Summary
Hepatocellular death is present in almost all types of human liver disease and is used as a sensitive parameter for the detection of acute and chronic liver disease of viral, toxic, metabolic, or autoimmune origin. Clinical data and animal models suggest that hepatocyte death is the key trigger of liver disease progression, manifested by the subsequent development of inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma. Modes of hepatocellular death differ substantially between liver diseases. Different modes of cell death such as apoptosis, necrosis, and necroptosis trigger specific cell death responses and promote progression of liver disease through distinct mechanisms. In this review, we first discuss molecular mechanisms by which different modes of cell death, damage-associated molecular patterns, and specific cell death responses contribute to the development of liver disease. We then review the clinical relevance of cell death, focusing on biomarkers; the contribution of cell death to drug-induced, viral, and fatty liver disease and liver cancer; and evidence for cell death pathways as therapeutic targets.
I. Introduction
The presence of hepatocyte death, reflected by increased levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), is the most widely used parameter to screen for and monitor patients with liver disease. Moreover, these markers drive therapeutic decisions; have prognostic value for patients with hepatitis B virus (HBV)1, 2, 3 and 4 and hepatitis C virus (HCV)5, 6, 7 and 8 infections, nonalcoholic steatohepatitis (NASH),9, 10 and 11 and autoimmune hepatitis12; and correlate with overall and liver-specific mortality in the general population.13, 14 and 15 These well-established facts emphasize the importance of cell death as the ultimate driver of liver disease progression and the development of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).
In the healthy liver, cell death controls organ homeostasis, with a tight equilibrium between the loss and replacement of hepatocytes.16 Turnover is low in the normal liver, with approximately 0.05% of hepatocytes at any given time being removed by apoptosis, mostly in zone 3.17 and 18 This is reflected by almost undetectable ALT levels in healthy subjects. Despite the fact that most hepatic cell types rest in G0 phase, the liver is endowed with an astounding ability to regenerate in response to massive hepatocellular death or loss of functional liver mass.19 This regenerative ability not only reflects essential metabolic functions of the liver but is also directly related to its high vulnerability to insults causing massive hepatic cell death, such as food-derived toxins or infections with hepatotropic viruses, bacteria, and parasites. As such, the wide range of metabolic and detoxifying functions predisposes hepatocytes to xenobiotic- and toxin-induced injury. Rapid regeneration represents an efficient mechanism to avoid the loss of key hepatic functions in this setting. Although acute liver failure caused by foodborne poisons and infections may have posed the biggest threat in former times, the bulk of modern liver diseases result from chronic disease processes such as chronic viral hepatitis, nonalcoholic fatty liver disease (NAFLD), and alcoholic liver disease (ALD). In these settings, the hepatic response to cell death, which is primarily geared toward restoring hepatic architecture and function in response to an acute threat to life (by providing extracellular matrix for mechanical stability and triggering hepatocyte regeneration to restore functional liver mass), becomes maladaptive and promotes the development of tissue fibrosis, cirrhosis, and HCC. The contribution of cell death to liver disease is cell-, stage- and context-specific. Although increased cell death may be a key driver of many chronic disease processes, including fibrogenesis and hepatocarcinogenesis (Table 1), loss or malfunction of programmed cell death (PCD) induction in subsets of epithelial cells contributes to the malignant transformation and constitutes a hallmark of cancer.20 Likewise, whereas increased cell death in hepatocytes contributes to fibrogenesis, cell death in fibrogenic cells is an important mechanism for resolution of liver fibrosis.21 Our review focuses on cell death, but it is also likely that cellular injury (not full-blown cell death) triggers stress responses that contribute to disease development. However, these aspects will not be covered in this review.
Table 1.
Evidence from animal model for cell death as a driver of liver disease
| Experimental evidence | Mode of cell death that promotes disease | References | |||
|---|---|---|---|---|---|
| Apoptosis | Necroptosis | Necrosis | |||
| Acetaminophen | Rip3 knockout protects from early liver injury | × | 40 | ||
| CsA inhibits acetaminophen hepatocyte toxicity in vitro and liver injury in vivo | × | 46,36 | |||
| Hepatic I/R injury | Cyclophilin D knockout inhibits necrotic cell death in hepatocytes and cardiac I/R injury | × | 33 | ||
| Fibrosis | Spontaneous fibrosis in Mcl1 hepatocyte ko mice | × | 54 | ||
| Bclxl hepatocyte knockout develops fibrosis | × | 55 | |||
| Necrotic injury models (CCI4, APAP) result in fibrosis | × | Common models in the literature | |||
| Tak1- and Nemo-hepatocyte-specific ko mice develop spontaneous liver fibrosis | × | 90, 152, 167 | |||
| Caspase inhibitor IDN-6556 inhibits fibrosis after bile duct ligation | × | 144 | |||
| NASH | Decreased inflammation and fibrosis in mice with ablation of RIP3 after MCD diet | × | 146 | ||
| Caspase inhibitor VX-166 inhibits inflammation and fibrosis in the MCD model | × | 145, 204 | |||
| ALD | Reduced steatosis, injury and inflammation in Rip3-deficient mice | × | 41 | ||
| HCC | Spontaneous HCC in Mcl1 hepatocyte ko mice | × | 56 | ||
| Spontaneous HCC in mice with Mcl1 or Bclxl hepatocyte ko, and inhibition of hepatocarcinogenesis by additional Bak ko | × | 150 | |||
| Spontaneous HCC development in mice with hepatocyte-specific Nemo or Tak1 knockout | × | 90, 152, 167 | |||
| Reduced HCC development by Caspase 8 ablation in Tak1 hepatocyte-specific ko mice, increase HCC development by Rip3 ko in Tak1 hepatocyte-specific ko mice | × | 105 | |||
In view of the fundamental role of cell death in virtually all hepatic diseases, precise knowledge of mechanisms regulating cell death and cell death responses is essential to understand the pathophysiology of liver disease and develop new therapeutic approaches.
II. Regulation of Cell Death in the Liver
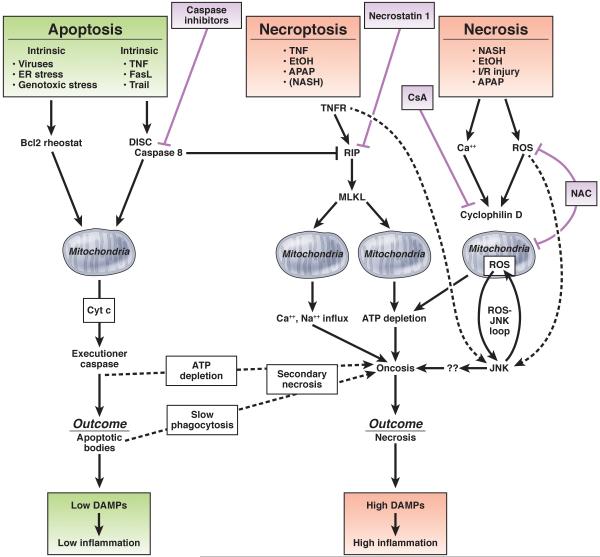
Cell death occurs not only as a passive response to physicochemical stress or noxious insults but may also be actively induced by the host via PCD. PCD plays an active role in development and organismal homeostasis.22 Accordingly, inhibition of PCD by genetic ablation of key cell death regulators leads to hepatic hyperplasia.23 Moreover, PCD is directly involved in the defense against pathogens, including hepatotropic viruses,24 and represents a key mechanism preventing malignant transformation.20 Traditionally, 2 distinct forms of cell death have been recognized: apoptosis as the mediator of PCD, actively induced by specific signaling cascades and occurring in a highly controlled fashion, and necrosis as the accidental form of cell death.25 and 26 However, recent evidence indicates that PCD can also trigger a specific form of necrosis, termed necroptosis.27 The regulated nature of multiple cell death modes not only affects our understanding of the underlying pathophysiology but also suggests the possibility of therapeutically interfering with regulatory mechanisms in diseases in which cell death was classically considered to be nontargetable (Figure 1).
Figure 1.
Necrosis
Necrosis is viewed as a largely unregulated consequence of physicochemical stress, characterized by mitochondrial impairment, depletion of adenosine triphosphate (ATP), and subsequent failure of ATP-dependent ion pumps. This results in rapid swelling of cells and cell organelles (“oncosis”) accompanied by the formation of membrane “blebs” and ultimately cellular rupture.25, 28 and 29 As a consequence, cellular constituents spill into the extracellular environment and elicit significant inflammatory responses, rendering necrosis an “immunogenic” form of cell death29 and 30 (Figure 1). Recent studies have highlighted that necrosis also contains regulated elements involving mitochondrial events. As such, the mitochondrial permeability transition (MPT) leads to the opening of a mitochondrial pore, triggering mitochondrial swelling and uncoupling of oxidative phosphorylation as a result of osmotic forces.31 The relevance of pathways regulating necrosis (“regulated necrosis”) is shown by the ability of drugs that target cyclophilin, a key contributor to the MPT,32, 33 and 34 or drugs targeting mediators downstream of mitochondria such as c-Jun N-terminal kinase (JNK) to inhibit hepatocyte necrosis.35, 36, 37 and 38 Furthermore, lack of ATP may convert apoptotic death into secondary necrosis (also sometimes referred to as “necraptosis” or “aponecrosis,” to be distinguished from “necroptosis”39). Accordingly, multiple forms of cell death, including necrosis, apoptosis, and necroptosis, commonly exist, most likely side by side, in relevant liver diseases such as ALD and NAFLD.40, 41, 42, 43 and 44 The therapeutic relevance of this finding has been highlighted by recent studies in renal ischemia-reperfusion in which combinations of regulated necrosis and necroptosis inhibitors exerted additive effects.45 Diseases with cell death that used to be considered largely a consequence of unregulated necrosis, such as acetaminophen-induced liver injury and ischemia-reperfusion injury, can be modulated by MPT inhibitor cyclosporin A or JNK inhibitors,35, 36, 46 and 47 suggesting an important role of regulated necrosis in these settings. With increasing data on pathways regulating hepatic necrosis, the question arises to what extent pure necrosis contributes to liver disease.
