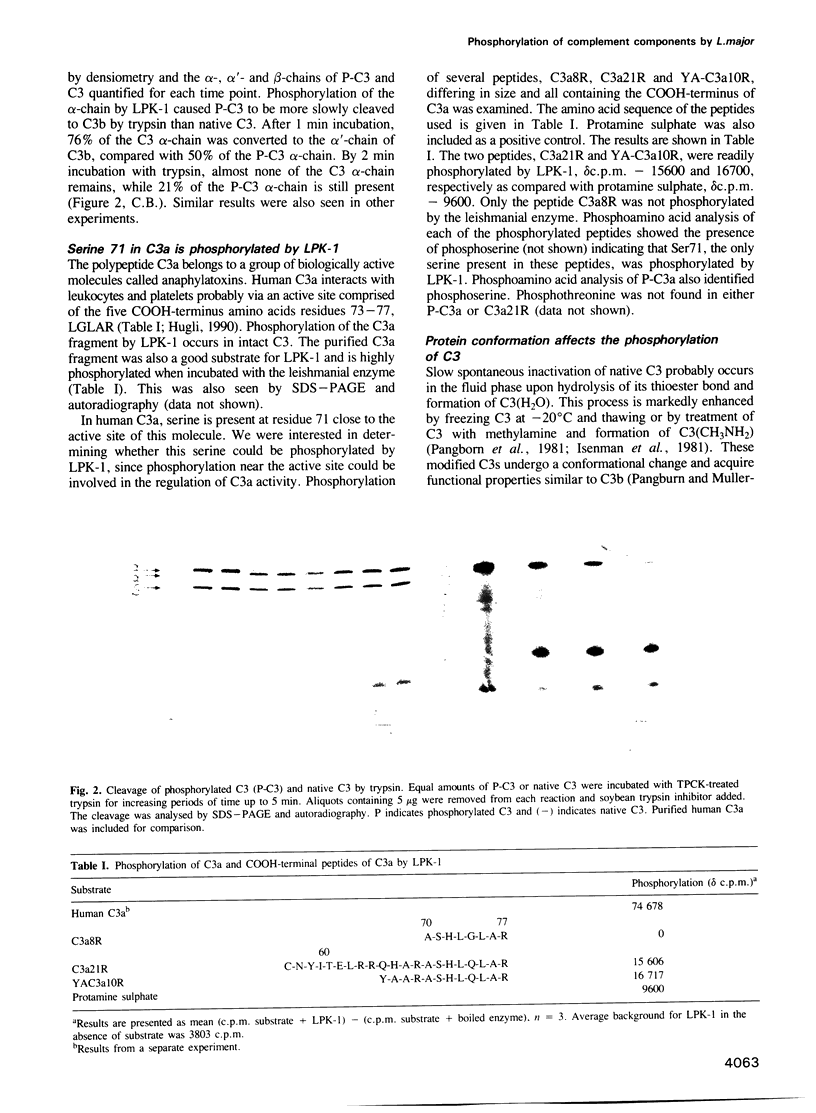
Abstract
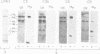
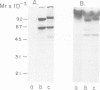
Externally oriented protein kinases are present on the plasma membrane of the human parasite, Leishmania. Since activation of complement plays an important role in the survival of these parasites, we examined the ability of protein kinases from Leishmania major to phosphorylate components of the human complement system. The leishmanial protein kinase-1 (LPK-1) isolated from promastigotes of L. major was able to phosphorylate purified human C3, C5 and C9. Only the alpha-chain of C3 and C5 was phosphorylated. The beta-chain appeared not to be a substrate for this enzyme. C3b which is formed by proteolytic cleavage of C3 was not phosphorylated by LPK-1. Trypsin treatment of phosphorylated C3 (P-C3) resulted in the disappearance of 32P from the alpha-chain. This was correlated with the conversion of the C3 alpha-chain to the alpha'-chain of C3b, and the appearance of a 9 kDa 32P fragment comigrating with the C3a fragment of C3. P-C3 was more resistant to cleavage by trypsin than nonphosphorylated C3. LPK-1 phosphorylated purified C3a and two synthetic peptides, C3a21R and YA-C3a10R, derived from its COOH-terminal end, which contain the C3a binding site to leukocytes and platelets. LPK-1 did not phosphorylate C3a8R. Phosphoamino acid analysis of the synthetic peptides indicated that serine 71 of C3a was phosphorylated by LPK-1. Treatment of C3 with either methylamine or freeze-thaw C3 (H2O) prevented phosphorylation by the LPK-1 suggesting that substrate conformation may be involved in recognition by the leishmanial enzyme.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970 Dec;49(12):2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri G., Chang K. P. Acid protease activity of a major surface membrane glycoprotein (gp63) from Leishmania mexicana promastigotes. Mol Biochem Parasitol. 1988 Jan 1;27(1):43–52. doi: 10.1016/0166-6851(88)90023-0. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Rowe J. G., Hugli T. E. A modified method for chemotaxis under agarose. J Immunol Methods. 1979;25(4):337–353. doi: 10.1016/0022-1759(79)90026-7. [DOI] [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- Das S., Saha A. K., Mukhopadhyay N. K., Glew R. H. A cyclic nucleotide-independent protein kinase in Leishmania donovani. Biochem J. 1986 Dec 15;240(3):641–649. doi: 10.1042/bj2400641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich Y. H., Garfield M. G., Davis T. B., Kornecki E., Chaffee J. E., Lenox R. H. Extracellular protein phosphorylation systems in the regulation of neuronal function. Prog Brain Res. 1986;69:197–208. doi: 10.1016/s0079-6123(08)61060-2. [DOI] [PubMed] [Google Scholar]
