Abstract
γ-Aminobutyric acid (GABA) plasma membrane transporters (GATS) influence synaptic neurotransmission by high-affinity uptake and release of GABA. The distribution and cellular localization of GAT-1, GAT-2, and GAT-3 in the rat retina have been evaluated by using affinity-purified polyclonal antibodies directed to the C terminus of each of these GAT subtypes. Small GAT-1–immunoreactive cell bodies were located in the proximal inner nuclear layer (INL) and ganglion cell layer (GCL), and processes were distributed to all laminae of the interplexiform layer (IPL). Varicose processes were in the optic fiber layer (OFL) and the outer plexiform layer (OPL). Weak GAT-1 immunostaining surrounded cells in the INL and GCL, and it was found in the OFL and OPL and in numerous processes in the outer nuclear layer (ONL) that ended at the outer limiting membrane. GAT-1 is therefore strongly expressed by amacrine, displaced amacrine, and interplexiform cells and weakly expressed by Müller cells. GAT-2 immunostaining was observed in the retina pigment epithelium and the nonpigmented ciliary epithelium. GAT-3 immunoreactivity was distributed to the OFL, to all laminae of the IPL, GCL and INL, and to processes in the ONL that ended at the outer limiting membrane. Small GAT-3–immunoreactive cell bodies were in the proximal INL and GCL. GAT-3 is therefore strongly expressed by Müller cells, and by some amacrine and displaced amacrine cells. Together, these observations demonstrate a heterologous distribution of GATs in the retina. These transporters are likely to take up GABA from, and perhaps release GABA into, the synaptic cleft and extracellular space. This suggests that GATs regulate GABA levels in these areas and thus influence synaptic neurotransmission.
Indexing terms: neurotransmitter transporter, amacrine cells, retina pigment epithelium, nonpigmented ciliary epithelium, glia cells
The action of γ-aminobutyric acid (GABA), the predominant inhibitory neurotransmitter of the nervous system, is regulated by specific GABA transporters (GATS) located in the plasma membrane of neurons and glial cells (Iversen and Kelly, 1975; Schousboe, 1981; Wood and Sidhu, 1987; Kanner and Bendahan, 1990; Radian et al., 1990; Brecha and Weigmann, 1994; Minelli et al., 1995; Ribak et al., 1996). These transporters exhibit high-affinity uptake for GABA and require Na+ and Cl− for transport. GATs have a number of functions that influence GABA neurotransmission (Iversen and Kelly, 1975; Kanner and Shuldiner, 1987; Isaacson et al., 1993; Mager et al., 1993). They terminate GABA neurotransmission by the uptake of GABA into neuronal or glial processes. The uptake of GABA into presynaptic processes allows for its subsequent concentration and storage in synaptic vesicles. In neurons and glial cells, GABA is rapidly metabolized to glutamate by GABA aminotransferase (Nagai et al., 1984). GATs may also release GABA into the synaptic cleft and extracellular space following membrane depolarization in a Ca2+-independent, nonvesicular manner (Schwartz, 1982, 1987; see Attwell et al., 1993; Levi and Raiteri, 1993, for reviews).
Three GATs have been isolated from the nervous system. They are designated GAT-1, GAT-2, and GAT-3 (Guastella et al., 1990; Nelson et al., 1990; Borden et al., 1992, 1994; Clark et al., 1992; Liu et al., 1993). A betaine glycine transporter (BGT-1), which also transports GABA with high-affinity, has been cloned from a Madin-Darby canine kidney cell line, and from mouse and human brain (Lopez-Corcuera et al., 1992; Yamauchi et al., 1992; Liu et al., 1993; Borden et al., 1995). The GATs and BGT-1 are members of a larger gene family, and they have similar structural features including 50–70% identity in their predicted amino acid sequence (see Amara and Kuhar, 1993; Schloss et al., 1994, for reviews). However, these transporters have distinct pharmacological properties and a differential distribution along the neuroaxis (Guastella et al., 1990; Brecha et al., 1992, 1995a; Borden et al., 1992; Clark et al., 1992; Keynan et al., 1992; Liu et al., 1993; Ikegaki et al., 1994; Durkin et al., 1995). In cortex and hippocampus, GAT-1 immunoreactivity is predominantly localized to preterminal axonal processes and terminals, and GAT-3 immunoreactivity is localized to astrocytic processes (Minelli et al., 1995, 1996; Ribak et al., 1996). In contrast, GAT-2 immunoreactivity and mRNA are found in the leptomeninges (Ikegaki et al., 1994; Brecha et al., 1995a; Durkin et al., 1995). Together these observations indicate that GABA transporters have distinct functional roles in the nervous system, including a role in synaptic transmission.
The retina has Na+-dependent high-affinity GABA uptake systems (Starr and Voaden, 1972; Goodchild and Neal, 1973; Iversen and Kelly, 1975; Neal, 1976; Redburn, 1977; see Yazulla, 1986, for review). The kinetic and pharmacology properties of these uptake systems are complex, and both neurons and Müller cells are reported to take up GABA and GABA analogs (Neal and Iversen, 1972; Brunn and Ehinger, 1974; Bruun et al., 1974; Marshall and Voaden, 1974; Iversen and Kelly, 1975; Ehinger, 1977; Neal and Bowery, 1977; Bauer and Ehinger, 1978; Cunningham et al., 1981; Agardh and Ehinger, 1982, 1983; Blanks and Roffler-Tarlov, 1982). High-affinity GABA uptake has also been reported in mouse retina pigment epithelium (RPE) (Blanks and Roffler-Tarlov, 1982) and in bovine RPE homogenates (Sivakami et al., 1992). Recent studies using polymerase chain reaction and Northern blot analysis indicate the presence of GAT-1, GAT-2, and GAT-3 mRNAs in retinal homogenates (Borden et al., 1992; Brecha and Weigmann, 1994). In situ hybridization studies demonstrate that GAT-1 mRNA is predominantly expressed by amacrine and ganglion cells, and weakly expressed by Müller cells (Brecha and Weigmann, 1994; Ruiz et al., 1994; Durkin et al., 1995). GAT-3 mRNA has also been mainly localized to cells in the inner nuclear layer (INL; Durkin et al., 1995; Johnson and Brecha, unpublished observations). Together these findings support the existence of multiple GABA transporters in the retina. However, their cellular expression patterns are poorly understood.
The present investigation describes the distribution and cellular localization of three GABA plasma membrane transporters, GAT-1, GAT-2, and GAT-3, in the rat retina by using newly developed, affinity-purified polyclonal antibodies. This study reports the localization of GAT-1 and GAT-3 immunoreactivities in the retina and GAT-2 immunoreactivity in RPE and nonpigmented ciliary epithelium. Brief descriptions of preliminary observations have been presented in abstract form (Brecha et al., 1995a,b; Johnson et al., 1995).
MATERIALS AND METHODS
Tissue preparation
Adult Sprague-Dawley and Long-Evans rats of either sex weighing 180–250 g were used. They were fed and housed under regular conditions with a 12-hour light-dark schedule. Care and handling of animals were approved by the Animal Research Committee of the VAMC-West Los Angeles in accordance with NIH guidelines.
Rats were deeply anesthetized with 30% chloral hydrate and perfused through the heart with 0.1 M phosphate-buffered saline (PBS; pH 7.4). In some cases, the PBS was followed by either 1) 2% or 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) or 2) 2% or 4% PFA with 1.37% polylysine and 0.214% sodium meta-periodate (PLP) in PB. The eyes were removed, the anterior segment was dissected, and the posterior eyecup containing the retina was immediately immersed in either the PFA or PLP fixative. The eyecup was stored in fixative for 1 to 2 hours at room temperature and then overnight in 25% sucrose in 0.1 M PB at 4°C. Sections of the retina were cut perpendicular or parallel to the vitreal surface with a cryostat or sliding microtome. Cryostat sections were cut at 12–16 μm, mounted onto gelatin-coated slides, air dried, and stored at −20°C. Sliding microtome sections were cut at 20–25 μm and stored in 0.1 M PB at 4°C.