Apoptosis
In contrast to necrosis, apoptosis is a highly controlled biochemical process in which the cell and many of its components are virtually “chopped” into pieces,48 mediated by the concerted action of aspartate-specific proteases, known as caspases49 (Figure 1). Accordingly, apoptosis is morphologically distinct from necrosis, with characteristic features such as cellular shrinkage, nuclear condensation, and fragmentation. Apoptosis is a common feature of viral, cholestatic, fatty, and alcoholic liver disease.44, 50, 51, 52 and 53 In viral hepatitis, apoptosis is readily seen in liver sections by the presence of characteristic Councilman bodies. Apoptosis is considered noninflammatory or low inflammatory due to the rapid removal of apoptotic cells, triggered “eat-me” signals such as phosphatidyl serine expressed on apoptotic bodies, which promotes engulfment by phagocytotic cells and thereby prevents leakage of cellular contents.54 Whereas low-grade leakage of specific proinflammatory mediators from apoptotic cells, including chemokines, ATP, uridine triphosphate, and sphingosine-1-phosphate mediators,55, 56 and 57 may be required for efficient detection of apoptotic cells by phagocytes (“find me” signals), activated caspases may also process intracellular contents in a way that renders them less inflammatory.58 Accordingly, patients with chronic HCV infection, a disease in which apoptosis is the dominant form of cell death,51 and 52 can display normal or minimally elevated serum ALT levels despite ongoing hepatitis.59 and 60 On the other hand, serum ALT levels can increase with higher numbers of apoptotic cells, supporting the hypothesis that either apoptotic hepatocytes leak some of their intracellular contents, possibly at higher levels when engulfment of apoptotic hepatocytes is saturated or not rapid enough, or some hepatocytes do not die from pure apoptosis. Thus, hepatocellular apoptosis may not be as inert as often assumed and might contribute to hepatic inflammation, fibrosis, and HCC (discussed in the following text).
Depending on whether the triggering event is cell intrinsic or cell extrinsic, apoptosis is distinguished into mechanistically largely separate intrinsic and extrinsic pathways (Figure 1). The intrinsic pathway commonly triggers apoptosis via members of the B-cell lymphoma 2 (Bcl-2) family, which control mitochondrial outer membrane permeabilization, cytochrome c release, and subsequently caspase activation. 61, 62, 63 and 64 Several intracellular triggers of apoptosis activate this pathway, including endoplasmic reticulum (ER) stress and p53 activation. ER stress is typically the result of accumulating unfolded or misfolded proteins in the ER, leading to the so-called unfolded protein response. Whereas mild ER stress is cytoprotective, profound or prolonged ER stress promotes cell death by activation of JNK and CHOP. 65 As such, increased ER stress induces cell death and disease progression in α1-antitrypsin deficiency. 66 Viral infections also commonly induce ER stress, and HCV infection triggers ER stress in culture and in infected patients. 67, 68 and 69 Exposure of hepatocytes to free fatty acids induces ER stress and an intrinsic cell death pathway termed lipoapoptosis in vitro and in vivo, 70 but it appears that lipoapoptosis is predominantly mediated by JNK activation in an ER stress–independent manner. 34, 71 and 72 p53 is another important regulator of the intrinsic death pathway and the central component of a continuously operative cell fate program that determines whether a cell should initiate DNA damage repair or die by apoptosis. In response to oncogene activation, DNA damage, and senescence, p53 becomes activated and, through mostly transcriptional regulation of specific target genes such as Bax, induces apoptosis. When damage is less severe, p53 induces cell cycle arrest through targets such as p21, allowing for cellular repair. Hence, p53 functions as the “guardian of the genome,” preventing malignant transformation of hepatocytes. Accordingly, HCCs commonly escape from this control mechanism by acquiring p53 mutations. 73
The extrinsic cell death pathway is typically triggered by members of the tumor necrosis factor (TNF) family of death receptor ligands, comprising TNF itself, Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL).74 Hepatocytes express high levels of death receptors, possibly allowing efficient eradication of hepatocytes infected by hepatotropic viruses.75 Death receptor–mediated apoptosis is a key feature of many types of liver diseases.75 The high susceptibility of hepatocytes to death receptor–induced cell death is highlighted by the fact that Fas agonistic antibodies trigger rapid apoptosis in murine and human hepatocytes as well as acute liver failure and death in mice.76 and 77 In the liver, the extrinsic and intrinsic pathways are linked, because hepatocytes as “type 2 cells” require mitochondrial amplification via cytochrome c release-mediated activation of caspase-3 for cell death execution 78 (Figure 2).
Figure 2.
TNF- and Fas ligand–induced cell death share many components of signal transduction such as formation of a death-inducing signaling complex after receptor oligomerization-induced recruitment of caspase-8, resulting in the activation of executioner caspases (reviewed in Muppidi et al,74 Wajant,79 and Reinehr and Haussinger80) (Figure 2). However, whereas the outcome of Fas activation is hepatocyte death, TNF receptor activation affects multiple cellular responses that besides cell death also include survival, inflammation, and proliferation.81 Transcription factor nuclear factor κB (NF-κB) represents a key cytoprotective pathway that up-regulates antiapoptotic genes such as Bcl-xl and c-FLIP and blocks prolonged activation of JNK,82 a key pathway through which TNF induces cell death. Prolonged JNK activation is inhibited by NF-κB–dependent up-regulation of antioxidant proteins such as ferritin and SOD2. Inhibition of NF-κB unmasks a TNF-induced self-amplifying pathway of mitochondrial oxidative stress that is mediated through the interaction of activated JNK and a protein in the outer membrane of mitochondria known as Sab (SH3BP5). This pathway sustains JNK activation, which then leads to degradation of cFLIP, inhibition of antiapoptotic members of the Bcl2 family, and activation of proapoptotic Bcl2 family members (Figure 2).83 Accordingly, TNF-mediated cell death requires inhibition of NF-κB or NF-κB target genes84 and 85 and can be blocked by JNK inhibition.82 and 86 JNK also promotes protective responses such as hepatocyte proliferation and liver regeneration.87
The dichotomous nature of TNF signaling and the dominance of antiapoptotic and proinflammatory signals over cell death induction have been shown in multiple disease models. Injection of TNF or lipopolysaccharide alone does not cause significant liver injury. However, blockage of NF-κB, through conditional deletion of Nemo, Tak1, NF-κB target genes like the caspase-8 inhibitor c-Flip, or both Ikkα and Ikkβ in hepatocytes or hepatic inhibition of transcription with D-galactosamine, sensitizes hepatocytes to TNF-induced apoptosis and liver failure. 88, 89, 90, 91 and 92
Necroptosis
The concept of apoptosis and its prevention by NF-κB seemed to sufficiently explain how TNF mediates cell death in the liver. However, this concept was recently challenged by a paradigmatic shift in our understanding of PCD based on evidence for a third form of cell death, necroptosis, which incorporates features of necrosis and apoptosis. Necroptosis uses the same upstream molecular machinery as apoptosis93 (Figure 2), supporting the hypothesis that it functions as a backup pathway to enable cell death in settings where apoptosis is inhibited (eg, by viruses expressing antiapoptotic genes).94 Despite sharing upstream mediators with apoptosis, the final outcome of necroptosis is cellular leakage as a result of organelle and cellular swelling (Figure 1). Necroptosis is best characterized in the setting of TNF-induced cell death, which has high relevance for many types of liver diseases but may also occur in other conditions, including ischemia-reperfusion injury.27
The decision whether death receptor activation induces apoptosis or necroptosis depends on 2 kinases: receptor-interacting protein (RIP) 1 and RIP3.95, 96, 97 and 98 Activation of caspase-8 shifts the balance toward apoptosis by cleaving RIP1 and RIP3, while inhibition of caspase-8 leads to assembly of RIP1/RIP3 complexes, forming the “necrosome,” a key transducer of the necroptotic signal.27 and 99 The exact mechanism of how necrosome activation executes necroptosis remains a matter of debate. Mixed lineage kinase domain-like protein (MLKL) is a key mediator of necroptosis. It has been suggested that MLKL increases mitochondrial reactive oxygen species (ROS) production through mitochondrial targets.100 Recent studies have shown that MLKL triggers cytotoxic influx of either calcium or sodium ions and that this requires MLKL to translocate to the plasma membrane.101 and 102
Methods for visualizing necroptosis, allowing determination of its role in human disease, are only starting to be developed, with phosphorylated MLKL representing a potential marker for necroptosis in both animal models and patients with drug-induced liver injury (DILI).103 So far, most data on the contribution of necroptosis to liver diseases and mechanistic insights are derived from murine studies. Interestingly, RIP3 is only weakly expressed in healthy murine livers compared with other organs such as lung or spleen104 but shows up-regulated protein levels in cells that are sensitized to undergoing necroptosis (eg, by ablation of caspase-8).105 Infection of mice with vaccinia virus induced assembly of RIP1/RIP3 complexes in the liver, suggesting necroptosis as a component of antiviral responses.95 RIP3-deficient mice show decreased hepatocyte death after acetaminophen intoxication and long-term ethanol feeding, suggesting an involvement of necroptosis in these diseases.40 and 41 Although inhibition of necroptosis by RIP3 deficiency, RIP1 blockade by necrostatin-1, or MLKL deficiency all reduce cell death at early time points,40 and 106 RIP3 deficiency was unable to abrogate acetaminophen-induced liver injury at 24 hours.40 Hence, additional pathways may contribute to cell death or compensate for the loss of RIP3. Finally, studies in a model of mice with liver-specific Tak1 deletion, a model of chronic liver injury, showed opposing roles of RIP3-mediated necroptosis and caspase-8–mediated apoptosis. In this model, necroptosis in mice with combined ablation of TAK1 and caspase-8 was associated with low proliferation of hepatocytes and cholangiocytes, leading to reduced hepatocarcinogenesis and development of cholestasis.105 Conversely, apoptosis was associated with high hepatocyte proliferation and hepatocarcinogenesis but absent cholestasis.105 The mutual relationship between apoptosis and necroptosis in hepatocytes, similar to findings in other organs,107 and 108 is further highlighted by RIP3-mediated spontaneous liver injury in mice with caspase-8 deletion in parenchymal liver cells. 105
Autophagy and Cell Death
Autophagy is the catabolic degradation of cellular components through the lysosome, involving different types of “cargo,” and serves as both a removal mechanism for dysfunctional cell content and an energy source.109 Autophagy is considered a predominantly cytoprotective pathway that protects from ALD,110 TNF-induced liver injury,111 acetaminophen-induced liver injury,112 ischemia-reperfusion injury,113 and high-fat diet-induced lipid accumulation.114 In specific circumstances, autophagy may be associated with cell death,109 but cell death–promoting functions still need to be better understood, particularly in the context of liver disease.113
III. Specific Considerations of Cell Death Regulation in the Liver
Cholestasis and Bile Acid–Induced Cell Death
Cholestasis is a common feature of acute and chronic liver disease, and accumulation of toxic bile acids contributes to hepatocyte death in this setting. Bile acid–induced cell death in cultured cells is largely apoptotic and mediated by ligand-independent activation of death receptors, including the Fas receptor.50 and 115 Cholestatic liver disease also often triggers necrotic cell death, reflected by the appearance of “bile infarcts” after bile duct ligation or in Mdr2ko mice, as well as in cholestatic liver disease in patients. However, at present it is not clear whether this is induced by bile acids or mediated by other mechanisms.