- Fishelson Z. Cell triggering by activated complement components. Immunol Lett. 1985;11(5-6):261–276. doi: 10.1016/0165-2478(85)90107-5. [DOI] [PubMed] [Google Scholar]
- Fishelson Z., Pangburn M. K., Müller-Eberhard H. J. Characterization of the initial C3 convertase of the alternative pathway of human complement. J Immunol. 1984 Mar;132(3):1430–1434. [PubMed] [Google Scholar]
- Forsberg P. O., Martin S. C., Nilsson B., Ekman P., Nilsson U. R., Engström L. In vitro phosphorylation of human complement factor C3 by protein kinase A and protein kinase C. Effects on the classical and alternative pathways. J Biol Chem. 1990 Feb 15;265(5):2941–2946. [PubMed] [Google Scholar]
- Fuhrman S. A., Joiner K. A. Complement evasion by protozoa. Exp Parasitol. 1989 May;68(4):474–481. doi: 10.1016/0014-4894(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Hall B. F., Joiner K. A. Strategies of obligate intracellular parasites for evading host defences. Immunol Today. 1991 Mar;12(3):A22–A27. doi: 10.1016/S0167-5699(05)80007-6. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of C3a anaphylatoxin. Curr Top Microbiol Immunol. 1990;153:181–208. doi: 10.1007/978-3-642-74977-3_10. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I., Cooper N. R., Müller-Eberhard H. J., Pangburn M. K. Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry. 1981 Jul 21;20(15):4458–4467. doi: 10.1021/bi00518a034. [DOI] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Kleine L. P., Whitfield J. F., Boynton A. L. Ca2+-dependent cell surface protein phosphorylation may be involved in the initiation of DNA synthesis. J Cell Physiol. 1986 Dec;129(3):303–309. doi: 10.1002/jcp.1041290306. [DOI] [PubMed] [Google Scholar]
- Kleine L. P., Whitfield J. F. Serum-activated T51B rat liver cells transiently accumulate cyclic AMP-dependent protein kinases on their surfaces during the G1 phase. J Cell Physiol. 1987 Aug;132(2):354–358. doi: 10.1002/jcp.1041320223. [DOI] [PubMed] [Google Scholar]
- Kweider M., Lemesre J. L., Darcy F., Kusnierz J. P., Capron A., Santoro F. Infectivity of Leishmania braziliensis promastigotes is dependent on the increasing expression of a 65,000-dalton surface antigen. J Immunol. 1987 Jan 1;138(1):299–305. [PubMed] [Google Scholar]
- Kübler D., Fehst M., Garcon T., Pyerin W., Burow E., Kinzel V. Substrates for the cell surface protein kinase in blood: a calf serum protein and its precursor in plasma. Biochem Int. 1987 Aug;15(2):349–357. [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Kinzel V. Protein kinase activity and substrates at the surface of intact HeLa cells. J Biol Chem. 1982 Jan 10;257(1):322–329. [PubMed] [Google Scholar]