Antibodies
Specific polyclonal antibodies were developed to the C-terminus of rat GAT-1, rat GAT-2, and rat GAT-3 (Guastella et al., 1990; Borden et al., 1992; Clark et al., 1992). The synthetic peptides, rat GAT-1588–599, rat GAT-2594–602, and rat GAT-3607–627, were coupled to keyhole limpet hemocyanin (KLH) with glutaraldehyde and dialyzed over 2–3 days in 0.15 M NaCl (Sternini and Brecha, 1986). For the initial immunization, 100 nmoles of the synthetic peptide conjugated to KLH were emulsified with complete Freund’s adjuvant (1:1). In subsequent booster immunizations, 50 nmoles of peptide conjugated to KLH were emulsified with incomplete Freund’s adjuvant (1:1). Two milliliters of the conjugate-Freund’s mixture was administered by multiple (20–30) intradermal and subcutaneous injections into the back of each rabbit. Plasma was harvested at regular intervals after each booster injection, and sera were tested for specific immunostaining. Selected crude sera were affinity purified by using either an Epoxy-Sepharose (Pharmacia Biotech, Piscataway, NJ) rat GAT-1, GAT-2, or GAT-3 C-terminal peptide affinity column that was prepared following the manufacturer’s instructions. Antibodies were eluted from the column with 3 M potassium thiocyanate, collected, and concentrated by 5K centrifugation with Centriprep-30 (Amicon, Beverly, MA). The antibodies were stored with 1%bovine serum albumin (BSA) and 0.1% sodium azide.
A rabbit polyclonal antiserum directed against the retinoid, cellular retinal-binding protein (CRALBP; Bunt-Milam and Saari, 1983) was used to identify Müller cells and the RPE. Specificity of this antiserum was previously demonstrated by preadsorption tests using CRALBP with both immunohistochemistry and Western blots (Bunt-Milam and Saari, 1983).
Immunohistochemistry
In initial studies, cryostat and free-floating sections were washed in 0.1 M PB and then pretreated with 1%sodium borohydrate in 0.1 M PB, 0.1 M glycine in 0.1 M PB, or 1% BSA in 0.1M PB followed by immunohistochemical processing. In subsequent studies, sections were washed in 0.1 M PB before immunohistochemical processing. Sections were incubated 12–48 hours in primary antibody containing 10% normal goat serum and 0.5%Triton X-100 at 4°C, and then washed in 0.1 M PB. Sections used for immunofluoresence studies were incubated in affinity-purified goat anti-rabbit IgG fluorescein isothiocyanate (FITC) or goat anti-mouse IgG FITC (American Qualex, La Mirada, CA or Jackson Immuno Labs, West Grove, PA) at a 1:50 dilution for 2 hours at room temperature or overnight at 4°C. Sections used for avidin-biotin peroxidase studies were incubated in affinity-purified biotinylated goat anti-rabbit IgG (Vector, Burlingame, CA) at a dilution of 1:100 for 2 hours at room temperature, washed in 0.1 M PB, and then incubated in avidin-biotin-peroxidase complex (ABC, Vector, Burlingame, CA) for 2 hours at room temperature. Sections were washed and incubated in 50–100 mg 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) in 0.1 M Tris for 5–10 minutes followed by DAB with 0.03% H2O2 for 5–10 minutes. Sections were mounted if free floating. Sections were coverslipped with a glycerol-phosphate or carbonate buffer containing 2% potassium iodide to retard fading for immunofluorescence studies, or coverslipped with Accu mount 60 (Baxter, McGaw Park, IL) for avidin-biotin-peroxidase studies.
Specificity of the antibody immunostaining was assessed by omitting the primary antibody, using preimmune serum in place of primary antibody, and by preadsorbing the antibody with synthetic peptides directed to the C-termini of known rodent GABA and glycine plasma membrane transporters (Table 1; Guastella et al., 1990, 1992; Borden et al., 1992; Liu et al., 1993).
TABLE 1.
Comparison of the C Terminus of GABA and Glycine Transporters1
| Rat GAT-1 588–599 | |
|---|---|
| Rat GAT-1 | Q A G S S A S K E A Y I |
| Rat GAT-2 | S L L R L T E L E S N C |
| Rat GAT-3 | T I S A I T E K E T H F |
| ms BET/GAT-2 | Q E L I A W E K E T H L |
| Rat GLYT-1 | S N G S S R L Q D S A I |
|
| |
| Rat GAT-2 594–602 | |
|
| |
| Rat GAT-2 | R L T E L E S N C |
| Rat GAT-1 | S S A S K E A Y I |
| Rat GAT-3 | A I T E K E T H F |
| ms BET/GAT-2 | I A W E K E T H L |
| Rat GLYT-1 | S S R L Q D S R I |
|
| |
| Rat GAT-2 607–627 | |
|
| |
| Rat GAT-3 | C E A K V K G D G T I S A I T E K E T H F |
| Rat GAT-1 | R P E N G P E Q P Q A G S S A S K E A Y I |
| Rat GAT-2 | L T S P A T P M T S L L R L T E L E S N C |
| ms BET/GAT-2 | Q N C S S S P A K Q E L I A W E K E T H L |
| Rat GLYT-1 | D K A Q I P I V G S N G S S R L Q D S R I |
Two or more common amino acids are in bold
GAT-1–and GAT-3–immunoreactive cell bodies were measured from horizontal sections processed by the ABC technique by using a Leitz Orthoplan and an eye piece reticule at ×1,000.
Confocal microscopy
GAT-1–and GAT-3–immunoreactive cell bodies were evaluated in horizontal sections of the retina processed by the immunofluorescence technique. Immunoreactive cells were examined using a Zeiss Axovert with a PlanApo × 100 1.4 objective and a Zeiss Laser Scanning Microscope 410 with a krypton/argon laser. Optical sections were taken with a z-axis resolution of 1 μm through the immunolabeled cell bodies. Images were collected with a magnification zoom of × 1.5. Images were processed and labeled using Adobe Photoshop 3.0.5 (Adobe Systems, Inc., Mountain View, CA).
RESULTS
Specificity of the antibodies
Initial studies showed that pretreatment of the sections with 1% sodium borohydrate, 0.1 M glycine, or 1% BSA in 0.1 M PB did not affect the immunostaining pattern for any of the antibodies. Furthermore, no differences in immunostaining patterns were observed in sections fixed with 2% PFA, 4% PFA, 2% PLP, or 4% PLP. No differences in immunostaining patterns were observed in the retinas of Sprague-Dawley albino and Long-Evans rats.
GAT-1 antibodies 341F, 346J, and 346M showed identical and specific immunostaining of the retina. GAT-1 immunostaining was prevented by preadsorption of the antibody with 10−5 M and 10−6 M rat GAT-1588–599, whereas preadsorption with 10−5M rat GAT-2594-602, rat GAT-3607–627, rat glycine plasma membrane transporter-1 (GLYT-1)625–633, or mouse GAT-2/BGT-1596–614 synthetic peptides did not affect immunostaining (Fig. 1).
Fig. 1.
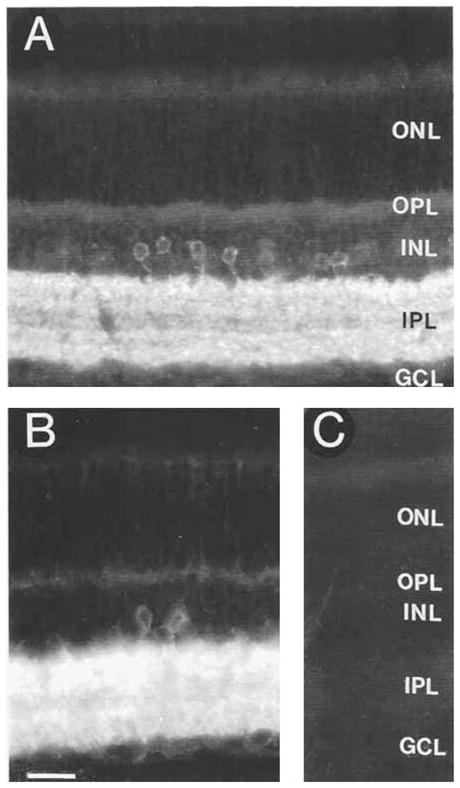
γ-aminobutyric acid plasma membrane transporter-1 (GAT-1) immunoreactivity in the rat retina. Strong GAT-1immunostaining is in cell bodies in the proximal inner nuclear layer (INL) and in processes distributed to all regions of the inner plexiform layer (IPL). The immunoreactive pattern indicates that GAT-1 is predominantly expressed by amacrine cells. A: GAT-1 immunoreactivity in a section incubated with the GAT-1 antibody preadsorbed with 10−5 M GAT-3607–627. Immunostaining is comparable to that observed with GAT-1 antibodies alone. B: Section incubated with GAT-1 antibody. C: Lack of GAT-1 immunostaining in a control section incubated with the GAT-1 antibody preadsorbed with 10−5 M GAT-1588–599. ONL, outer nuclear layer; OPL, outer plexiform layer; GCL, ganglion cell layer. Scale bar = 25 μm.