Cell Death in Non-hepatocyte Populations
Cell death in non-hepatocyte populations is also an essential feature of chronic liver disease. Cell death of cholangiocytes is not as well characterized as hepatocyte death, both in terms of mechanisms and its contribution to liver disease. TNF receptor family-mediated apoptosis, mediated by activation of Fas, TRAIL receptor 2, or CD40, appears to be the most common form of cholangiocyte death.116, 117, 118, 119 and 120 Cholangiocyte apoptosis occurs in immune-mediated and drug-induced cholangiopathies120 and may contribute to disease progression and ductopenia.117 In contrast to hepatocyte death as a common trigger of wound healing and fibrosis, it is believed that cholangiocyte proliferation rather than cholangiocyte death promotes disease, for example, by contributing to the development of peribiliary fibrosis seen in cholangiopathies.120 Hepatic stellate cell (HSC) death is a mechanism that achieves removal of activated myofibroblasts and resolution of hepatic fibrosis21 and therefore is generally considered beneficial in chronic liver disease. Death of liver sinusoidal endothelial cells occurs after ischemia-reperfusion injury (eg, after liver transplantation), but its relative contribution to organ dysfunction remains controversial.121 and 122 Likewise, the regulation and contribution of macrophage death in the liver is not well understood. Finally, the liver also serves as the “graveyard” of specific immune cell subsets such as activated CD8+ T cells,123 which may contribute to the high immune tolerance of the liver.
IV. Response Pathways to Cell Death
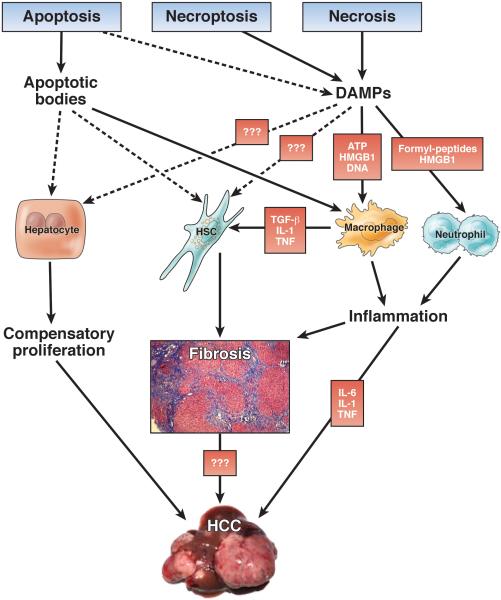
Although massive liver cell death can impair liver function in acute or acute-on-chronic liver disease, in most chronic liver diseases only a small percentage of hepatocytes die at the same time. In this setting, cell death has no direct significant impact on liver function. Instead, hepatic responses to cell death, often persisting over decades, dictate the development of long-term consequences and clinical outcomes (Figure 3).
Figure 3.
Damage-Associated Molecular Patterns as a Link Between Cell Death and Inflammation in Chronic Liver Disease
Pattern recognition is a cornerstone of innate immunity.124 Matzinger suggested that abnormal molecular patterns play a role not only in pathogen recognition by the host but also in the recognition of “danger” associated with tissue injury.125 Damage-associated molecular patterns (DAMPs), released from dying cells, are believed to trigger sterile inflammation occurring after tissue injury.125, 126 and 127 DAMP release is believed to occur mainly after necrosis and necroptosis due to the loss of membrane integrity, thus explaining the inflammatory nature of these cell death modes.30, 126, 127, 128 and 129 Some DAMPs such as high-mobility group box 1 (HMGB1) may even be actively retained during apoptosis.129 In the liver, several DAMPs and DAMP receptors, including HMGB1 (via the receptor for advanced glycation end products or Toll-like receptor 4), formyl peptides (via FPR1), or ATP (via P2X7), trigger inflammatory cell recruitment130, 131 and 132 and contribute to inflammation and exacerbation of injury in acute liver diseases such as hepatic ischemia-reperfusion injury130 and acetaminophen intoxication.133 and 134 The contribution of DAMP-induced sterile inflammation to chronic disease processes is much less understood. A recent study reported that RAGE, one of the receptors for HMGB1, is required for oval cell proliferation and hepatocarcinogenesis, suggesting that this receptor might provide a mechanistic link between DAMPs and hepatocarcinogenesis in chronic liver disease.135 Given that DAMPs represent a novel and potentially highly relevant class of therapeutic targets which may be involved in driving many of the complications of chronic liver injury, further studies are needed to examine if low-grade release of DAMPs from apoptotic cells may contribute to inflammation in relevant chronic liver diseases such as viral hepatitis, NAFLD, and ALD. Moreover, secretion of mediators from stressed cells may additionally mediate hepatic responses to injury. As such, IL-33 is released from stressed hepatocytes to promote fibrogenesis and bile duct proliferation via innate lymphoid cells.136 and 137
Hepatic Fibrogenesis in Response to Cell Death
Fibrosis is one of the clinically most relevant consequences of chronic liver disease. Development of fibrosis is seen in all animal models with significant hepatocyte injury and correlates with elevated ALT levels in various liver diseases1, 7, 8 and 138 as well as elevated levels of cleaved keratin 18 (K18), a marker of caspase activation.139 HSCs are the dominant contributors to fibrosis in the liver,140 but the molecular links between hepatocyte death and HSC activation remain poorly understood. Although the hypothesis that DAMPs provide a direct link between hepatocyte death and HSC activation is attractive, it has not been rigorously tested and there is no convincing evidence for DAMPs that directly activate HSCs. Alternatively, DAMPs could act on other cell types that in turn release fibrogenic mediators such as transforming growth factor β or platelet-derived growth factor to activate HSCs. Among these, hepatic macrophages are best known to interact with HSCs and to promote HSC fibrogenesis.141, 142 and 143 Moreover, it needs to be considered that cell death in many noncholestatic liver diseases (eg, viral hepatitis) is predominantly apoptotic and that DAMP release may be low in these conditions. There is ample evidence that apoptosis may trigger fibrogenesis. Mouse models with selective increases of hepatocyte apoptosis by hepatocyte-specific deletion of Nemo, Mcl-1, or Bcl-xl develop liver fibrosis,62, 63 and 90 thus providing a link between apoptosis and fibrogenesis. Whether this is pure apoptosis or also involves necrotic cell death remains uncertain because these models are typically accompanied by elevated ALT levels. Whereas the pan-caspase inhibitor IDN-6556 reduced the development of bile duct ligation–induced liver fibrosis,144 the pan-caspase inhibitor VX166 exerted only little effect on NASH-induced liver fibrosis despite improved injury and inflammation.145 In contrast, we recently showed that inhibition of necroptosis through ablation of RIP3 protects mice from NASH-induced liver fibrosis,146 highlighting the hypothesis that necroptosis rather than apoptosis controls liver fibrosis on metabolic injury. The mechanistic link between apoptosis or necroptosis and HSC activation is not fully understood. Several studies have proposed that phagocytosis of apoptotic bodies by HSCs links cell death to HSC activation, with several in vitro studies showing increased HSC activation, migration, and survival after phagocytosis of apoptotic bodies.147, 148 and 149 Phagocytosis of apoptotic hepatocytes by hepatic macrophages results in increased secretion of profibrogenic mediators, suggesting that apoptosis may promote HSC activation and fibrosis through indirect mechanisms involving professional phagocytic cells.150
Cell Death and Compensatory Proliferation
Partial hepatectomy is classically used to investigate liver regeneration and model regeneration after surgery in patients. In contrast, regeneration in human disease may be direct response to acute hepatocyte death (eg, after acetaminophen intoxication) or persistent cell death in chronic liver diseases rather than a direct consequence of loss of anatomical or functional liver mass. Hence, it is not clear whether compensatory regeneration after cytotoxic loss of hepatocytes differs mechanistically from liver regeneration after partial hepatectomy. The relevance of compensatory proliferation for hepatocarcinogenesis is well established across animal models.105 and 151 In particular, the aforementioned Tak1 and Nemo liver-specific knockout mice show massive compensatory proliferation, which is believed to contribute to the spontaneous development of HCC.90 and 152 In both models, apoptosis appears to be the driving force triggering proliferation,105 but it is presently unclear which mediators and cell types mechanistically link these processes.