- Laumas S., Abdel-Ghany M., Leister K., Resnick R., Kandrach A., Racker E. Decreased susceptibility of a 70-kDa protein to cathepsin L after phosphorylation by protein kinase C. Proc Natl Acad Sci U S A. 1989 May;86(9):3021–3025. doi: 10.1073/pnas.86.9.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D. S., Hermoso T., Jaffe C. L. Extracellular phosphorylation in the parasite, Leishmania major. Biochim Biophys Acta. 1990 May 2;1052(2):293–298. doi: 10.1016/0167-4889(90)90224-2. [DOI] [PubMed] [Google Scholar]
- Lin M. F., Lee P. L., Clinton G. M. Characterization of tyrosyl kinase activity in human serum. J Biol Chem. 1985 Feb 10;260(3):1582–1587. [PubMed] [Google Scholar]
- Martin S. C. Phosphorylation of complement factor C3 in vivo. Biochem J. 1989 Aug 1;261(3):1051–1054. doi: 10.1042/bj2611051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Burke S. K., Coutavas E. E., Wedgwood J. F., Edelson P. J. Leishmania species: mechanisms of complement activation by five strains of promastigotes. Exp Parasitol. 1986 Dec;62(3):394–404. doi: 10.1016/0014-4894(86)90048-2. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N. K., Saha A. K., Lovelace J. K., Da Silva R., Sacks D. L., Glew R. H. Comparison of the protein kinase and acid phosphatase activities of five species of Leishmania. J Protozool. 1988 Nov;35(4):601–607. doi: 10.1111/j.1550-7408.1988.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes S. M., Da Silva R. P., Sacks D. L., Hammer C. H., Joiner K. A. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol. 1990 Dec 15;145(12):4311–4316. [PubMed] [Google Scholar]
- Puentes S. M., Dwyer D. M., Bates P. A., Joiner K. A. Binding and release of C3 from Leishmania donovani promastigotes during incubation in normal human serum. J Immunol. 1989 Dec 1;143(11):3743–3749. [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter Y., Fishelson Z. Targeting of complement to tumor cells by heteroconjugates composed of antibodies and of the complement component C3b. J Immunol. 1989 Apr 15;142(8):2771–2777. [PubMed] [Google Scholar]
- Sacks D. L., Brodin T. N., Turco S. J. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):225–233. doi: 10.1016/0166-6851(90)90165-i. [DOI] [PubMed] [Google Scholar]
- Sacks D. L. Metacyclogenesis in Leishmania promastigotes. Exp Parasitol. 1989 Jul;69(1):100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Kocher H. P., Luzio J. P., Jackson P., Tschopp J. The sequence and topology of human complement component C9. EMBO J. 1985 Feb;4(2):375–382. doi: 10.1002/j.1460-2075.1985.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Lemons R. S., Le Beau M. M., Barnum S. R., Noack D., Tack B. F. Molecular analysis of human complement component C5: localization of the structural gene to chromosome 9. Biochemistry. 1988 Mar 8;27(5):1474–1482. doi: 10.1021/bi00405a012. [DOI] [PubMed] [Google Scholar]