GAT-2 antibodies 363E and 365E showed identical and specific immunostaining of the RPE and nonpigmented ciliary epithelium. GAT-2 immunostaining was prevented by preadsorption with 10−5 M rat GAT-2594–602 (Fig. 8B), whereas preadsorption with 10−5 M rat GAT-1588–599, rat GAT-3607–627, rat GLYT-1625–633, or mouse GAT-2/BGT-1596–614 had no effect on immunostaining.
Fig. 8.
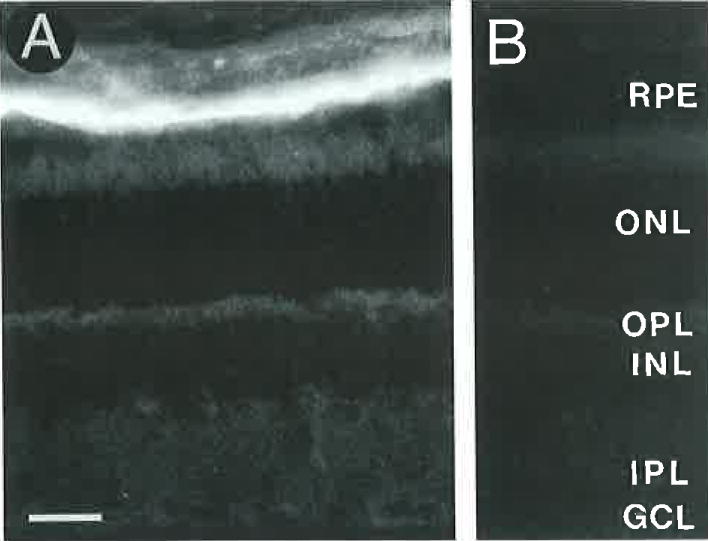
A: Localization of GAT-2 immunoreactivity to the retina pigment epithelium (RPE). The GAT-2 immunolabeling pattern appears to be identical with the cellular retinal-binding protein (CRALBP) immunolabeling of the RPE (see Fig. 14). B: Lack of GAT-2 immunostaining in a control section incubated with the GAT-2 antibody preadsorbed with 10−5 M GAT-2594-602. Scale bar = 25 μm.
GAT-3 antibodies 369D and 374E showed identical and specific immunostaining of the retina. GAT-3 immunostaining was prevented by preadsorption with 10−5 M rat GAT-3607–627, whereas preadsorption with 10−5 M rat GAT-1588–599, rat GAT-2594–602, rat GLYT-1625–633, or mouse GAT-2/BGT-1596–614 synthetic peptides did not affect immunostaining (Fig. 10C).
Fig. 10.
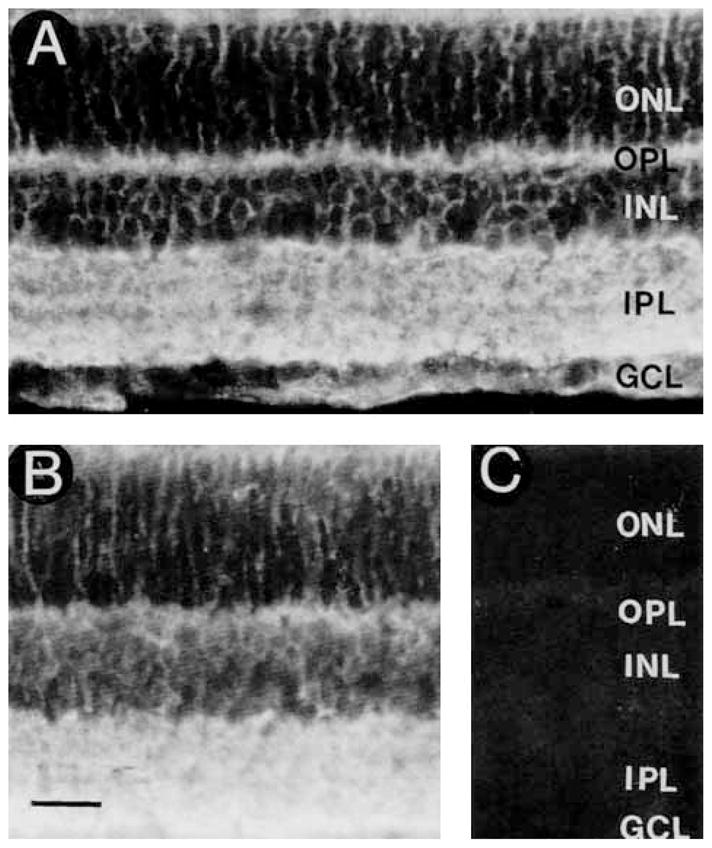
A: GAT-3 immunoreactivity is distributed to all regions of the retina. The immunostaining pattern indicates that it is predominantly expressed by Müller cells. B: No change in GAT-3 immunostaining in a section incubated with GAT-3 antibody preadsorbed with 10−5 M GAT-1588–599. C: Lack of GAT-3 immunostaining in a control section incubated with the GAT-3 antibody preadsorbed with 10−5 M GAT-3607–627. Scale bar = 25 μm.
GAT-1
The strongest immunostaining was observed in the inner plexiform layer (IPL) (Figs. 1, 2). Immunostaining was prominent in the proximal inner nuclear layer (INL) where it was mainly localized to small cell bodies (Figs. 1, 2). Weak immunostaining was observed around cell bodies in the INL and ganglion cell layer (GCL) and in the optic fiber layer (OFL), which is located between the GCL and vitreous, and in the outer retina (Fig. 2). At high antibody concentrations, the immunostaining was more easily visualized in the OFL, outer plexiform layer (OPL), and outer nuclear layer (ONL) (Fig. 2). GAT-1 immunoreactivity was not detected in the RPE and the ciliary body.
Fig. 2.

GAT-1 immunostaining in a section incubated with a high concentration of GAT-1 antibody to better visualize the immunostaining in the ONL and in the optic fiber layer (OFL), which is just below the GCL. GAT-1–immunoreactive cell bodies are mainly found in the proximal INL. Strong GAT-1 immunoreactivity is found in the IPL. Weak-to-moderate GAT-1 immunoreactivity is localized to Müller cell processes in the OFL and to processes (arrows) in the ONL that terminate at the outer limiting membrane (OLM). GAT-1–immunoreactive Müller cell endfeet are in the OFL. Abbreviations as in Figure 1. Scale bar = 25 μm.
In all retinal regions, specific GAT-1 immunoreactivity was expressed by numerous small ovoid cell bodies that were in the proximal INL and GCL (Figs. 1,2,3,4,5,6). In the proximal INL, immunostained cell bodies were usually in the first two or three cellular rows adjacent to the IPL. They usually gave rise to single primary processes which entered the IPL. Strong immunostaining outlined amacrine cell bodies. The predominant plasma membrane localization of GAT-1 immunoreactivity was further established using confocal microscopy (Fig. 5). Immunostained cells in the proximal INL measured 8.6 ± 0.06 μm (X ± SD, N = 25) in diameter in horizontally sectioned retinas processed by the avidin-biotin-peroxidase technique. GAT-1–immunostained cells in the GCL were of similar size to those in the INL (Figs. 3, 4). Medium and large GAT-1–immunoreactive cell bodies were not observed in the GCL. On the basis of cell body size, location, and appearance, the immunoreactive cells are amacrine and displaced amacrine cells.
Fig. 3.
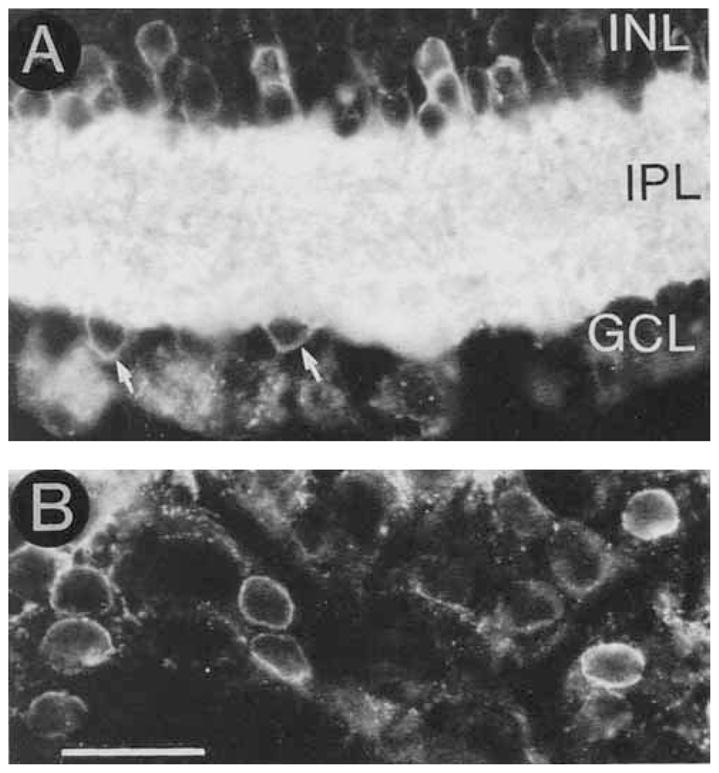
GAT-1 immunoreactivity in the INL and GCL. A: Transverse section of the retina illustrating small GAT-1–immunoreactive cell bodies in the proximal INL and GCL. Arrows indicate GAT-1–containing displaced amacrine cells. B: Horizontal section of the retina through the GCL. GAT-1 immunostaining is predominantly localized to the plasma membrane of many small cell bodies. These small cells are displaced amacrine cells. Some diffuse immunostaining of Müller cell processes between these cells can he seen in this figure. Scale bar = 25 μm.