Cell Death and Hepatocarcinogenesis
Hepatocarcinogenesis is tightly linked to cell death. However, one needs to carefully distinguish between cell death occurring in nontransformed hepatocytes and cell death occurring in transformed hepatocytes because these have opposite functional consequences. Cell death in nontransformed hepatocytes represents a tumor-promoting mechanism, mediated by increased compensatory regeneration, fibrogenesis, and inflammation. The tumor-promoting effects of hepatocyte apoptosis have been clearly shown by hepatocyte-specific deletion of the antiapoptotic proteins Mcl-1 or Bcl-xl, which not only increased the rate of hepatocyte apoptosis62, 63 and 64 but also resulted in spontaneous development of HCC.64 and 153 In the Bcl-xl liver knockout model, hepatocarcinogenesis could be suppressed by additional knockout of Bak, thus providing a direct link between hepatocyte apoptosis and HCC.153 Similar evidence comes from models in which NF-κB inhibition through conditional deletion of Nemo in hepatocytes leads to massively increased cell death and spontaneous development of HCC. 151 Increasing carcinogen-induced apoptosis and compensatory proliferation through hepatocyte-specific IKKβ deletion promotes formation of HCC, 151 whereas decreasing it through knockout of Bcl-2 family member p53 up-regulated modulator of apoptosis (PUMA) decreases it. 154 The key role of apoptosis is highlighted by the fact that development of HCC in these latter models can be prevented by codeletion of the proapoptotic genes Fadd 90 or caspase-8. 105 and 155 Likewise, antibody-mediated neutralization of Fas ligand prevented not only hepatocyte apoptosis but also hepatocarcinogenesis in a transgenic hepatitis B surface antigen HCC mouse model. 156
In contrast to the tumor-promoting effect of cell death in non-transformed hepatocytes, cell death in transformed hepatocytes limits development of HCC. Accordingly, tumor cells often undergo a selection process that allows them to successfully evade apoptosis, such as mutations of p53.73 and 157 Down-regulation of proapoptotic Bax and Bcl-XS30 and 158 and up-regulation of antiapoptotic proteins such as Bcl-XL, Mcl-1, survivin, and X-linked inhibitor of apoptosis protein (XIAP) endows transformed hepatocytes with increased survival properties.27, 40, 41, 159, 160 and 161 Development of genetic resistance to cell death is favored in settings of chronic cell death and high cell turnover by selection of clones that are fittest to survive and proliferate. In addition to the preciously described mechanisms, infection with hepatotropic viruses may also lead to increased resistance to apoptosis.162 Additional survival signals may derive from cell-extrinsic sources, such as the altered hepatic microenvironment,163 or a leaky gut that increases Toll-like receptor signaling in the liver.164 NF-κB represents a key antiapoptotic pathway that promotes survival of transformed hepatocytes,165 as shown by reduced development of cancer in Mdr2-knockout mice in which NF-κB is inhibited by expression of an IκB superrepressor.166
Distinct modes of cell death differentially affect carcinogenesis in the liver. Despite the widely held belief that apoptosis represents an “unreactive” form of cell death, aforementioned evidence from mouse models with increased hepatocyte apoptosis64, 90, 152, 153 and 167 and interventions that reduce apoptosis105, 153, 155 and 156 strongly suggest that apoptosis is the primary driver of HCC development. Accordingly, co-deletion experiments in TAK1-knockout mice showed that apoptosis but not necroptosis promoted carcinogenesis in this model.105 Further studies are required to confirm these findings in additional hepatocarcinogenesis models. We currently do not understand how this classically “areactive” form of cell death promotes cancer. It is possible that the engulfment of apoptotic bodies triggers HCC-promoting compensatory proliferation or fibrosis more efficiently than necrosis or that secondary necrosis associated with apoptosis promotes HCC. Alternatively, it could be envisioned that proapoptotic executioner enzymes, including DNAses, induce collateral damage in neighboring cells or that some cells survive the “apoptotic attack” but show genetic alterations that ultimately lead to cancer.
V. Biomarkers of Cell Death
ALT and AST
Serum ALT is the most common and best-established biomarker for diagnosis and monitoring of acute and chronic liver disease168 (Table 2). As a reflection of its nearly hepatocyte-specific expression, serum ALT levels in the general population are associated with overall and liver-specific mortality13, 14 and 15 but not with mortality from other causes.15 Elevations of other markers of liver disease, such as γ-glutamyl transpeptidase and AST, are associated with overall mortality but do not specifically reflect liver-related mortality.13 and 15 Elevated ALT levels also correlate with clinical progression to fibrosis and cirrhosis in patients with HBV infection,1, 2, 3 and 4 HCV infection,5, 6, 7 and 8 and NASH138 (Table 2). Whereas ALT seems to be the best-established marker for predicting disease progression in chronic liver disease, there appear to be more sensitive markers that outperform ALT in acute liver disease, such as microRNA (miR)-122, HMGB1, and K18. In contrast to ALT, AST is expressed in a wider range of tissues, including cardiac and skeletal muscle, kidney, and blood.168 Hence, elevated serum AST levels are less specific than elevated serum ALT levels. Higher AST levels and in particular an AST/ALT ratio >2 are indicative but not specific to severe ALD.169 However, AST has only low sensitivity for detecting alcohol intake and ALD.170 An increased AST/ALT ratio is also associated with increased risk of development of fibrosis in NASH.9, 10 and 11
Table 2.
Biomarkers of cell death and their prognostic value for acute and chronic liver disease
| Biomarker | Cell type, cellular localization and function | Relevant diseases | Key findings | References |
|---|---|---|---|---|
| ALT | Almost hepatocyte-specific; cytoplasmic enzyme that catalyzes the transfer of amino groups | All liver diseases | Association with overall and liver-related mortality | 13 – 15 |
| Hepatitis B | Association with progression to fibrosis, HCC and mortality | 1 – 4 | ||
| Hepatitis C | Association with progression to fibrosis, HCC and mortality | 5 – 8 | ||
| Autoimmune hepatitis | Abnormal ALT 6 months after diagnosis associated with mortality or need for transplant | 12 | ||
| AST and AST/ALT ratio | Highly expressed in hepatocytes but also in many other cell types; mitochondrial enzyme that catalyzes the transfer of amino groups | All liver diseases | Association with overall mortality | 13 |
| NASH | Increased AST/ALT ratio associated with progression to fibrosis | 9 – 11 | ||
| K18 | Highly expressed in epithelial cells, in the liver mainly in hepatocytes; intermediate filament; cleaved by caspases into 44 kd, 29kD and 23kD fragments; full length K18 indicates overall cell death, neoepitopes on fragments are indicative of apoptosis | NAFLD/NASH | Associated with presence of NASH in NAFLD patients; correlates with histological disease activity | 173,174, 175 |
| Hepatitis C | Increased K18 levels can occur in patients with normal ALT, and increase risk for fibrosis in these | 139 | ||
| Alcoholic liver disease | Association with presence of Mallory-Denk bodies, hepatocyte ballooning and liver fibrosis | 176 | ||
| Acetaminophen intoxication | Increased K18 correlates with worse prognosis | 178 | ||
| HMGB1 | Present in all cells, nuclear DNA binding protein that is released from necrotic cells and functions as inflammatory DAMP | Acetaminophen intoxication | Increased total and acetylated HMGB1 in patients, and correlated with worse prognosis (outperforming ALT) | 178 |
| miR-122 | MicroRNA with liver specific expression, most abundant microRNA in the liver, expressed in hepatocytes; relevant functions in lipid metabolism and HCV infection | Acetaminophen intoxication | Increased miR-122 detects liver injury earlier than serum ALT and is more accurate in predicting liver injury | 179, 180 |
K18
K18 is a 48-kilodalton intermediate filament protein highly expressed in epithelial cells. When released into the extracellular space, K18 can be used as a serum marker for epithelial cell death. K18 is cleaved sequentially by caspases to first generate 44-kilodalton and 4-kilodalton fragments, with a second caspase digestion occurring within the 44-kilodalton fragment to generate 29-kilodalton and 23-kilodalton fragments.171 The M30 antibody, which has been used in numerous clinical studies, recognizes exposed neoepitopes on the C-terminus of the 44-kilodalton and 23-kilodalton fragments; additional antibodies, such as the M65 antibody, are used in some studies as a necrosis readout even though it recognizes both uncleaved and caspase (or other protease) cleaved K18. Although there is an ongoing debate whether antibody-based assays reliably reflect caspase-cleaved K18 and apoptosis, numerous studies have shown the usefulness of K18 in quantifying cell death and predicting clinical outcomes.172 The strongest evidence for K18 as a biomarker comes from studies in NAFLD, where it has high sensitivity and specificity in diagnosing NASH among patients with NAFLD173 and positively correlates with clinical parameters and the histological activity score (Table 2).174 and 175 Studies in HCV infection showed that 50% of patients with normal aminotransferase levels exhibited elevated serum K18 levels and, among these, 30% showed advanced stages of fibrosis.139 Mallory–Denk bodies, a hallmark of ALD, largely consist of ubiquitinated K8 and K18 aggregates. Accordingly, full-length K18 and K18 fragments correlate with the presence of Mallory–Denk bodies, hepatocyte ballooning, and liver fibrosis in patients with ALD.176 Despite these encouraging data on K18 as a biomarker for several hepatic diseases, additional data are required to recommend its use for routine clinical practice.
HMGB1
HMGB1 is a nonhistone DNA binding protein that is present in virtually all eukaryotic cells.177 HMGB1 is passively released from necrotic cells and may, after hyperacetylation, also be actively secreted from inflammatory cells.177 Apoptotic cells only release little HMGB1 due to retention by cruciform DNA.129 HMGB1 has largely been studied in liver disease with necrotic cell death. Total and hyperacetylated HMGB1 are increased in patients with acetaminophen intoxication.178 Total HMGB1 concentrations were found to be superior to serum ALT levels in identifying acute liver injury within 8 hours of acetaminophen overdose. Increases in total and acetylated HMGB1 were associated with worse prognosis after acetaminophen intoxication, a finding not reflected by serum ALT levels.178
microRNAs
microRNAs are small noncoding RNAs with important roles in the regulation of gene expression. miR-122 is the most abundant microRNA in hepatocytes and is released into serum after liver injury. After acetaminophen intoxication, miR-122 levels are increased and detect liver injury earlier than serum ALT levels179 and outperform serum ALT for the prediction of subsequent acute liver injury.180 In chronic liver disease, miR-122 levels correlate inversely with the severity of liver fibrosis, probably reflecting the loss of functional hepatocyte mass rather than the rate of hepatocyte cell death.181 HSC activation and fibrosis, as responses to hepatic cell death, are reflected by increased serum levels of miR-29, miR-133, miR-571, and miR-652.182, 183 and 184
Carbamoyl Phosphate Synthetase 1
Carbamoyl phosphate synthetase 1 is the most abundant protein in liver mitochondria and more liver specific than ALT.185 A recent study showed carbamoyl phosphate synthetase 1 in acute liver injury triggered by acetaminophen intoxication, Wilson disease, and ischemic liver injury.185 Because of its very short half-life, carbamoyl phosphate synthetase 1 may more accurately predict when liver injury terminates than ALT.
VI. Cell Death in Clinical Liver Disease
Considerable evidence for a role for cell death in promotion of liver disease in animals (Table 1) and in numerous clinical studies has provided the basis to move cell death–based therapies for acute and chronic liver disease toward clinical studies (Table 3). In the following section we review the contribution of cell death to specific liver diseases and approaches to therapeutically target cell death in patients.
Table 3.
