Abstract
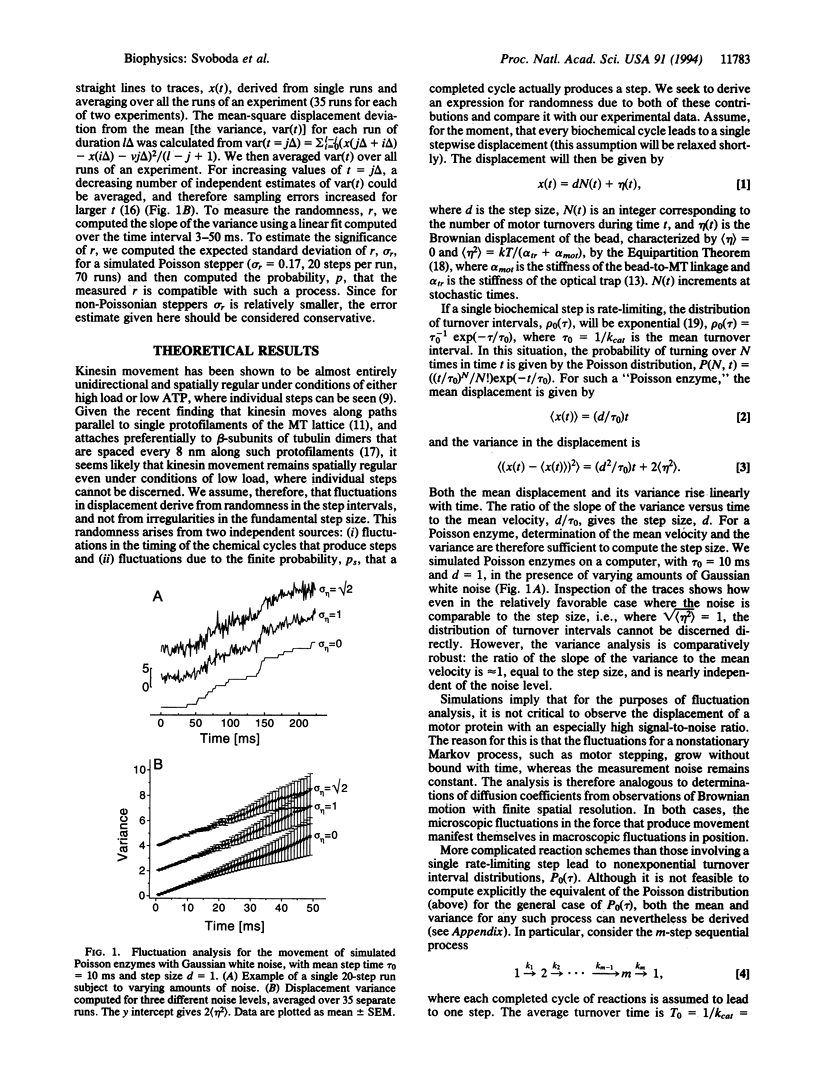
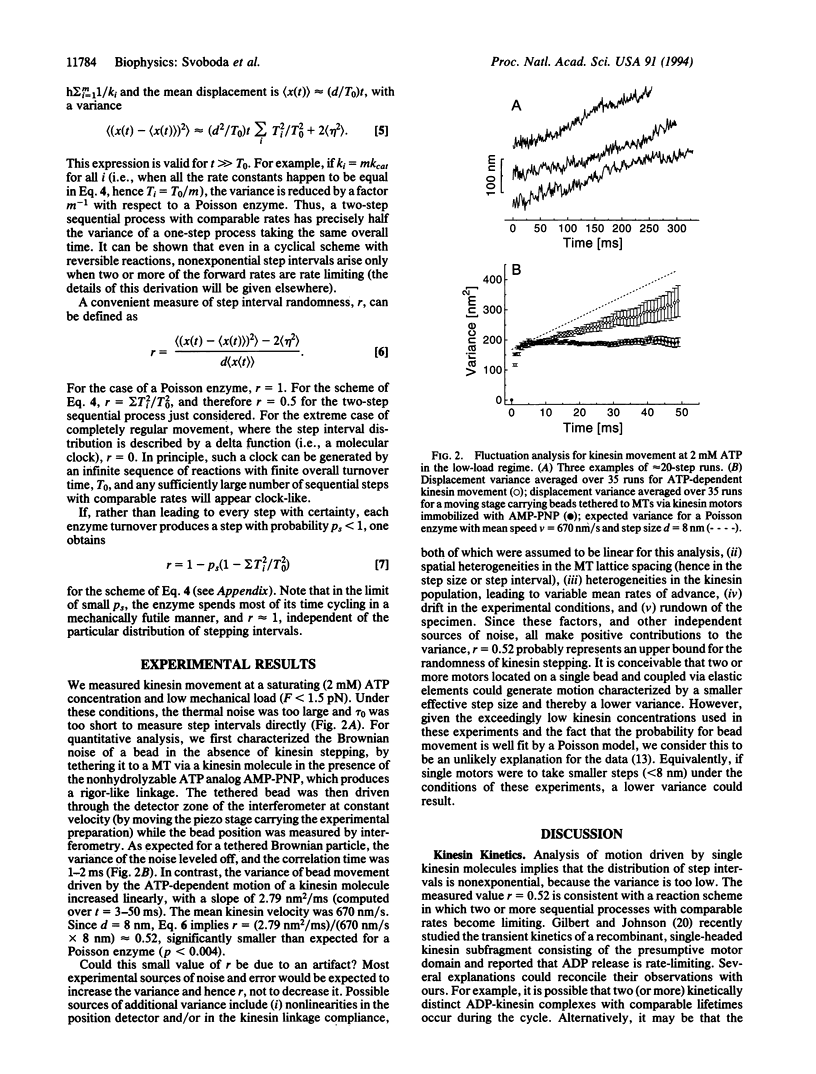
We studied fluctuations in the displacement of silica beads driven by single molecules of the motor protein kinesin, moving under low mechanical loads at saturating ATP concentrations. The variance in position was significantly smaller than expected for the case of stepwise movement along a regular lattice of positions with exponentially distributed intervals. The small variance suggests that two or more sequential processes with comparable reaction rates dominate the biochemical cycle. The low value is inconsistent with certain recently proposed thermal ratchet models for motor movement as well as with scenarios where the hydrolysis of a single ATP molecule leads to a cluster of several steps. Fluctuation analysis is a potential powerful tool for studying kinetic behavior whenever the output of a single enzyme can be monitored.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astumian RD, Bier M. Fluctuation driven ratchets: Molecular motors. Phys Rev Lett. 1994 Mar 14;72(11):1766–1769. doi: 10.1103/PhysRevLett.72.1766. [DOI] [PubMed] [Google Scholar]
- Block S. M., Berg H. C. Successive incorporation of force-generating units in the bacterial rotary motor. 1984 May 31-Jun 6Nature. 309(5967):470–472. doi: 10.1038/309470a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Draber S., Schultze R. Correction for missed events based on a realistic model of a detector. Biophys J. 1994 Jan;66(1):191–201. doi: 10.1016/S0006-3495(94)80756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Johnson K. A. Pre-steady-state kinetics of the microtubule-kinesin ATPase. Biochemistry. 1994 Feb 22;33(7):1951–1960. doi: 10.1021/bi00173a044. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D., Levitt J. D., Wagner D. D. Characterization of alpha 2 beta 2 and alpha 2 forms of kinesin. Biochem Biophys Res Commun. 1991 Jan 31;174(2):810–815. doi: 10.1016/0006-291x(91)91490-4. [DOI] [PubMed] [Google Scholar]