Fig. 4.

GAT-1–immunoreactive amacrine cell bodies in a horizontal section through the proximal INL. Strong GAT-1 immunostaining is predominantly localized to the plasma membrane of the immunolabeled amacrine cell bodies. Horizontal section through the proximal INL. Scale bar = 25 μm.
Fig. 5.
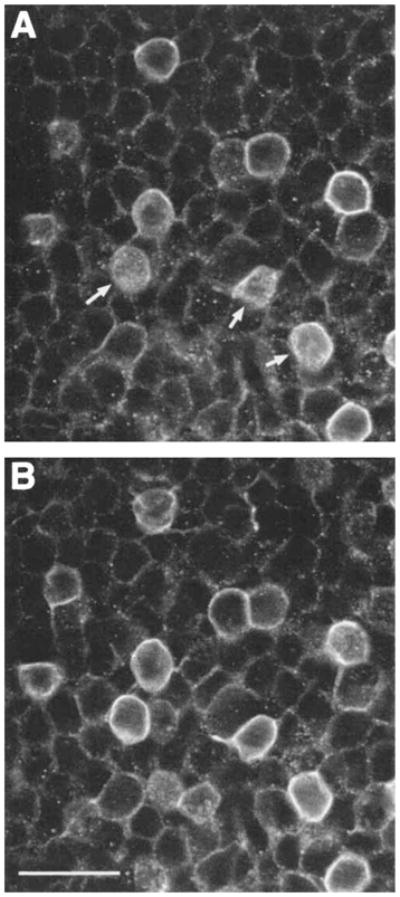
Confocal images of GAT-1–immunoreactive amacrine cell bodies in a horizontal section through the proximal INL. A and B are adjacent 1-μm optical sections that show the predominant localization of GAT-1 to the plasma membrane. The plasma membrane of several cells (arrows) are sectioned tangenital to the plane of focus. Scale bar = 20 μm.
Fig. 6.
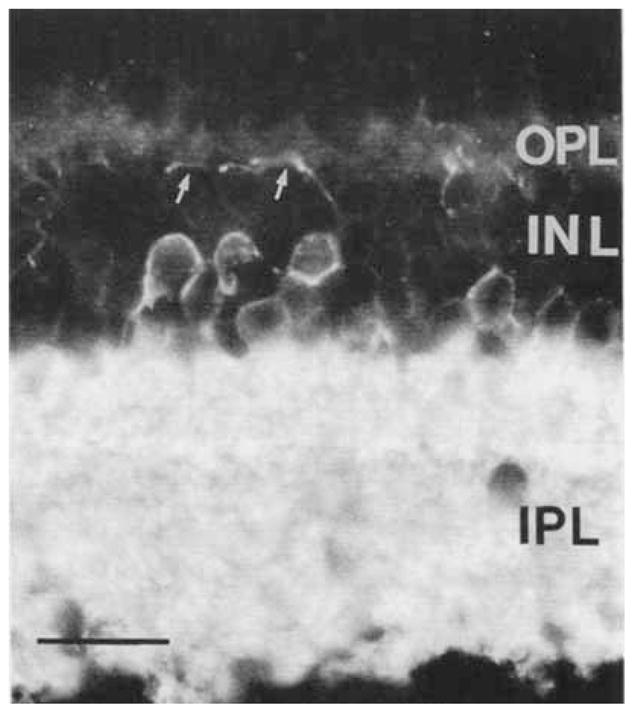
GAT-1–immunoreactive amacrine and interplexiform cell bodies. The GAT-1–immunoreactive interplexiform cell body is located in the INL, and it gives rise to a process (arrows) that crosses the distal INL and ramifies in the OPL. Scale bar = 25 μm.
Immunoreactive cell bodies were not found in the distal INL or ONL. It was not possible to determine if cell bodies in the IPL expressed GAT-1 immunoreactivity because of the high density of immunoreactive processes in the IPL (Figs. 1, 2, 6).
Immunoreactivity was quite abundant in the IPL, and it was characterized by intensely stained puncta, varicosities, and processes (not shown). In horizontal sections, some of the processes were observed to transverse large regions of the IPL. There were differences in the density of immunostaining in different regions of the IPL (Fig. 1A). The distal and proximal regions of the IPL were the most densely immunostained when low antibody concentrations were used to stain the retina. However, overall the lamination pattern was difficult to evaluate because of strong immunostaining in the IPL.
In the OPL, GAT-1–immunostained processes were visualized in both transverse and horizontal sections (Figs. 6, 7). These fibers were observed to originate from immunostained cell bodies in the proximal INL (Fig. 6). Immunostained fibers were characterized by varicosities and occasional small branches. These fibers often branched several times in the OPL before ending in the OPL (Fig. 7). Immunostained fibers were observed in all retinal regions, and they formed a sparse network in the OPL. This immunostaining pattern indicates the presence of interplexiform cells.
Fig. 7.
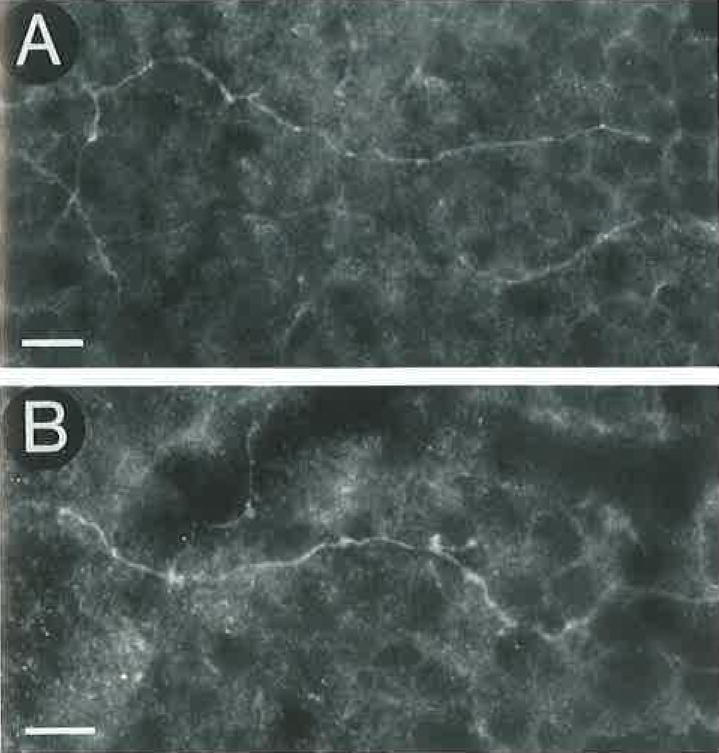
A,B: GAT-1–immunoreactive processes in the OPL. GAT-1–immunostained processes are found in all retinal regions. These fibers are characterized by numerous varicosities and occasional small branches. Horizontal sections through the OPL. Scale bar = 10 μm.
In the OFL there were rare varicose GAT-1–immunoreactive fibers. Immunoreactive fibers were not present in the optic nerve head.
Finally, weak-to-moderate GAT-1 immunostaining was also observed in the OFL, around cell bodies in the GCL and INL, in the OPL, and in Müller cell processes in the ONL (Figs. 2, 3, 5). In the OFL, immunoreactivity was diffuse and localized to Müller cell endfeet. In the GCL and INL, weak immunoreactivity surrounded unlabeled and labeled cell bodies. In the OPL there was weak and diffuse immunoreactivity. In the ONL, immunostained processes were oriented perpendicular to the vitreal surface, and they extended from the OPL through the ONL to end at the outer limiting membrane (Fig. 2). Immunostaining appeared to be continuous at the outer limiting membrane. Together, these observations indicate that GAT-1 is weakly expressed by Müller cells.