Clinical studies with cell death based interventions in liver disease
| Treatment | Cell death Intervention | Study Design | Results | Year of completion | Phase | Patient number | NCT Identifier or reference |
|---|---|---|---|---|---|---|---|
| Acute liver failure | |||||||
| N-acetylcysteine in acetaminophen-induced liver failure | Replenishment of hepatic GSH and reduction of oxidative stress | Non-randomized | Reduction of mortality, early treatment more effective than late treatment | 1985 | 2540 | 187 | |
| Late N-acetylcysteine in acetaminophen-induced liver failure (10–36h after intoxication) | Replenishment of hepatic GSH and reduction of oxidative stress | Randomized | Improved survival, and less edema in patients receiving N-acetylcysteine | Not described. | 50 | 189 | |
| N-acetylcysteine in acute liver failure (not acetaminophen induced) | Replenishment of hepatic GSH and reduction of oxidative stress | Randomized | Improved transplant-free survival | 2009 | 3 | 173 | NCT00004467 239 |
| N-acetylcysteine in acute liver failure (not acetaminophen induced) in pediatric patients | Replenishment of hepatic GSH and reduction of oxidative stress | Randomized | No improvement in 1-year survival, 1-year transplant-free survival significantly lower than placebo (!) | 2011 | 3 | 184 | NCT00248625 240. |
| Emricasan (PF-03491390 / IDN-6556) in patients with acute on chronic liver failure | Caspase-Inhibition, anti-apoptotic | Randomized | pending | 2014 | 2 | 60 | NCT01937130 |
| ALF-5755 in patients with severe acute hepatitis and early acute liver failure (not acetaminophen) | Prevents apoptosis and oxidative stress, promotes regeneration | Randomized | Not fully published | 2013 | 2 | 60 | NCT01318525 |
| Viral Hepatitis | |||||||
| Combined oral and i.v. antioxidants in chronic HCV patients after failure of interferon | Reduction of oxidative stress | Randomized | Decline in ALT levels | 2007 | 2 | 100 | 241 |
| Vitamin E for children with chronic Hepatitis B | Reduction of oxidative stress | Randomized | Non-significant trend to higher HBeAg-seroconversion | 2008 | 2 | 76 | 242 |
| Mitoquinone mesylate in patients with chronic HCV | Caspase-inhibition, mitochondria-targeted antioxidant, anti-apoptotic | Randomized | Reduction in ALT and AST levels | 2010 | 2 | 30 | NCT00433108 243 |
| GS-9450 in patients with chronic HCV | Caspase-inhibition, anti-apoptotic | Randomized | Reduction in ALT levels | 2010** | 2 | 32 | NCT00725803 244 |
| Emricasan (PF-03491390 / IDN-6556) in chronic HCV | Caspase-Inhibition, anti-apoptotic | Randomized | Reduction in ALT and AST levels | 2010 | 2 | 204 | NCT00088140 245 |
| Pentoxifylline added to PEG-Interferon and Ribavirin in chronic HCV | Inhibition of TNF, protection of hepatocytes | Randomized | Increased SVR rates | 2013 | 2 | 72 | 246 |
| Peginterferon Alfa-2b (SCH 054031) vs Glycyrrhizin in Interferon (IFN)-Treated Patients With Chronic Hepatitis C | Glycyrrhizin is a natural compound of licorice with HMGB1 inhibitory activity | Randomized | Significantly higher ALT reduction by glyzyrrhizin compared to placebo after 12 weeks and improved necroinflammation and fibrosis after 52-weeks | NCT00686881 247 | |||
| Alcoholic liver disease | |||||||
| Pentoxifylline in severe alcoholic hepatitis | Inhibition of tumor necrosis factor (TNF) | Randomized | Improved 1-month-survival, reduced fraction of patients with hepatorenal syndrome | 2000 | 101 | 248 | |
| Pentoxifylline vs. prednisolone in severe alcoholic hepatitis | Inhibition of TNF | Randomized | Improved 3-month-survival and lower 1-month-MELD-score | 2009 | 2 | 68 | 249 |
| Pentoxifylline plus Anakinra plus zink (vs. methylprednisolone) in alcoholic hepatitis | Inhibition of TNF and Interleukin-receptor signaling | Randomized | pending | 2015 | 2/3 | 130 | NCT01809132 |
| Combined antioxidant treatment (alone or with corticosteroids) | Reduction of oxidative stress | Randomized | No improvement in 6-month-survival | 2007 | 2 | 70 | 250 |
| Eternacept in moderate to severe alcoholic hepatitis | TNF inhibition | Randomized | Increased mortality and increased infections in eternacept group | 2007 | 48 | 230 | |
| N-acetylcysteine in severe alcoholic hepatitis | Replenishment of hepatic GSH and reduction of oxidative stres | Randomized | No benefits in survival and biological parameters | 2010 | 3 | 52 | NCT00962442 251 |
| N-acetylcysteine plus (plus corticoids) in severe alcoholic hepatitis | Replenishment of hepatic GSH and reduction of oxidative stress (anti-inflammatory) | Randomized | Combination did not improve 6 months-survival compared to corticoids alone (primary endpoint), improved 1-months-survival | 2011 | 3 | 174 | NCT00863785 231 |
| Emricasan (PF-03491390 / IDN-6556) in patients with alcoholic hepatitis and contraindications to steroids | Caspase-Inhibition, anti-apoptotic | Randomized | pending | 2018 | 2 | 60 | NCT01912404 |
| Pentoxifylline and prednisolone versus prednisolone in severe alcoholic hepatitis | Randomized | No improvement in survival by the combination of pentoxifylline and prednisolone vs. prednisolone alone | 2010 | 3 | 270 | NCT01214226. 252 | |
| NAFLD | |||||||
| Pentoxifylline in NASH | Inhibition of TNF | Randomized | Improved NAFLD activity score | 2011 | 3 | 55 | NCT00590161 253 |
| Vitamin E (or Pioglitazone) in non-diabetic NASH patients (PIVENS) | Reduction of oxidative stress | Randomized | Histological improvement in NASH (not Pioglitazone), Reduction in AST/ALT, Reduced steatosis, no improvement in fibrosis score | 2010 | 3 | 247 | NCT00063622 219 |
| Vitamin E (or Metformin) in children and adolescents with NAFLD (TONIC) | Reduction of oxidative stress | Randomized | No benefit of Vit. E and Metformin on ALT/AST; improved hepatocyte ballooning score, NAFLD activity score and proportion of NASH resolution with Vit. E | 2011 | 3 | 173 | 218 |
| Vitamin E in NASH: Effects of Dose reduction (200mg, 400mg, 800mg) | Reduction of oxidative stress | Randomized | pending | 2018 | 2 | 90 | NCT01792115 |
| Obeticholic acid in patients with NASH and type II diabetes | FXR agonism | Randomized | Improved insulin sensitivity, ALT, GGT, and liver fibrosis markers in 20 mg OCA dose group; improved GGT in 50 mg OCA dose group | 2009 | 2 | 64 | 220 |
| Phyllanthus urinaria (Hepaguard®) in NASH | Reduction of oxidative stress | Randomized | No significant histological or serological improvement compared to placebo | 2013 | Not provided | 60 | NCT01210989 254 |
| Anthocyanin in non-diabetic NAFLD patients | Reduction of oxidative stress | Randomized | pending | 2014 | 0 | Not indicated | NCT01940263 |
| Metadoxin (and Metformin) in prediabetic NAFLD patients | Reduction in oxidative stress | Randomized | pending | 2015 | 4 | 100 | NCT02051842 |
| GS-9450 in patients with NASH | Caspase-inhibition, anti-apoptotic | Randomized | Reduction in ALT levels | 2012 | 2 | 124 | NCT00740610 216. |
| Emricasan (PF-03491390 / IDN-6556) in NAFLD patients with elevated ALT | Caspase-Inhibition, anti-apoptotic | Randomized | pending | 2014 | 2 | 40 | NCT02077374 |
| HCC | |||||||
| AEG35156 in combination with Sorafenib in HCC | XIAP antisense to promote apoptosis of HCC cells | randomized | PFS 4.0 months (combination) vs. 2.4 months (Sorafenib alone) Higher benefit for patients needing dose reductions | 2012 | 2 | 51 | NCT00882869 255 |
| Resminostat (4SC-201) alone or in combination with Sorafenib in 2nd Line HCC Treatment | Histone -Deacetylase inhibition to induce apoptosis and growth arrest | Open label, two arms | Median OS 8.1 Months in patients treated with Resminostat and Sorafenib in 2nd line | 2013 | 2 | 45 | NCT00943449 256 |
| Panobinostat (LBH589) in combination with Sorafenib in HCC | Histone-Deacetylase inhibition to induce apoptosis | Non-randomized | pending | Not reported | 1 | 18 | NCT00823290 |
| JX-594 in low or high dose | Oncolytic and immunotherapeutic vaccinia virus | Randomized | Median survival of 14.1 months compared to 6.7 months on the high and low dose | 2013 | 2 | 30 | 238 |
| JX-594 in combination with sorafenib | Oncolytic and immunotherapeutic vaccinia virus followed by treatment with mulitkinasse | Non-randomized | Pending | 2014 | 2 | 25 | NCT01171651 |
DILI
DILI is the major cause of acute liver failure in Western countries and an important cause of acute hepatitis or cholestasis in clinical practice. The most common cause of DILI is acetaminophen toxicity, which induces dose-related necrosis of hepatocytes through a mechanism involving conversion of a small fraction of the dose to a reactive metabolite that depletes glutathione and covalently binds to proteins, with the mitochondria being the critical target organelle, resulting in cell death promotion by sustained JNK activation.35, 83 and 186 N-acetylcysteine (NAC) is a highly effective antidote for early acetaminophen overdose and successfully prevents toxicity by replenishing glutathione, thereby preventing covalent binding of the reactive metabolite.187 and 188 However, after acetaminophen is metabolized and glutathione is depleted, the role of NAC is limited.189 Anecdotal reports suggest that late administration of NAC may have some efficacy in dampening toxicity in humans and it is widely used in clinical practice, even up to 48 hours after overdose or when subacute unintentional poisoning is identified. In this setting, additional treatments that target downstream pathways such as JNK may be beneficial. Although delayed JNK inhibition works well in animal models, the effectiveness of NAC in early stages, theoretical concerns about interference with the late protective role of JNK-dependent regenerative signaling, and the inability to identify the critical window in patients with acetaminophen intoxication make it unlikely that direct JNK inhibitors will be used in clinical settings. Although necroptosis is important in the early phase of acetaminophen toxicity, the failure of RIP3 inhibition to block late liver injury makes the necrosome an unlikely therapeutic target for acetaminophen intoxication.
