between age groups after adjusting for sex (P = 0.270).
Figure 4.

Distribution of myocloniform and non-myocloniform movements across sleep stages. The panel shows the distribution of the movement indices for myocloniform (M) and non-myocloniform (NM) movements in total sleep, NREM sleep, and REM sleep. Individuals are presented as circles (women) or squares (men). The horizontal lines represent the 50th and 90th percentiles for the different indices. Note that the difference of both myocloniform and non-myocloniform movement indices between NREM sleep and REM sleep was significant (P < 0.001).
Only a few (0.1%) movements were accompanied by vocalization. Seven of the 8 vocalizations had an apparent neutral expression.
Topographical Distribution
The topographical distribution of involved body parts for total sleep, NREM and REM sleep is given in Figure 5. During total sleep, the most involved body parts were the lower extremities (51.6%), followed by the upper extremities (36.3%), trunk (4.8%), neck (4.7%), and head (2.6%). This order was also true for both NREM and REM sleep. Men had higher indices than women with reference to involvement of upper and lower extremities (P = 0.020 and P = 0.022, respectively). This difference withstood adjusting for age (upper extremities: standardized coefficient = −0.259, P = 0.008; lower extremities: standardized coefficient = −0.215, P = 0.037), but did not withstand correction for multiple comparisons according to Bonferroni. No significant difference was found between age groups after adjusting for sex (upper extremities: P = 0.106; lower extremities: P = 0.844).
Figure 5.
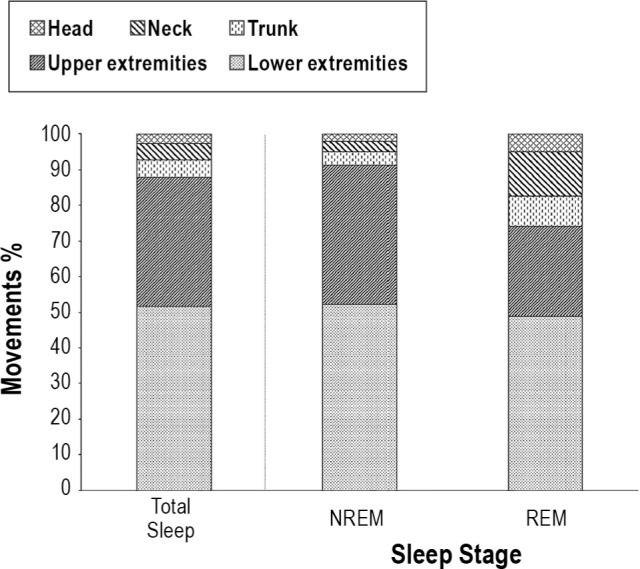
Involved body parts across the different sleep stages. The figure shows the percentages of the involved body parts (head, neck, trunk, upper extremities, lower extremities) across total sleep, NREM sleep, and REM sleep.
Most movements involved only one body region (mean number of involved body parts was 1 for all sleep stages).
According to spatial distribution, in total sleep a median of 4.8 movements/h (IQR 2.8–9.4; 90th percentile 19.2) was classified as focal; median of 2.4 movements/h (IQR 1.2–6.3; 90th percentile 14.3) as segmental; median of 0.5 movements/h (IQR 0.2–1.2; 90th percentile 3) as multifocal; and median of 0.1 movements/h (IQR 0–0.3; 90th percentile 0.7) as generalized. Similar findings were found for NREM and REM sleep. There was no statistically significant difference between age groups after adjusting for sex. Spatial distribution showed a sex difference with higher indices for focal and segmental movements in men (P = 0.035 and P = 0.033, respectively). Those differences remained statistically significant after adjusting for age (focal: standardized coefficient = −0.251, P = 0.014; segmental: stan -dardized coefficient = −0.217, P = 0.036), but not after Bonferroni correction for multiple comparisons.
The distribution of distal, proximal, and axial movements is shown in Table 1. REM sleep exhibited higher axial movement rates than NREM sleep (P < 0.001). There was a moderate correlation between the number of axial movements and the number of head jerks in REM sleep (Spearman rho 0.549, P < 0.001).
Laterality
The majority of movements in total sleep were unilateral (71.5%, median 6.3/h, IQR 3.2–10.5; 90th percentile 26.9); 28.5% (median 2.3/h, IQR 0.8–4.7; 90th percentile 10.8) were bilateral. A similar distribution was found between NREM (unilateral: 72.3%; median 5.1/h, IQR 2.6–10.9; 90th percentile 28.3) and REM sleep (unilateral: 67.4%; median 7.9/h, IQR 2.9–14.1; 90th percentile 21.9; P = 0.8).