Overall, these findings provide evidence that amacrine, displaced amacrine, and interplexiform cells are the predominant cell types that express GAT-1. In addition, GAT-1 is weakly expressed by Müller cells.
GAT-2
GAT-2 immunostaining was prominent in the RPE (Fig. 8) and the nonpigmented ciliary epithelium (Fig. 9). All cells in these epithelia were immunostained. Immunoreactivity in the nonpigmented ciliary epithelium was more prominent than in the RPE at similar antibody dilutions. In the RPE, the appearance of GAT-2 immunoreactivity was similar to the appearance of CRALBP immunoreactivity (Fig. 14). GAT-2 immunoreactivity was not observed in the neural retina or in the pigmented ciliary epithelium and ciliary body stroma.
Fig. 9.

A,B: GAT-2 immunoreactivity in the nonpigmented ciliary epithelium. GAT-2 immunoreactivity is not observed in the pigmented ciliary epithelium or the ciliary body stroma. GAT-2 immunostaining appears to mainly be localized to the plasma membrane on the basolateral side of the nonpigmented ciliary epithelium. Scale bar = 25 μm.
Fig. 14.
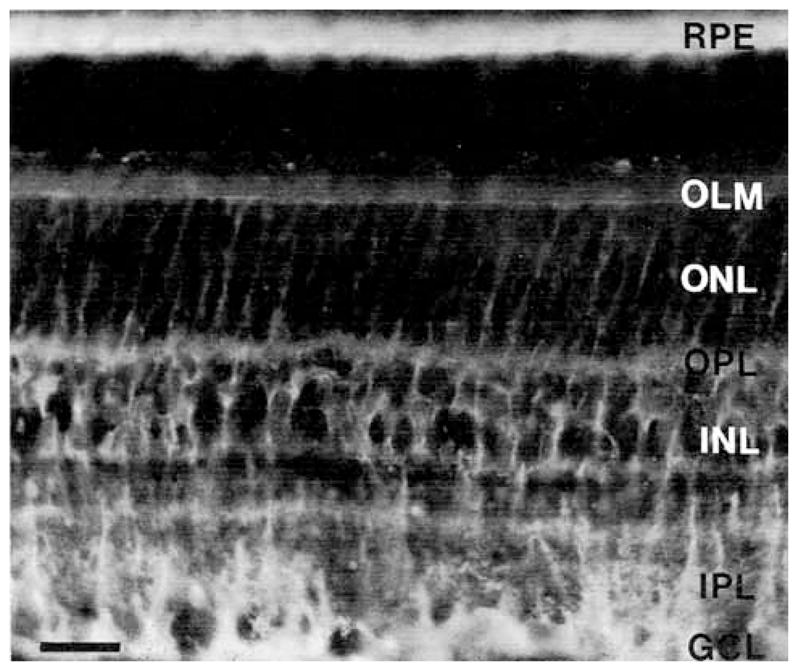
Localization of cellular retinal-binding protein (CRALBP) immunostaining to Müller cells and the retinal pigment epithelium (RPE). Immunoreactivity is prominent in the OFL just below the GCL, in processes in the IPL and ONL and at the OLM. Immunoreactive cell bodies are in the INL. Abbreviations as in Figures 1 and 2. Scale bar = 25 μm.
GAT-2 immunostaining appeared to be mainly localized to the plasma membrane of the basolateral surface, and not to the apical surface of these epithelia. However, a more detailed evaluation using thin sections and electron microscopy is necessary to establish if GAT-2 is primarily or exclusively expressed on the basolateral surface of these epithelia.
GAT-3
GAT-3 immunoreactivity was abundant, and it completely surrounded all the cell bodies in the cellular layers (Figs. 10,11). Immunostaining was prominent in the OFL, OPL, and in processes in the ONL that ended at the outer limiting membrane, but it was not detected in the RPE and the ciliary body. This immunoreactive pattern indicates that GAT-3 is prominently expressed by Müller cells. In the IPL there was a dense plexus of immunoreactive processes. In the proximal INL and GCL, the plasma membrane of some small cell bodies was immunostained (Figs. 11, 12). This immunostaining pattern indicates that amacrine and displaced amacrine cells also express GAT-3.
Fig. 11.
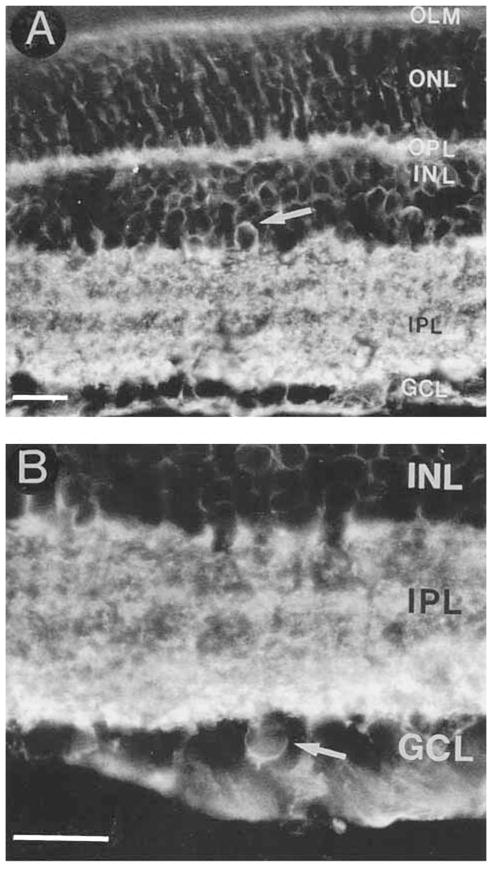
A: GAT-3 is predominantly expressed by Müller cells, but it is also expressed by some amacrine and displaced amacrine cells. A GAT-3–immunoreactive amacrine cell (arrow) in the proximal INL. B: A GAT-3–immunostained displaced amacrine cell (arrow) in the GCL. Abbreviations as in Figure 1. Scale bar = 25 μm.
Fig. 12.

GAT-3 immunoreactivity in a horizontal section through the proximal INL. A: GAT-3 immunostaining surrounds all cell bodies, and it is also localized to the plasma membrane of some amacrine cell bodies. B: Proximal INL adjacent to the IPL showing two GAT-3–immunoreactive cell bodies and the immunoreactive processes (arrows) of one of these cells. Overall, there are fewer GAT-3–immunoreactive amacrine cells than GAT-1–immunoreactive amacrine cells. Abbreviations as in Figure 1. Scale bar = 25 μm.
Immunostained cell bodies were usually located in the proximal INL at the border of the IPL, and these cells gave rise to processes that enter the IPL. GAT-3–immunoreactive amacrine cells occurred less frequently than GAT-1–immunoreactive amacrine cells at similar retinal locations. However, it was difficult to firmly establish the number of GAT-3–immunoreactive cells owing to the presence of pericellular GAT-3 immunoreactivity that often obscured the GAT-3 plasma membrane immunostaining. The predominant localization of GAT-3 immunoreactivity to the plasma membrane was confirmed by confocal microscopy (Fig. 13). GAT-3–immunoreactive cells in the INL measured 8.6 ± 0.06 μm (X ± SD; N = 26) in diameter in horizontally sectioned retinas processed by the avidin-biotin-peroxidase technique. Cells in the GCL were similar in size to the immunostained cells in the INL. Medium and large GAT-3–immunoreactive cell bodies were not seen in the GCL.
Fig. 13.

Confocal image of a GAT-3–immunoreactive amacrine cell body in a horizontal section through the proximal INL. One-micron optical section showing the predominant localization of GAT-3 to the plasma membrane of the cell body and its process. Scale bar = 20 μm.
GAT-3–immunoreactive cells were not observed in the distal INL or ONL. In addition, it was not possible to determine if cell bodies in the IPL expressed GAT-3 owing to the high density of immunoreactive processes in this layer.
In the inner retina, strong diffuse immunostaining was observed in the OFL just below the GCL, which was strongest at the vitreal surface (Figs. 10A, 11). Immunoreactivity was also strong in the IPL, and it was characterized by a high density of intensely stained puncta and varicosities (not shown). The highest densities of immunoreactivity were in the most distal and proximal regions of the IPL, corresponding best to laminae 1 and 5 of the IPL. In addition, a high density of immunostaining was in the middle of the IPL, corresponding best to lamina 3 of the IPL. The lowest density of immunostaining corresponded best to laminae 2 and 4 of the IPL. The laminar distribution of immunoreactivity was best seen when low antibody concentrations were used to stain the retina. GAT-3–immunoreactive fibers were not observed in the optic nerve head.