Aside from acetaminophen, most DILI is idiosyncratic (IDILI), meaning that only a small proportion of patients treated with an IDILI drug develop liver injury, and the cell death appears to be mediated by the adaptive immune system, as evidenced by striking recently described HLA associations that imply a genetic risk.190 Current concepts suggest that IDILI drugs induce hepatocellular stress, which may provide a danger signal to the immune system of genetically susceptible individuals. At present, there is no specific treatment for IDILI. Drugs that inhibit immune-mediated cell death mechanisms might have benefit in early stages of overt or in prolonged IDILI but have not been formally studied. Once acute liver failure develops, such an approach has been unsuccessful.191
Viral Hepatitis
Most acute and chronic infections with HBV or HCV elicit an active antiviral immune response, resulting in active killing of infected hepatocytes.192 and 193 Elimination of infected hepatocytes occurs largely through CD8+ effector T cells and natural killer cells.192 and 193 In patients who do not clear the HBV or HCV virus, there is persistent T cell– and natural killer cell–mediated hepatocyte apoptosis. Although apoptosis of infected hepatocytes is primarily a protective response, it is often not sufficient to eliminate the virus in patients with chronic viral hepatitis and then becomes a key driver of liver disease development. T cell– and natural killer cell–induced hepatocyte apoptosis is largely mediated by members of the TNF receptor family (including Fas and TRAIL receptor 2) and, to a lesser extent, by granzyme B and perforin.52 and 194 Based on aforementioned studies in mice, in which apoptosis is sufficient to trigger fibrosis and HCC,62, 63, 64, 90, 152, 153 and 167 it is likely that apoptosis, induced by persistent elimination of infected hepatocytes in chronic HBV and HCV infection, constitutes the main contributor to disease progression. Based on this principle, several studies have tested caspase inhibitors IDN-6556 and GS-9450 in HCV-infected patients (Table 3), but no positive results have yet been reported. In view of the advent of direct antivirals with cure rates of more than 90% in HCV-infected patients195 and high rates for hepatitis B e antigen seroconversion and/or suppression of viral replication in HBV-infected patients,196 strategies to target cell death (eg, by caspase inhibitors) are unlikely to represent a clinically relevant avenue in viral hepatitis in the future. Glycyrrhizin, an ingredient of licorice that inhibits HMGB1,197 improved necroinflammation and fibrosis in HCV-infected patients without changing serum HCV RNA,198 suggesting that glycyrrhizin may be used to suppress HMGB1-mediated cell death responses in viral hepatitis and potentially other chronic liver diseases.
NAFLD
NAFLD represents the most common chronic liver disease in the Western world.199 and 200 NASH is a more aggressive disease entity within the spectrum of NAFLD that is distinguished from simple steatosis by the presence of hepatocyte death and subsequent cell death responses, visible as hepatocyte ballooning, an inflammatory infiltrate, and/or collagen deposition, and promotes the development of fibrosis, cirrhosis, and HCC.201 Despite clinical trials of multiple molecular targets in patients with NASH,202 no effective pharmacological strategy against NASH-induced liver fibrosis and HCC has yet been established in clinical practice, highlighting the need to identify signaling pathways regulating the transition from NAFLD to NASH. This transition is typically accompanied by elevated ALT levels,173 and 203 indicating the pivotal role of cell death in this process. Of all cell death pathways, apoptosis is the best characterized form in this context.145, 204 and 205 Accordingly, plasma K18 fragment levels correlated with the magnitude of hepatocyte apoptosis and independently predicted the presence of NASH in several large trials.206 and 207 Beyond the putative role of apoptosis, we recently showed that human NASH livers express high levels of RIP3,146 suggesting that necroptosis might represent an alternative target for clinical studies in NASH.
Multiple cell-intrinsic mechanisms have been suggested to trigger cell death and the progression to NASH. As such, saturated fatty acids are more hepatotoxic than unsaturated fatty acids.115 Fatty acid accumulation stimulates ROS generation in the liver, presumably due to enhanced β-oxidation and the subsequent electron overflow in the mitochondrial electron transfer chain.120 Increased ROS directly injure DNA, proteins, and lipids and promote cell death through activation of stress-related signaling pathways such as JNK or p38 mitogen-activated protein kinase.81 JNK induction by fatty acid has been suggested to be a key factor in this setting,71 and 72 whereas the role of the ER stress response remains controversial because ablation of CHOP worsens NASH.208 and 209 In contrast to these intrinsic apoptosis pathways, the function of extrinsic death receptor– mediated apoptosis pathways in NASH is less clear. Although adipose tissue expansion in patients with NASH is associated with the release of proinflammatory cytokines such as TNF,116 their contribution to cell death and clinical relevance remain unknown. In a murine NASH model, TNF receptor knockout mice were protected from hepatic steatosis and fibrosis.210 Moreover, TRAIL receptor 2 has a role in hepatocyte lipoapoptosis, but its contribution to NASH remain untested.211
Bariatric surgery successfully treats the underlying cause and achieves sustained amelioration of insulin resistance, hepatic steatosis and inflammation, serum ALT and K18 levels, and hepatic fibrosis.212, 213 and 214 Pharmacological strategies have focused on insulin resistance as the most relevant underlying pathophysiological mechanism and have found beneficial effects of the thiazolidinediones pioglitazone and rosiglitazone on serum ALT levels and histology during the first year of therapy.215 and 216 However, these treatments are not considered first-line therapies by current guidelines for various reasons.217 In contrast, targeting cellular stress and cell death pathways has led to promising findings and changed clinical standards. Based on the prominent role of ROS in cell death and NASH pathophysiology, the PIVENS trial tested the effect of the antioxidant vitamin E in nondiabetic patients with NASH.218 and 219 The observed decrease in inflammation and serum ALT and AST levels, but not fibrosis, in nondiabetic patients taking vitamin E in this trial219 provided the basis for the recent NAFLD guideline recommending vitamin E as a first-line treatment for nondiabetic patients with biopsy-proven NASH.217 Investigating farnesoid X receptor as yet another target, a recent study reported improved insulin sensitivity, weight loss, ALT level, γ-glutamyl transpeptidase level, and liver fibrosis markers in patients with type 2 diabetes mellitus and NASH treated with the FXR receptor agonist obeticholic acid.220 However, some effects, such as improved ALT levels and fibrosis markers, were only observed at the lower dose of obeticholic acid in this trial. A recent phase 2 study reported normalization of ALT levels in 35% of patients with NASH treated with the selective caspase inhibitor GS-9450,216 the effects on liver histology are unknown, and reduction of another relevant biomarker, K18, was not significant. In summary, there is increasing evidence that targeting cell death and oxidative stress may be beneficial in NASH, but larger trials with longer observation intervals will be needed to determine effects on relevant clinical outcomes.
ALD
ALD represents one of the most common causes of liver-related morbidity and mortality worldwide.221 Moreover, its relative contribution to liver disease is expected to further increase due to a worldwide increase of alcohol abuse121 and the declining prevalence of hepatitis B222 and 223 and hepatitis C.122 Alcoholic steatohepatitis is the severe form of ALD and may progress to fibrosis, cirrhosis, and HCC.132 Similar to NASH, alcoholic steatohepatitis is characterized by hepatic steatosis, cell injury, and inflammation.63 In ALD, there is evidence for both apoptosis and necrosis.42, 44 and 224 Cytotoxic effects of alcohol are at least in part caused by its metabolite acetaldehyde, which causes excess ROS production leading to lipid peroxidation, mitochondrial damage, and cell death.221 and 225 Accordingly, treatment with antioxidants ameliorates experimental alcoholic liver injury.62 An increased gut permeability, resulting in increased levels of portal vein lipopolysaccharide, contributes to the production of inflammatory cytokines TNF and IL-6, ROS, and liver injury, as evidenced by improved liver injury in Toll-like receptor 4–deficient mice or mice receiving nonabsorbable antibiotics for gut sterilization.226, 227 and 228 TNF induces liver injury on alcohol consumption,147 and anti-TNF treatment prevented alcohol-induced hepatic cell death in rats.149 A recent study using RIP3-deficient mice suggested that necroptosis is involved in TNF-dependent cell death in alcoholic liver injury.41 However, the clinical relevance of these pathways for the treatment of ALD and/or prevention of progression to HCC remains to be established.
As continued alcohol ingestion is the single most important risk factor for disease progression, and abstinence represents the most effective measure.221 and 225 Pharmacological treatments for patients with ALD are largely restricted to the setting of severe acute hepatitis, a life-threatening disease with high mortality.219 However, it is not clear whether hepatocellular death, presumably triggered by ROS, TNF, and bacterial pathogen-associated molecular patterns, is a main contributor to acute hepatitis. Corticosteroids improve short-term survival in acute hepatitis in the majority of studies,153 making this the first-line treatment in responders as defined by the Lille score.229 The prominent role of TNF in alcoholic steatohepatitis–dependent cell death in murine studies provided the basis for clinical trials assessing TNF inhibitors such as etanercept and pentoxifylline, an inhibitor of TNF synthesis. However, a recent large trial did not show a benefit of combined pentoxifylline and corticosteroids versus corticosteroids alone.156 Moreover, etanercept increased mortality and infections in patients with acute hepatitis.230 Likewise, the effect of antioxidants such as NAC remains uncertain because the combination of NAC and corticosteroids increased 1-month but not 6-month survival in comparison to corticosteroids alone.231
HCC
HCC represents the common end stage of chronic liver diseases, including viral, alcoholic, and nonalcoholic fatty liver disease. Hepatocarcinogenesis is multifactorial, and the relative contribution of cell death to the development of HCC depends on the underlying disease. For example, HCC can develop in patients with HBV infection or NAFLD in the absence of chronic liver injury and/or fibrosis,232, 233 and 234 suggesting that cell death–independent signals (eg, HBV-induced signals or altered metabolism) may be sufficient to trigger carcinogenesis in disease-specific contexts. Nonetheless, 80% of HCCs develop in fibrotic or cirrhotic livers, which in turn develop in the setting of chronic hepatocellular death. Moreover, aforementioned data from murine models with increased hepatocellular death64, 90, 152, 153 and 167 strongly support the notion that the presence of chronic cell death in the liver is sufficient to trigger development of HCC (Table 1). As such, numerous clinical studies have shown correlations between biomarkers of liver cell death such as ALT and the risk of cancer development. For example, HBV- and HCV-infected patients with persistent ALT levels >45 U/L have a 10-fold and 7-fold higher risk, respectively, of developing HCC than patients with persistently normal ALT hepatitis.59 and 60 Measurement of ALT levels is thus part of surveillance and treatment guidelines.235
Interference with pathways that modulate cell death in the liver appears to be a promising strategy for the prevention or treatment of HCC but is likely to require stage-, disease- and compartment-specific approaches. HCC prevention strategies need to inhibit cell death in early stages to stop the HCC-promoting cell death/inflammation/regeneration/fibrosis cascade. In addition to treating the underlying disease, this could possibly be achieved by DAMP inhibitors such as glycyrrhizin,236 interruption of the gut microbiome-liver axis by nonabsorbable antibiotics such as rifaximin,164 or other modifiers of cell death, inflammation, or fibrosis.236
In contrast, treatment of established HCC requires promotion of cell death in cancerous tissue but not healthy liver. The only approved drug for treatment of HCC, sorafenib, affects HCC proliferation and angiogenesis but not cell death.237 Besides ablative strategies that induce HCC death with low selectivity, such as radiofrequency ablation and transarterial chemoembolization, more HCC-specific approaches are being developed. Oncolytic viruses can induce selective cell death in HCC, and a recent phase 2 trial showed dose-dependent improvement of survival by oncolytic and immunotherapeutic vaccinia virus JX-594.238 Studies testing combinations of JX-594 and sorafenib are ongoing (Table 3). Another study is targeting cell death in liver cancer using XIAP antisense in combination with sorafenib to lower the apoptosis threshold of cancer cells (Table 3).