The distributions of movement indices across women and men are summarized in Table 2, and the distributions across age groups are summarized in Table S1 (supplemental material).
Table 2.
Distribution of movement indices in women and men.
RBE
None of our subjects was RBE positive as defined by a minimum of two isolated RBEs.13 One subject showed a single definitive RBE during the whole night, and one subject showed a single possible RBE (divergent judgement between both senior raters).
Correlation between EMG Activity during REM Sleep and Visible REM-Related Movements
The correlation between REM-related movements/h and “any” EMG activity in the mentalis muscle was lower (Spearman rho 0.387, P < 0.001), than the correlation between movements/h in REM sleep and the SINBAR index (Spearman rho 0.473, P < 0.001).
DISCUSSION
This is the first study to perform a systematic video analysis of the rates, types, and distribution of movements and their relation to different sleep stages in healthy adults using contemporary v-PSG technology. The major findings were that brief, predominantly small distal movements are frequent during physiological sleep, that myocloniform movements predominate during REM sleep, and that there is a gender difference in movement indices.
Elementary Movements are Frequent during Physiological Sleep
We found that the vast majority of movements are small elementary movements. This finding is in accordance to data reported by several historical studies.2,6,25–27 Moreover, we confirmed the association between rates of movements and sleep stages (N1 > REM > N2 > N3), as shown by two previous studies in small samples of both healthy subjects and patients with narcolepsy.11,26
Of note, a previous video-analysis in patients with severe RBD revealed that the majority of movements were elementary, too, but that the proportion of complex movements was higher.8 The larger amount of complex movements in RBD patients compared to healthy subjects might support the hypothesis of a cortical generation of movements in this condition.28 However large-amplitude movements represented only a small fraction of movements even in RBD patients (13.5%8 and 30.8%9), and violent movements represented only the tip of the iceberg (3.6%8 and 7.5%9). Nevertheless, there is also a relevant difference in small movements when compared to our data in healthy subjects, which could allow a good distinction between normal and pathological movements, even if only small movements are captured during PSG in a patient with RBD.
In healthy controls, the lower extremities were more often involved than the upper extremities; in patients with RBD, the opposite has been found by various groups.8,28 The reason for the difference in the movement pattern between healthy subjects and RBD patients is not clear. Given the hypothesis of a cortical generation of RBD movements,28 one might argue that in RBD movements the upper limbs are more often involved than the lower limbs because the upper limbs are more largely represented in the cerebral cortex.
Myocloniform Movements Predominate during REM Sleep
In contrast to movements during NREM sleep, movements during REM sleep were shorter and mostly myocloniform. Some historical studies have focused on differences between NREM and REM sleep. They found a prevalence of small jerky muscle movements of the extremities in REM sleep.2,6,25,27,29 Muscular twitches during this stage may occur due to glutamatergic inputs to trigeminal and spinal motoneurons, which are not completely suppressed by phasic inhibitory drives responsible for motoneuron inhibition during REM sleep.30,31
An alternative explanation to the traditional hypothesis of a cortical origin of REM twitches as motor expression of dreams, is the hypothesis of Blumberg et al., who showed that REM-related twitches are important for the development of the sensorimotor system and are due to pure, non-random brainstem generation without corollary discharge.32,33
Effects of Gender and Age
There was a gender difference for many movement indices, with a preference for men, whereas no difference between age groups was observed. To our knowledge, no previous study has analyzed gender differences in movements during sleep in healthy subjects. A previous study of our group18 found a sex preference for men for fragmentary myoclonus and neck myoclonus indices in the same cohort. However, fragmentary myoclonus is usually not observed in the video, and neck myoclonus alone does not explain the gender difference in several movement indices. In this context, it is tempting to speculate that those differences might at least in part explain the apparent lower or milder incidence of RBD in women, in addition to the reported difference in dream content compared to men.34,35
First Normative Data for Movements across the Lifespan
For the diagnosis of RBD, normative values are needed. Presently, normative data on REM sleep without atonia (RWA) are available from 3 studies,18,24,36 whereas normative data on sleep-related movements are missing. The presence of small movements occasionally during REM sleep is physiological. However, a continuum from RWA to RBD is likely, and the transition phase might be prone to be overlooked without a detailed video-analysis. In the present study, we provide for the first time normative values for movements which might turn out to be useful for increasing the diagnostic accuracy of RBD. In addition, we demonstrated that true complex or violent movements were absent in healthy subjects, which further helps us classify a patient with definitive RBD in case of RWA and one complex or violent movement in the v-PSG night in the absence of a corresponding history.