In the outer retina, diffuse GAT-3 immunostaining was observed in the OPL (Figs. 10, 11A). Immunoreactive processes were not observed in either transverse or horizontal sections. In the ONL, numerous strongly immunostained processes had a radial orientation and extended from the OPL to the outer limiting membrane. GAT-3 immunostaining was prominent at the outer limiting membrane, and immunostained processes extended into the interphotoreceptor space.
CRALBP antibodies were used to aid in the evaluation of the GAT-3–immunoreactive pattern, as CRALBP immunoreactivity is restricted to Müller cells and RPE and is not expressed by neurons (Bunt-Milam and Saari, 1983). Like earlier observations, there was strong CRALBP immunostaining in Müller cell bodies in the middle of the INL and in Müller cell processes in both the inner and outer retina. Müller cell endfeet were well stained in the OFL, and immunoreactivity extended to the vitreal surface (Fig. 14). In the IPL, immunoreactive processes were radially oriented, and nonimmunostained regions were present between the processes. Immunostaining was strongest in laminae 1, 3, and 5 of the IPL. In the outer retina, diffuse immunostaining was in the OPL. In the ONL, CRALBP-immunoreactive processes had a radial orientation and ended at the outer limiting membrane.
GAT-3 and CRALBP immunostaining patterns closely matched in the OFL and the outer retina. However, there was a poor match of the immunostaining patterns in the inner retina. Specifically, GAT-3 was observed in some small cell bodies in the proximal INL and GCL, and dense immunoreactivity was in all IPL laminae (Figs. 10–12). In contrast, CRALBP immunoreactivity was not observed in cell bodies in the proximal INL, but in cell bodies in the middle of the INL (Figs. 14). Furthermore, CRALBP immunoreactivity was mainly confined to radially oriented processes and several laminae in the IPL.
Overall, these findings indicate that Müller cells are the predominant cell type to express GAT-3. In addition, the presence of immunoreactive cells in the proximal INL and GCL and the dense GAT-3 immunostaining in all IPL laminae suggest that amacrine and displaced amacrine cells also express GAT-3.
DISCUSSION
These investigations showed that the GABA plasma membrane transporters GAT-1, GAT-2, and GAT-3 are expressed in the rat retina. GAT-1 and GAT-3 immunoreactivities were localized to amacrine, interplexiform, and Müller cells. GAT-2 immunoreactivity was localized to epithelial cells. These findings extend earlier investigations that have indicated the existence of multiple Na+ and C1−-dependent high-affinity GABA uptake systems in the rodent retina (Neal and Iversen, 1972; Starr and Voaden, 1972; Goodchild and Neal, 1973; Bruun and Ehinger, 1974; Marshall and Voaden, 1974; Iversen and Kelly, 1975; Neal, 1976; Blanks and Roffler-Tarlov, 1982; Borden et al., 1992; Brecha and Weigmann, 1994). The present findings thus suggest that multiple GABA transporters regulate GABA levels in the retina.
The overall distribution and cellular localization of GAT-1 and GAT-3 immunoreactivities are similar to previous descriptions of GABA and GAD immunoreactivity in the rat retina. GABA and GAD immunostaining has been reported in amacrine and interplexiform cells (Vaughn et al., 1981; Brandon, 1985; Mosinger et al., 1986; Versaux-Botteri et al., 1989) as well as in Müller cells (Neal et al., 1989). Furthermore, the lack of GAT-1, GAT-2, or GAT-3 immunoreactivity in horizontal cells indicates that these cells in the adult rodent retina are not likely to have a high-affinity uptake system for GABA. This conclusion is in agreement with previous GAT-1 and GAT-3 in situ hybridization and GABA uptake-autoradiographic findings (Neal and Iversen, 1972; Bruun and Ehinger, 1974; Blanks and Roffler-Tarlov, 1982; Brecha and Weigmann, 1994; Ruiz et al., 1994; Durkin et al., 1995). However, the possibility that these GATS or a novel GAT are transiently expressed by horizontal cells of the developing rodent and rabbit retina (Schnitzer and Rusoff, 1984; Redburn and Madtes, 1986; Versaux-Botteri et al., 1989) and by the adult cat and primate retina (Grünert and Wässle, 1990; Koontz and Hendrickson, 1990; Vardi et al., 1994) remains an unresolved issue.
GAT-1 is expressed in neurons and Müller cells
GAT-1–immunoreactive cells are likely to be amacrine, displaced amacrine, and interplexiform cells based on their appearance, size, and distribution in the proximal INL and GCL, as well as the localization of their processes in the IPL and OPL. Immunoreactive interplexiform cells gave rise to a single process that crossed the INL and ramified in the OPL. A similar cell type also contains GABA and GAD immunoreactivity, and it is likely that these cells also express GAT-1 immunoreactivity (Brandon, 1985; Mosinger et al., 1986). Furthermore, the GAT-1–immunoreactive interplexiform cells are likely to be the same as the previously described tyrosine hydroxylase- (TH) immunoreactive cells, since TH-immunoreactive cells are located in the proximal INL, give rise to processes that ramify in the OPL, and contain GABA immunoreactivity (Nguyen-Legros et al., 1982; Foster et al., 1985; Wulle and Wagner, 1990). Finally, these findings extend earlier observations that GAT-1 mRNA is localized to amacrine and displaced amacrine cells (Brecha and Weigmann, 1994; Ruiz et al., 1994; Durkin et al., 1995).
The neuronal component of the GAT-1 immunostaining pattern is strikingly similar to the overall GABA and GAD immunostaining patterns, although interestingly it more closely matches in detail the GAD than the GABA immunostaining pattern in the IPL (Vaughn et al., 1981; Brandon, 1985; Mosinger et al., 1986; Versaux-Botteri et al., 1989). The prominent localization of GAT-1 immunoreactivity to neuronal processes in the retina is consistent with immunohistochemical and in situ hybridization studies of rat cortex and hippocampus which report a neuronal localization of GAT-1 and the presence of immunoreactivity on preterminal axons and axon terminals (Minelli et al., 1995; Ribak et al., 1996). In retina, GAT-1 immunoreactivity is also localized near synaptic profiles (Marshak, unpublished observations). Together, these findings indicate that GAT-1 participates in the uptake or release of GABA at synapses in the IPL and OPL.
GAT-1 immunoreactivity was also heavily localized to the plasma membrane of amacrine and interplexiform cell bodies. This localization is unique, because a somatic localization of GAT-1 immunoreactivity has not been observed elsewhere in the nervous system (Ikegaki et al., 1994; Minelli et al., 1995; Brecha et al., 1995a; Ribak et al., 1996). These findings suggest that GABA may also be accumulated or released from the cell bodies of these cells.
GAT-1 immunoreactivity was not detected in ganglion cell bodies or axons in the OFL and optic nerve head, although earlier studies reported GAT-1 mRNA and GABA immunoreactivity in ganglion cells (Caruso et al., 1989; Brecha and Weigmann, 1994). It is possible that GAT-1 mRNA-containing ganglion cells express GAT-1 at preterminal axons and axonal terminals in retinal-recipient nuclei in the central nervous system. The lack of GAT-1 immunoreactivity in ganglion cell bodies and dendrites and its presence in axon terminals would be congruent with general observations elsewhere in the nervous system (Ikegaki et al., 1994; Brecha et al., 1995a; Minelli et al., 1995; Ribak et al., 1996).
GAT-1 immunoreactivity is present in Müller cells, but at low levels, as these cells can be detected best using higher concentrations of antibody than that needed for the visualization of GAT-1 immunoreactivity in neurons. The GAT-1 immunostaining pattern is consistent with that expected for Müller cells based on other studies using antibodies to CRALBP (Bunt-Milam and Saari, 1983) and to glial fibrillary acidic protein or vimentin (Bignami and Dahl, 1979; Schnitzer, 1985). Furthermore, weak-to-moderate GAT-1 immunoreactivity in Müller cells is consistent with a previous report from our laboratory describing low levels of GAT-1 mRNA in Müller cells compared to adjacent neurons in dissociated retinal preparations (Brecha and Weigmann, 1994). Finally, the expression of GAT-1 immunoreactivity in Müller cells is consistent with other studies indicating the localization of this transporter to astrocytic processes in the cortex and hippocampus (Minelli et al., 1996; Ribak et al., 1996) and of GAT-1 mRNA to Bergmann glia of the rat cerebellum (Rattray and Priestley, 1993; Swan et al., 1994) and glia in the electromotor nucleus of Torpedo californica (Swanson et al., 1994). The localization of GAT-1 to Müller cells suggests that this transporter also functions to take up GABA from the extracellular space.