- Hawkes A. G., Jalali A., Colquhoun D. Asymptotic distributions of apparent open times and shut times in a single channel record allowing for the omission of brief events. Philos Trans R Soc Lond B Biol Sci. 1992 Sep 29;337(1282):383–404. doi: 10.1098/rstb.1992.0116. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Kabata H., Kurosawa O., Arai I., Washizu M., Margarson S. A., Glass R. E., Shimamoto N. Visualization of single molecules of RNA polymerase sliding along DNA. Science. 1993 Dec 3;262(5139):1561–1563. doi: 10.1126/science.8248804. [DOI] [PubMed] [Google Scholar]
- Magnasco MO. Forced thermal ratchets. Phys Rev Lett. 1993 Sep 6;71(10):1477–1481. doi: 10.1103/PhysRevLett.71.1477. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Prost J, Chauwin JF, Peliti L, Ajdari A. Asymmetric pumping of particles. Phys Rev Lett. 1994 Apr 18;72(16):2652–2655. doi: 10.1103/PhysRevLett.72.2652. [DOI] [PubMed] [Google Scholar]
- Qian H., Sheetz M. P., Elson E. L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991 Oct;60(4):910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Meyhöfer E., Milligan R. A., Howard J. Kinesin follows the microtubule's protofilament axis. J Cell Biol. 1993 Jun;121(5):1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Gelles J., Sheetz M. P., Landick R. Transcription by single molecules of RNA polymerase observed by light microscopy. Nature. 1991 Aug 1;352(6334):444–448. doi: 10.1038/352444a0. [DOI] [PubMed] [Google Scholar]
- Song Y. H., Mandelkow E. Recombinant kinesin motor domain binds to beta-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1671–1675. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Force and velocity measured for single kinesin molecules. Cell. 1994 Jun 3;77(5):773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Cell motility. Variations on the theme of movement. Nature. 1993 Jan 14;361(6408):115–116. doi: 10.1038/361115a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Harada Y., Ishijima A. Nano-manipulation of actomyosin molecular motors in vitro: a new working principle. Trends Biochem Sci. 1993 Sep;18(9):319–324. doi: 10.1016/0968-0004(93)90064-t. [DOI] [PubMed] [Google Scholar]