VII. Conclusions
Cell death is at the center of virtually every acute and chronic liver disease. The discovery of novel modes of cell death such as necroptosis and specific pathways that regulate cell death and cell death responses has greatly improved our understanding of the pathophysiology of acute and chronic liver disease. Although revolutionary progress in HCV therapy allows prevention of many of the deadly complications of chronic liver disease in this specific cohort of patients, we cannot halt progression in most other relevant liver diseases. Despite new insights into key pathways through which cell death drives liver disease, we are still awaiting their clinical translation. The discovery of necroptosis as a key backup pathway to apoptosis provides a convincing explanation why caspase inhibitors have not achieved broad application in clinical hepatology. Hence, it may be necessary to simultaneously block multiple pathways or those that are considered more reactive or detrimental (ie, possibly necroptosis rather than apoptosis). In view of these dilemmas and the possibility that blocking one cell death pathway may lead to activation of another, future studies should focus on understanding the regulation of cell death response pathways in the liver. These pathways might hold important clues for tailored treatment of acute and chronic liver disease. Activation of beneficial cell death response pathways (eg, those that trigger hepatocyte proliferation) may be beneficial in acute liver disease, whereas blocking profibrogenic, proliferative, and proinflammatory cell death response pathways may inhibit the progression of chronic liver disease. Finally, novel biomarkers of cell death may allow more accurate predictions for outcome and treatment decisions than transaminases, on which we have relied for the past 6 decades.
References
- 1.Fattovich G, Olivari N, Pasino M, et al. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 2.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Tai DI, Lin SM, Sheen IS, et al. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotrans- ferase levels over time. Hepatology. 2009;49:1859–1867. doi: 10.1002/hep.22878. [DOI] [PubMed] [Google Scholar]
- 4.Tseng TC, Liu CJ, Yang HC, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carci- noma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149.e3. doi: 10.1053/j.gastro.2012.02.007. quiz e13–e14. [DOI] [PubMed] [Google Scholar]
- 5.Ghany MG, Kleiner DE, Alter H, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 6.Hui CK, Belaye T, Montegrande K, et al. A comparison in the progression of liver fibrosis in chronic hepatitis C between persistently normal and elevated transaminase. J Hepatol. 2003;38:511–517. doi: 10.1016/s0168-8278(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 7.Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: a 20-year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- 8.Wiese M, Fischer J, Lobermann M, et al. Evaluation of liver disease progression in the German hepatitis C virus (1b)-contaminated anti-D cohort at 35 years after infection. Hepatology. 2014;59:49–57. doi: 10.1002/hep.26644. [DOI] [PubMed] [Google Scholar]
- 9.Amarapurka DN, Amarapurkar AD, Patel ND, et al. Nonalcoholic steatohepatitis (NASH) with diabetes: pre- dictors of liver fibrosis. Ann Hepatol. 2006;5:30–33. [PubMed] [Google Scholar]
- 10.Angulo P, Keach JC, Batts KP, et al. Independent pre- dictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 11.McPherson S, Henderson E, Burt AD, et al. Serum immu- noglobulin levels predict fibrosis in patients with non- alcoholic fatty liver disease. J Hepatol. 2014;60:1055–1062. doi: 10.1016/j.jhep.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Feld JJ, Dinh H, Arenovich T, et al. Autoimmune hepati- tis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Kim WR, Benson JT, et al. Serum aminotrans- ferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- 14.Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhl CE, Everhart JE. Elevated serum alanine amino- transferase and gamma-glutamyl transferase and mortality in the United States population. Gastroenterology. 2009;136:477–485.e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- 17.Columbano A, Ledda-Columbano GM, Coni PP, et al. Occurrence of cell death (apoptosis) during the involution of liver hyperplasia. Lab Invest. 1985;52:670–675. [PubMed] [Google Scholar]
- 18.Benedetti A, Jezequel AM, Orlandi F. Preferential distribution of apoptotic bodies in acinar zone 3 of normal human and rat liver. J Hepatol. 1988;7:319–324. doi: 10.1016/s0168-8278(88)80004-7. [DOI] [PubMed] [Google Scholar]
- 19.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 20.Yanai H, Matsuda A, An J, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci U S A. 2013;110:20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura M. Active participation of cell death in development and organismal homeostasis. Dev Growth Differ. 2011;53:125–136. doi: 10.1111/j.1440-169X.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 23.Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 24.Ashida H, Mimuro H, Ogawa M, et al. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011;195:931–942. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 27.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker NI, Harmon BV, Gobe GC, et al. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- 29.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Lemasters JJ, Theruvath TP, Zhong Z, et al. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 34.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 35.Gunawan BK, Liu ZX, Han D, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 36.Masubuchi Y, Suda C, Horie T. Involvement of mito- chondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Theruvath TP, Zhong Z, Pediaditakis P, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldmeier PC, Zimmermann K, Qian T, et al. Cyclophilin D as a drug target. Curr Med Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 39.Lemasters JJ. Dying a thousand deaths: redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology. 2005;129:351–360. doi: 10.1053/j.gastro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran A, McGill MR, Xie Y, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roychowdhury S, McMullen MR, Pisano SG, et al. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanji AA, Hiller-Sturmhofel S. Apoptosis and necrosis: two types of cell death in alcoholic liver disease. Alcohol Health Res World. 1997;21:325–330. [PMC free article] [PubMed] [Google Scholar]
- 43.Joka D, Wahl K, Moeller S, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55:455–464. doi: 10.1002/hep.24734. [DOI] [PubMed] [Google Scholar]
- 44.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 45.Linkermann A, Brasen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kon K, Kim JS, Jaeschke H, et al. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 47.Lemasters JJ, Nieminen AL, Qian T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 48.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 49.Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. doi: 10.1034/j.1600-065x.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 50.Faubion WA, Guicciardi ME, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bantel H, Schulze-Osthoff K. Apoptosis in hepatitis C virus infection. Cell Death Differ. 2003;10(suppl 1):S48–S58. doi: 10.1038/sj.cdd.4401119. [DOI] [PubMed] [Google Scholar]
- 52.Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13:4865–4872. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Natori S, Rust C, Stadheim LM, et al. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 54.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gude DR, Alvarez SE, Paugh SW, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullen SP, Henry CM, Kearney CJ, et al. Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell. 2013;49:1034–1048. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Martin SJ, Henry CM, Cullen SP. A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell. 2012;46:387–397. doi: 10.1016/j.molcel.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 59.Chen CF, Lee WC, Yang HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240–1248. 1248.e1–2. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 60.Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 61.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takehara T, Tatsumi T, Suzuki T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Vick B, Weber A, Urbanik T, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber A, Boger R, Vick B, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greene CM, McElvaney NG. Z alpha-1 antitrypsin deficiency and the endoplasmic reticulum stress response. World J Gastrointest Pharmacol Ther. 2010;1:94–101. doi: 10.4292/wjgpt.v1.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asselah T, Bieche I, Mansouri A, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264–274. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- 68.Merquiol E, Uzi D, Mueller T, et al. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS One. 2011;6:e24660. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cazanave SC, Gores GJ. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5:71–85. doi: 10.2217/clp.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holzer RG, Park EJ, Li N, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56:192–198. doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin Liver Dis. 2007;27:327–338. doi: 10.1055/s-2007-991510. [DOI] [PubMed] [Google Scholar]
- 76.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 77.Galle PR, Hofmann WJ, Walczak H, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 80.Reinehr R, Haussinger D. CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch Biochem Biophys. 2012;518:2–7. doi: 10.1016/j.abb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 82.Tang G, Minemoto Y, Dibling B, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 83.Win S, Than TA, Han D, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 85.Wallach D, Varfolomeev EE, Malinin NL, et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 86.Schwabe RF, Uchinami H, Qian T, et al. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 87.Schwabe RF, Bradham CA, Uehara T, et al. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 88.Leist M, Gantner F, Bohlinger I, et al. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 89.Luedde T, Assmus U, Wustefeld T, et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115:849–859. doi: 10.1172/JCI23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 91.Luedde T, Heinrichsdorff J, De Lorenzi R, et al. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proc Natl Acad Sci U S A. 2008;105:9733–9738. doi: 10.1073/pnas.0800198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schattenberg JM, Zimmermann T, Worns M, et al. Ablation of c-FLIP in hepatocytes enhances death-receptor mediated apoptosis and toxic liver injury in vivo. J Hepatol. 2011;55:1272–1280. doi: 10.1016/j.jhep.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 94.Han J, Zhong CQ, Zhang DW. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12:1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 95.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 98.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 99.Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 101.Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen X, Li W, Ren J, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Luedde M, Lutz M, Carter N, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 105.Vucur M, Reisinger F, Gautheron J, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4:776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 106.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonnet MC, Preukschat D, Welz PS, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 108.Welz PS, Wullaert A, Vlantis K, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 109.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 110.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amir M, Zhao E, Fontana L, et al. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20:878–887. doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ni HM, Bockus A, Boggess N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czaja MJ, Ding WX, Donohue TM, Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Higuchi H, Bronk SF, Takikawa Y, et al. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem. 2001;276:38610–38618. doi: 10.1074/jbc.M105300200. [DOI] [PubMed] [Google Scholar]
- 116.Alabraba EB, Lai V, Boon L, et al. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008;47:552–562. doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 117.Harada K, Ozaki S, Gershwin ME, et al. Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology. 1997;26:1399–1405. doi: 10.1002/hep.510260604. [DOI] [PubMed] [Google Scholar]
- 118.Takeda K, Kojima Y, Ikejima K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ueno Y, Ishii M, Yahagi K, et al. Fas-mediated cholangiopathy in the murine model of graft versus host disease. Hepatology. 2000;31:966–974. doi: 10.1053/he.2000.5764. [DOI] [PubMed] [Google Scholar]
- 120.Xia X, Demorrow S, Francis H, et al. Cholangiocyte injury and ductopenic syndromes. Semin Liver Dis. 2007;27:401–412. doi: 10.1055/s-2007-991516. [DOI] [PubMed] [Google Scholar]
- 121.Gao W, Bentley RC, Madden JF, et al. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- 122.Huet PM, Nagaoka MR, Desbiens G, et al. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology. 2004;39:1110–1119. doi: 10.1002/hep.20157. [DOI] [PubMed] [Google Scholar]