Absence of REM Sleep Behavioral Events
None of the participating healthy subjects in this study was positive for REM sleep behavioral events. In contrast, Sixel-Döring et al.13 reported that 15% of the healthy controls in their study were RBE-positive. This difference could be due to the presence of older controls in the Sixel-Döring study (mean age: 65 ± 7 years) compared to our study (median age: 43 years). In this context, it is also worthwhile noting that in the present work, in one of the two RBE candidate events, two expert scorers did not reach the same conclusion, which might point to subjectivity of the interpretation of RBE events. Studies investigating inter-rater reliability of RBE are therefore warranted.
Strengths and Limitations of the Present Study
The major strengths of this study were the highly scrutinized selection of healthy subjects, the analysis method with a detailed and objective classification of movements using qualitative as well as quantitative parameters, and a meticulous investigation of identified video sequences during the whole sleep recording. A potential limitation of this study is that we only investigated video sequences in case of an EMG activity increase of at least 100 msec. in the mentalis, the FDS and the AT muscles. Based on this approach, we cannot rule out that some movements, especially facial movements or movements of very proximal body muscles might have been underestimated. Since, however, a previous study of our group showed that by combining the mentalis muscle with a muscle of the upper and lower extremities, 94% of all REM-related video events were detected,37 we feel confident that the majority of movements were also captured in the present study. Moreover, only movements visible in the video were classified. Detection of very small movements was often difficult because of the infrared light and the limited resolution of the recordings. Moreover, as volunteers slept with blankets, several small movements might be hidden by the blanket, and the rates of video events might be even higher than those provided in this study. To minimize those biases, all videos were looked through a minimum of two times with regard to different body parts. On the other hand, our procedure reflects the usual real-life setting in a sleep laboratory and allows therefore a direct comparison with video data gathered from routine patients in these settings.
CONCLUSION
Our study demonstrated that movements during sleep are frequent in healthy subjects, albeit of small amplitude and predominantly involving the lower limbs. In addition, men seemed to have higher movement indices than women. Furthermore, movements during REM sleep were shorter and more myocloniform compared to NREM sleep, which might be explained by the different mechanisms responsible for motor regulation of these two states of sleep. These video normative values might be useful for differentiation between normal and pathological movements during sleep, which is a basic prerequisite when quantitatively assessing sleep-related movement disorders and parasomnias.
DISCLOSURE STATEMENT
This study was supported by the Oesterreichische Nationalbank (Oesterreichische Nationalbank Anniversary Fund, project number: 15127) and the Austrian Science Fund (project KLI 236). The recruitment of healthy volunteers was supported by the intramural funding program of Innsbruck Medical University (MUI START 2 grant 2010012005). The work was performed at the Department of Neurology, Inns-bruck Medical University, Innsbruck, Austria. Dr. Stefani is supported by the Oesterreichische Nationalbank (Oesterreichische Nationalbank Anniversary Fund, project number: 15127). Dr. Gabelia is supported by the Austrian Science Fund (project KLI 236) and has received travel support from Habel Medizintechnik and Vivisol. Dr. Mitterling has received travel support from AOP Orphan. Dr. Poewe reports the following support: AbbVie, Allergan, AstraZeneca, Boehringer-Ingelheim, Boston Scientific, GlaxoSmithKline, Ipsen, Lundbeck, Medtronic, MSD, Merck-Serono, Merz Pharmaceuticals, Novartis, Orion Pharma, Teva, UCB, and Zambon (consultancy and lecture fees) and the following royalties: Thieme, Wiley Blackwell, Oxford University Press, Cambridge University Press. Dr. Högl has received personal fees from UCB, Mundipharma, Pfizer, Respironics, Sanofi, and non-financial support from Habel Med.-Technik, Vienna and Vivisol, Vienna, outside the submitted work. Dr. Frauscher is supported by the Austrian Science Fund (Schrödinger fellowship abroad J3485).
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AT
anterior tibialis
- ECG
electrocardiography
- EEG
electoencephalography
- EMG
electromyography
- EOG
electrooculography
- FDS
flexor digitorum superficialis
- ICSD
International Classification of Sleep Disorders
- IQR
interquartile range
- MI
movement index
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RBE
REM behavioral event
- RWA
REM sleep without atonia
- SINBAR
Sleep Innsbruck-Barcelona group
- v-PSG
video-polysomnography
SUPPLEMENTAL MATERIAL
Classification method. The figure summarizes the classification method used in this study to classify individual movements.