GAT-2 is expressed in epithelia
GAT-2 immunoreactivity was strongly expressed by the RPE and the nonpigmented epithelium of the ciliary body. GAT-2 appeared to be mainly localized to the basolateral surface of these epithelia. This localization extends an earlier uptake-autoradiographic study reporting GABA uptake by the RPE in mouse retina (Blanks and Roffler-Tarlov, 1982). An epithelial distribution of GAT-2 has also been observed in the brain where it is restricted to the pia and arachnoid (Ikegaki et al., 1994; Durkin et al., 1995; Brecha et al., 1995a).
A previous pharmacological study has described a highaffinity, Na+ and Cl−-dependent GABA carrier that is enriched in apical membrane vesicles prepared from bovine RPE (Sivakami et al., 1992). This carrier is characterized by high-affinity taurine uptake. Furthermore, excess taurine completely inhibits GABA uptake (Sivakami et al., 1992). This carrier is not likely to be GAT-2, because GABA uptake by COS cells transfected with GAT-2 is not inhibited by excess taurine (Borden et al., 1992).
The expression of a GABA transporter by the RPE and nonpigmented ciliary epithelium is unexpected, because the extracellular levels of GABA near these epithelia, especially the nonpigmented epithelium, are likely to be quite low. The present observations and the earlier GABA uptake study (Blanks and Roffler-Tarlov, 1982) suggest that GAT-2 takes up GABA from the extracellular space. Furthermore, considering the prominent level of expression of GAT-2 immunoreactivity and the organization and position of these epithelia, it seems likely that they form an effective barrier to the diffusion of GABA. Finally, GAT-2 may also transport other molecules or osmolytes between the RPE and choroid capillaries, or between the nonpigmented ciliary epithelium and vitreous, as this transporter has been demonstrated to take up a variety of amino acids (Borden et al., 1992; Liu et al., 1993).
GAT-3 is expressed in neurons and Müller cells
The diffuse distribution of GAT-3 immunostaining in all nuclear and plexiform layers from the inner limiting to the outer limiting membrane indicates that this transporter is principally expressed by Müller cells. Comparison of GAT-3 immunostaining with the immunostaining patterns of several Müller cell–selective markers (Bignami and Dahl, 1979; Bunt-Milam and Saari, 1983; Schnitzer, 1985) supports the interpretation that Müller cells are the predominant cell type expressing this transporter. This observation is also supported by the predominant localization of GAT-3 mRNA to the INL (Durkin et al., 1995; Johnson and Brecha, unpublished observation). It is likely that GAT-3 functions to accumulate GABA from the extracellular space. In addition, the abundance of GAT-3–containing Müller cell processes at the vitreal surface suggests that these cells could transport GABA between the retina and vitreous.
The presence of small GAT-3–immunoreactive cell bodies in the proximal INL and GCL, and of a dense plexus of processes in the IPL indicates that GAT-3 is also expressed by amacrine and displaced amacrine cells. In addition, the differences between the GAT-3 and CRALBP immunostaining patterns in the inner retina support this conclusion. Furthermore, the laminar distribution of GAT-3 immunoreactivity in the IPL is due to the presence of GAT-3–immunostained processes from amacrine, displaced amacrine, and Müller cells. The neuronal localization of GAT-3 suggests that this transporter is involved in the neuronal uptake or release of GABA at synaptic sites, as well as from the extracellular space, like the GAT-1 transporter.
The absence of GAT-3–immunoreactive processes in the OPL suggests that interplexiform cells do not express this transporter. It is not possible to determine if GAT-3 is expressed by some ganglion cells, from the present investigation. That is, the failure to visualize this transporter in medium and large cell bodies in the GCL as well as in processes in the OFL and optic nerve head suggests that GAT-3 is not expressed by ganglion cells, although it could be present in ganglion cell axonal terminals like GAT-1 in the forebrain (Minelli et al., 1995; Ribak et al., 1996). In addition, GABA-containing ganglion cells have been described (Caruso et al., 1989), and more definite experiments such as retrograde transport to identify ganglion cells with in situ hybridization to detect GAT-3 mRNA are needed to establish if these cells do or do not express this transporter.
The expression of GAT-3 immunoreactivity by Müller cells, and to a lesser extent by neurons, is consistent with other studies of the central nervous system. For instance, in cortex and hippocampus, GAT-3 immunoreactivity is localized exclusively to astrocytic processes (Brecha et al., 1995a; Minelli et al., 1996; Ribak et al., 1996). Furthermore, these observations are consistent with GABA uptake studies, which demonstrate that astrocytes in primary culture and heterologous cells transfected with GAT-3 have similar pharmacological properties (Clark and Amara, 1994). However, GAT-3 mRNA is also reported to be exclusively localized to neurons in the rat mesencephalon (Clark et al., 1992) and to both glia and neurons along the neuroaxis (Durkin et al., 1995). Together, these findings with the present observations provide evidence that GAT-3 is primarily expressed by astrocytes and that in some regions, such as the retina, it is also expressed by neurons.
Pharmacology of GATs in the neural retina
There are numerous studies reporting the presence of Na+-dependent, high-affinity uptake transporters for GABA in the rodent retina (Starr and Voaden, 1972; Goodchild and Neal, 1973; Marshall and Voaden, 1974; Iversen and Kelly, 1975; Neal, 1976; see Yazulla, 1986, for review). Most reports describe 1) the predominant accumulation of GABA by Müller cells using either in vivo or in vitro preparations (Neal and Iversen, 1972; Brunn and Ehinger, 1974; Marshall and Voaden, 1974) and 2) that GABA uptake is partially blocked by competitive GABA uptake inhibitors such as L-2,4-diaminobutyric acid (L-DABA) and β-alanine (Blanks and Roffler-Taylor, 1982). L-DABA and β-alanine are also taken up by both Müller and amacrine cells (Brunn and Ehinger, 1974; Bauer and Ehinger, 1978; Cunningham et al., 1981). In addition, an uptake study using the GABA analog cis-aminocyclohexane carboxylic acid (ACHC) reports the exclusive labeling of Müller cells (Cunningham et al., 1981), whereas another study using the GABA agonist isoguvacine reports the labeling of amacrine cells (Agardh and Ehinger, 1983). Finally, an uptake study using the GABA agonist muscimol only labels amacrine cells (Blanks and Roffler-Tarlov, 1982). These pharmacological observations from the rodent retina can be best accounted for by the coexpression of GAT-1 and GAT-3 by amacrine cells and by all Müller cells, because GABA uptake by GAT-1 is most sensitive to ACHC and L-DABA, and GABA uptake by GAT-3 is most sensitive to β-alanine, moderately sensitive to L-DABA, and insensitive to ACHC in heterologous cells transfected with either GAT-1 or GAT-3 cDNAs (Guastella et al., 1990; Borden et al., 1992; Clark et al., 1992; Liu et al., 1993). There is also low-affinity uptake of muscimol by heterologous cells transfected with GAT-1 (Ruiz et al., 1994).
However, there remain some differences between the observed and predicted uptake patterns of GABA and GABA analogs in the rat retina based on the expression of GAT-1 and GAT-3 in neurons and Müller cells. These include the labeling of amacrine cells and not Müller cells with isoguvacine (Agardh and Ehinger, 1983) or the exclusive labeling of Müller cells by ACHC when amacrine cells should presumably also be labeled (Cunningham et al., 1981). These discrepancies between observed and predicted uptake patterns may be due to several factors including the presence of diffusion barriers and gradients in the retina, the cellular localization and distribution of the GABA transporters, and the presence of other transporters that can take up GABA or its analogs.
Function of GATs in the retina
The ubiquitous distribution of GAT-1 and GAT-3 in the retina and of GAT-2 in the RPE allows for considerable regulation of GABA levels in the retina. For instance, the expression of GATs by Müller cells would markedly influence GABA levels in all retinal regions, as these cells span the entire neural retina and both GAT-1 and GAT-3 were localized to Müller cell processes. Perhaps these transporters regulate the levels of GABA in the extracellular space. Furthermore, these transporters could restrict the spread of GABA from GABA synapses (Isaacson et al., 1993). In contrast, GATs expressed by neurons are likely to both remove GABA from the synaptic cleft following depolarization and perhaps release GABA by a nonvesicular, Ca2+- independent mechanism. Transmitter uptake at the synapse would thus regulate the action of GABA at GABA synapses (Isaacson et al., 1993; Mager et al., 1993). There is also good evidence for carrier-mediated release of GABA by nonmammalian horizontal cells (Schwartz, 1982, 1987; Yazulla and Kleinschmidt, 1983; Ayoub and Lam, 1984; Kamermans and Werblin, 1992; Cammack and Schwartz, 1993). In addition, the acetylcholine- and GABA-containing starburst amacrine cells of the rabbit retina (Brecha et al., 1988) are likely to release GABA in a nonvesicular, Ca2+- independent manner following depolarization (O’Malley et al., 1992). The stoichiometry of GAT-1 transport has proven to be complex, and both uptake and release operate in an asymmetric and variable manner (Cammack et al., 1994). Finally, GAT-1 activity is regulated by protein kinase C and protein phosphatases (Corey et al., 1994; Tian et al., 1994). These represent other control points for GABA regulation.