- 123.Crispe IN, Dao T, Klugewitz K, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 124.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 125.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 126.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 129.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 130.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 132.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Hoque R, Sohail MA, Salhanick S, et al. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pusterla T, Nemeth J, Stein I, et al. Receptor for advanced glycation endproducts (RAGE) is a key regulator of oval cell activation and inflammation-associated liver carcinogenesis in mice. Hepatology. 2013;58:363–373. doi: 10.1002/hep.26395. [DOI] [PubMed] [Google Scholar]
- 136.McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li J, Razumilava N, Gores GJ, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 139.Bantel H, Lugering A, Heidemann J, et al. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078–1087. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- 140.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 144.Canbay A, Feldstein A, Baskin-Bey E, et al. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 145.Anstee QM, Concas D, Kudo H, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–550. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 146.Gautheron J, Vucur M, Reisinger F, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Canbay A, Taimr P, Torok N, et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 148.Jiang JX, Mikami K, Venugopal S, et al. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–148. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhan SS, Jiang JX, Wu J, et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 150.Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 151.Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 152.Bettermann K, Vucur M, Haybaeck J, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 153.Hikita H, Kodama T, Shimizu S, et al. Bak deficiency inhibits liver carcinogenesis: a causal link between apoptosis and carcinogenesis. J Hepatol. 2012;57:92–100. doi: 10.1016/j.jhep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 154.Qiu W, Wang X, Leibowitz B, et al. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology. 2011;54:1249–1258. doi: 10.1002/hep.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liedtke C, Bangen JM, Freimuth J, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176–2187. doi: 10.1053/j.gastro.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 156.Nakamoto Y, Kaneko S, Fan H, et al. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-fas ligand antibody therapy. J Exp Med. 2002;196:1105–1111. doi: 10.1084/jem.20020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 158.Beerheide W, Tan YJ, Teng E, et al. Downregulation of proapoptotic proteins Bax and Bcl-X(S) in p53 over-expressing hepatocellular carcinomas. Biochem Biophys Res Commun. 2000;273:54–61. doi: 10.1006/bbrc.2000.2891. [DOI] [PubMed] [Google Scholar]
- 159.Shi YH, Ding WX, Zhou J, et al. Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology. 2008;48:497–507. doi: 10.1002/hep.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sieghart W, Losert D, Strommer S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 161.Takehara T, Liu X, Fujimoto J, et al. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
- 162.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 163.Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luedde T, Schwabe RF. NF-kappaB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 167.Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kew MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. 2000;355:591–592. doi: 10.1016/S0140-6736(99)00219-6. [DOI] [PubMed] [Google Scholar]
- 169.Botros M, Sikaris KA. The De Ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 170.Liangpunsakul S, Qi R, Crabb DW, et al. Relationship between alcohol drinking and aspartate aminotransferase:alanine aminotransferase (AST: ALT) ratio, mean corpuscular volume (MCV), gamma-glutamyl transpeptidase (GGT), and apolipoprotein A1 and B in the U. S. population. J Stud Alcohol Drugs. 2010;71:249–252. doi: 10.15288/jsad.2010.71.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ku NO, Omary MB. Effect of mutation and phosphorylation of type I keratins on their caspase-mediated degradation. J Biol Chem. 2001;276:26792–26798. doi: 10.1074/jbc.M103315200. [DOI] [PubMed] [Google Scholar]
- 172.Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60:1063–1074. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 173.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 174.Feldstein AE, Alkhouri N, De Vito R, et al. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013;108:1526–1531. doi: 10.1038/ajg.2013.168. [DOI] [PubMed] [Google Scholar]
- 175.Tsutsui M, Tanaka N, Kawakubo M, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. J Clin Gastroenterol. 2010;44:440–447. doi: 10.1097/MCG.0b013e3181bdefe2. [DOI] [PubMed] [Google Scholar]
- 176.Lavallard VJ, Bonnafous S, Patouraux S, et al. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLoS One. 2011;6:e17599. doi: 10.1371/journal.pone.0017599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 178.Antoine DJ, Jenkins RE, Dear JW, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 179.Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Trebicka J, Anadol E, Elfimova N, et al. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013;58:234–239. doi: 10.1016/j.jhep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 182.Roderburg C, Urban GW, Bettermann K, et al. MicroRNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 183.Roderburg C, Luedde M, Vargas Cardenas D, et al. miR-133a mediates TGF-beta-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J Hepatol. 2013;58:736–742. doi: 10.1016/j.jhep.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 184.Roderburg C, Mollnow T, Bongaerts B, et al. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One. 2012;7:e32999. doi: 10.1371/journal.pone.0032999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weerasinghe SV, Jang YJ, Fontana RJ, et al. Carbamoyl phosphate synthatase-1 (CPS1) is a rapid turnover biomarker in mouse and human acute liver injury. Am J Physiol Gastrointest Liver Physiol. 2014;307:G355–G364. doi: 10.1152/ajpgi.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 188.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303:1026–1029. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaplowitz N, DeLeve L. Drug-induced liver injury: introduction and overview. In: Kaplowitz N, editor. Drug-induced liver injury. 3rd ed Elsevier; San Diego, CA: 2013. pp. 3–14. [Google Scholar]
- 191.Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology. 2014;59:612–621. doi: 10.1002/hep.26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014;146:1193–1207. doi: 10.1053/j.gastro.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 194.Kafrouni MI, Brown GR, Thiele DL. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J Immunol. 2001;167:1566–1574. doi: 10.4049/jimmunol.167.3.1566. [DOI] [PubMed] [Google Scholar]
- 195.Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 196.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142:1360–1368.e1. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 197.Mollica L, De Marchis F, Spitaleri A, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 198.Manns MP, Wedemeyer H, Singer A, et al. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat. 2012;19:537–546. doi: 10.1111/j.1365-2893.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725.e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 200.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 201.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cao W, Zhao C, Shen C, et al. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS One. 2013;8:e82092. doi: 10.1371/journal.pone.0082092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Witek RP, Stone WC, Karaca FG, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 205.Hatting M, Zhao G, Schumacher F, et al. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–2201. doi: 10.1002/hep.26271. [DOI] [PubMed] [Google Scholar]
- 206.Wu J, Huang Z, Ren J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Soon RK, Jr, Yan JS, Grenert JP, et al. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology. 2010;139:1730–1739. 1739.e1. doi: 10.1053/j.gastro.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Malhi H, Kropp EM, Clavo VF, et al. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem. 2013;288:18624–18642. doi: 10.1074/jbc.M112.442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cazanave SC, Mott JL, Bronk SF, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286:39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mathurin P, Hollebecque A, Arnalsteen L, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 213.Diab DL, Yerian L, Schauer P, et al. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Burza MA, Romeo S, Kotronen A, et al. Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PLoS One. 2013;8:e60495. doi: 10.1371/journal.pone.0060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 216.Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 218.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 221.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ni YH, Chang MH, Huang LM, et al. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796–800. doi: 10.7326/0003-4819-135-9-200111060-00009. [DOI] [PubMed] [Google Scholar]
- 223.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 224.Ziol M, Tepper M, Lohez M, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254–260. doi: 10.1016/s0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 225.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 226.Adachi Y, Moore LE, Bradford BU, et al. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 227.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Uesugi T, Froh M, Arteel GE, et al. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 229.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 230.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 232.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 233.Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 235.European Association for the Study of the Liver EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 236.Morgan TR. Chemoprevention of hepatocellular carcinoma in chronic hepatitis C. Recent Results Cancer Res. 2011;188:85–99. doi: 10.1007/978-3-642-10858-7_7. [DOI] [PubMed] [Google Scholar]
- 237.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 238.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. 864.e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Squires RH, Dhawan A, Alonso E, et al. Intravenous N-acetylcysteine in pediatric patients with non-acetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57:1542–1549. doi: 10.1002/hep.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gabbay E, Zigmond E, Pappo O, et al. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol. 2007;13:5317–5323. doi: 10.3748/wjg.v13.i40.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gerner P, Posselt HG, Krahl A, et al. Vitamin E treatment for children with chronic hepatitis B: a randomized placebo controlled trial. World J Gastroenterol. 2008;14:7208–7213. doi: 10.3748/wjg.14.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gane EJ, Weilert F, Orr DW, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 244.Manns MP, Lawitz E, Hoepelman AIM, et al. Short term safety, tolerability, pharmacokinetics and preliminary activity of GS-9450, a selective Caspase inhibitor, in patients with chronic HCV infection. J Hepatol. 2010;52:S114–S115. [Google Scholar]
- 245.Shiffman ML, Pockros P, McHutchison JG, et al. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor—a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:969–978. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 246.Jimenez-Luevano MA, Lerma-Diaz JM, Hernandez-Flores G, et al. Addition of pentoxifylline to pegylated interferon-alpha-2a and ribavirin improves sustained virological response to chronic hepatitis C virus: a randomized clinical trial. Ann Hepatol. 2013;12:248–255. [PubMed] [Google Scholar]
- 247.Manns MP, Wedemeyer H, Singer A, et al. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat. 2012;19:537–546. doi: 10.1111/j.1365-2893.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 249.De BK, Gangopadhyay S, Dutta D, et al. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613–1619. doi: 10.3748/wjg.15.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stewart S, Prince M, Bassendine M, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277–283. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 251.Moreno C, Langlet P, Hittelet A, et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. J Hepatol. 2010;53:1117–1122. doi: 10.1016/j.jhep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 252.Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–1041. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 253.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wong VW, Wong GL, Chan AW, et al. Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: a randomized trial. J Gastroenterol Hepatol. 2013;28:57–62. doi: 10.1111/j.1440-1746.2012.07286.x. [DOI] [PubMed] [Google Scholar]
- 255.Lee AS, Zee BCY, Cheung FY, et al. Randomized phase II study of the X-linked inhibitor of apoptosis (XIAP) antisense AEG35156 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC) Am J Clin Oncol. 2014 Jun 23; doi: 10.1097/COC.0000000000000099. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 256.Bitzer M, Ganten TM, Woerns MA, et al. Resminostat in advanced hepatocellular carcinoma (HCC): overall survival subgroup analysis of prognostic factors in the SHELTER trial (abstr) J Clin Oncol. 2013;31(suppl):e15088. [Google Scholar]