Age-related distribution of movement indices.
REFERENCES
- 1.Frauscher B, Comella CL. Sleep-related movement disorders. In: Poewe W, Jankovic J, editors. Movement disorders in neurologic and systemic disease. Cambridge University Press; 2014. pp. 314–32. [Google Scholar]
- 2.Gardner R, Grossman WI, Roffwarg HP, Weiner H. The relationship of small limb movements during REM sleep to dreamed limb action. Psychosom Med. 1975;37:147–59. doi: 10.1097/00006842-197503000-00005. [DOI] [PubMed] [Google Scholar]
- 3.De Lisi L. Su di un fenomeno motorio constante del sonno normale: le mioclonie ipniche fisiologiche. Riv Pat Nerv Ment. 1932;39:481–96. [Google Scholar]
- 4.Johnson HM, Swan TH. Sleep. Psychol Bull. 1930;27:1–39. [Google Scholar]
- 5.Gardner R, Grossman WI. Normal motor patterns in sleep in man. Adv Sleep Res. 1975;2:67–107. [Google Scholar]
- 6.Baldridge BJ, Whitman RM, Kramer M. The concurrence of fine muscle activity and rapid eye movements during sleep. Psychosom Med. 1965;27:19–26. doi: 10.1097/00006842-196501000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Zucconi M, Oldani A, Ferini-Strambi L, Bizzozero D, Smirne S. Nocturnal paroxysmal arousals with motor behaviors during sleep: frontal lobe epilepsy or parasomnia? J Clin Neurophysiol. 1997;14:513–22. doi: 10.1097/00004691-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22:1464–70. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 9.Manni R, Terzaghi M, Glorioso M. Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep. Sleep. 2009;32:241–5. doi: 10.1093/sleep/32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Högl B. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behaviour disorder. Sleep Med. 2009;10:174–81. doi: 10.1016/j.sleep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20:514–21. doi: 10.1111/j.1365-2869.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 12.Franceschini C, Ferri R, Pizza F, et al. Motor events during REM sleep in patients with narcolepsy-cataplexy: a video-polysomnographic pilot study. Sleep Med. 2011;12(Suppl 2):S59–63. doi: 10.1016/j.sleep.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep. 2014;37:431–8. doi: 10.5665/sleep.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behavior disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 16.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 18.Frauscher B, Gabelia D, Mitterling T, et al. Motor events during healthy sleep: a quantitative polysomnographic study. Sleep. 2014;37:763–73. doi: 10.5665/sleep.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 20.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Jankovic J, Fahn S. Dystonic disorders. In: Jankovic J, Tolosa E, editors. Parkinson's disease and movement disorders. 3rd ed. Baltimore: Lippincott Williams & Wilkins; 1998. pp. 513–51. [Google Scholar]
- 23.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35:835–47. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugaresi E. Some aspects of sleep in man. Proc Soc Med. 1972;65:173–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Wilde-Frenz J, Schulz H. Rate and distribution of body movements during sleep in humans. Percept Mot Skills. 1983;56:275–83. doi: 10.2466/pms.1983.56.1.275. [DOI] [PubMed] [Google Scholar]
- 27.Aaronson ST, Rashed S, Biber MP, Hobson JA. Brain state and body position. a time-lapse video study of sleep. Arch Gen Psychiatry. 1982;39:330–5. doi: 10.1001/archpsyc.1982.04290030062011. [DOI] [PubMed] [Google Scholar]
- 28.De Cock VC, Vidailhet M, Leu S, et al. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007;130:450–6. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 29.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 30.Peever J, Luppi PH, Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37:279–88. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–45. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals development origins of motor synergies. Curr Biol. 2013;23:2100–9. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with ‘unexpected’ sensory consequences. Curr Biol. 2014;24:2136–41. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. J Womens Health. 2009;18:1955–63. doi: 10.1089/jwh.2008.1348. [DOI] [PubMed] [Google Scholar]
- 35.Bjornara KA, Dietrichs E, Toft M. REM sleep behavior disorder in Parkinson's disease--is there a gender difference? Parkinsonism Relat Disord. 2013;19:120–2. doi: 10.1016/j.parkreldis.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 37.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12:284–8. doi: 10.1016/j.sleep.2010.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Classification method. The figure summarizes the classification method used in this study to classify individual movements.
Age-related distribution of movement indices.