In summary, the GATs have distinct distributions in the retina, with GAT-1 and GAT-3 located to Müller and neuronal cells, and GAT-2 located to surrounding epithelia. GAT-1 and GAT-3 likely take up and perhaps release GABA from neuronal terminals. These same transporters expressed in Müller cells may take up GABA from the extracellular space. Finally, the exclusive epithelial distribution of GAT-2 indicates a role in controlling GABA and osmolyte concentrations in the blood–retina and vitreous–retina boundaries.
Acknowledgments
We thank Duane Keith, Jr., Katherine Wen, Dennis Su. Doris Peter, and Dr. Giovanni Casini for their help. We thank Dr. C. Sternini for her helpful comments and discussions regarding this investigation. This work was supported by NIH grant EY 04067 and VA Medical Research Funds.
LITERATURE CITED
- Agardh E, Ehinger B. [3H]-muscimol, [3H]-nipecotic acid and [3H]-isoguvacine as autoradiographic markers for GABA neurotransmission. J Neural Transm Gen Sect. 1982;54:1–18. doi: 10.1007/BF01249274. [DOI] [PubMed] [Google Scholar]
- Agardh E, Ehinger B. Retinal GABA neuron labeling with [3H]-isoguvacine in different species. Exp Eye Res. 1983;36:215–229. doi: 10.1016/0014-4835(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Ayoub GS, Man-Kit Lam D. The release of γ-aminobutyric acid from horizontal cells of the goldfish (carassius auratus) retina. J Physiol (Land) 1984;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Ehinger B. Retinal uptake and release of [3H]DABA. Exp Eye Res. 1978;26:275–289. doi: 10.1016/0014-4835(78)90075-1. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28:63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Roffler-Tarlov S. Differential localization of radioactive gamma-aminobutyric acid and muscimol in isolated and in vivo mouse retina. Exp Eye Res. 1982;35:573–584. doi: 10.1016/s0014-4835(82)80071-7. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the γ-aminobutyric acid (GABA) transport system. J Biol Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Branchek TA, Gluchowski C, Weinshank RL. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Receptors & Channels. 1994;2:207–213. [PubMed] [Google Scholar]
- Borden LA, Smith KE, Gustafson EL, Branchek TA, Weinshank RL. Cloning and expression of a betaine/GABA transporter from human brain. J Neurochem. 1995;64:977–984. doi: 10.1046/j.1471-4159.1995.64030977.x. [DOI] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res. 1985;344:286–295. doi: 10.1016/0006-8993(85)90806-6. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminohutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994;345:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha NC, Sternini C, Humphrey MF. Cellular distribution of L-glutamate decarboxylase (GAD) and γ-aminobutyric acidA (GABAA) receptor mRNAs in the retina. Cell Mol Neurobiol. 1991;11:497–509. doi: 10.1007/BF00734812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha N, Weigmann C, Messersmith E. Expression of GABA transporter mRNA in the rat central nervous system. Soc Neurosci Abstr. 1992;18:475. [Google Scholar]
- Brecha N, Johnson D, Peichl L, Wässle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and γ-aminobutyrate immunoreactivity. Proc Natl Acad Sci USA. 1988;85:6187–6191. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha N, Johnson J, Chen T, Conti F, Minelli A, DeBiassi S, Ribak C. Presynaptic Mechanisms Neurotransmission. San Diego, USA: 1995a. GABA transporter expression in the rat nervous system Abstr; p. 124. [Google Scholar]
- Brecha N, Johnson J, Khuon T, Evans C, Rickman D. Multiple GABA plasma membrane transporters are expressed in the vertebrate retina. Invest Ophthalmol Vis Sci. 1995b;36:214. [Google Scholar]
- Bruun A, Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974;19:435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- Bruun A, Ehinger B, Forsberg A. In vitro uptake of β-alanine into rabbit retinal neurons. Exp Brain Res. 1974;19:239–247. doi: 10.1007/BF00233232. [DOI] [PubMed] [Google Scholar]
- Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J Physiol (Lond) 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Caruso DM, Owczarzak MT, Goebel DJ, Hazlett JC, Pourcho RG. GABA-immunoreactivity in ganglion cells of the rat retina. Brain Res. 1989;476:129–134. doi: 10.1016/0006-8993(89)91544-8. [DOI] [PubMed] [Google Scholar]
- Clark JA, Amara SG. Stable expression of a neuronal γ-aminobutyric acid transporter, GAT-3, in mammalian cells demonstrates unique pharmacological properties and ion dependence. Mol Pharmacol. 1994;46:550–557. [PubMed] [Google Scholar]
- Clark JA, Deutch AY, Gallipoli PZ, Amara SG. Functional expression and CNS distribution of a β-alanine-sensitive neuronal GABA transporter. Neuron. 1992;9:337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Corey JL, Davidson N, Lester HA, Brecha N, Quick MW. Protein kinase C modulates the activity of a cloned γ-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J Biol Chem. 1994;269:14759–14767. [PubMed] [Google Scholar]
- Cunningham J, Marshall J, Neal MJ. The radioautographical localization in the vertebrate retina of [3H]-(±)-cis-aminocyclohexane caroboxylic acid (ACHC); a selective inhibitor of neuronal GABA transport. Exp Eye Res. 1981;32:445–450. doi: 10.1016/s0014-4835(81)80023-1. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Glial and neuronal uptake of GABA, glutamatic acid, glutamine and glutathione in the rabbit retina. Exp Eye Res. 1977;25:221–234. doi: 10.1016/0014-4835(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Foster GA, Schultzberg M, Goldstein M, Hökfelt T. Differential ontogeny of three putative catecholamine cell types in the postnatal rat retina. Brain Res. 1985;354:187–196. doi: 10.1016/0165-3806(85)90170-1. [DOI] [PubMed] [Google Scholar]
- Goodchild M, Neal MJ. The uptake of 3H-γ-aminobutyric acid by the retina. Br J Pharmacol. 1973;47:529–542. doi: 10.1111/j.1476-5381.1973.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert U, Wässle H. GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990;297:509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Guastella J, Brecha N, Weigmann C, Lester HA, Davidson N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc Natl Acad Sci USA. 1992;89:7189–7193. doi: 10.1073/pnas.89.15.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaki N, Saito N, Hashima M, Tanaka C. Production of specific antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and their application to immunocytochemistry. Mol Brain Res. 1994;26:47–54. doi: 10.1016/0169-328x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Kelly JS. Uptake and metabolism of γ-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975;24:933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johnson J, Chen TK, Evans C, Rickman D, Brecha N. Multiple GABA plasma membrane transporters are expressed in the vertebrate retina. Soc Neurosci Abstr. 1995;21:2062. [Google Scholar]
- Kamermans M, Werblin F. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J Neurosci. 1992;12:2451–2463. doi: 10.1523/JNEUROSCI.12-07-02451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Bendahan A. Two pharmacologically distinct sodium- and chloride-coupled high-affinity γ-aminobutyric acid transporters are present in plasma membrane vesicles and reconstituted preparations from rat brain. Proc Natl Acad Sci USA. 1990;87:2550–2554. doi: 10.1073/pnas.87.7.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–39. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Keynan S, Suh YJ, Kanner BI, Rudnick G. Expression of a cloned γ-aminobutyric acid transporter in mammalian cells. Biochemistry. 1992;31:1974–1979. doi: 10.1021/bi00122a011. [DOI] [PubMed] [Google Scholar]
- Koontz MA, Hendrickson AE. Distribution of GABA-immunoreactive amacrine cell synapses in the inner plexiform layer of macaque monkey retina. Vis Neurosci. 1990;5:17–28. doi: 10.1017/s0952523800000043. [DOI] [PubMed] [Google Scholar]
- Levi G, Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993;16:415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Liu QR, López-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. J Biol Chem. 1993;268:2106–2112. [PubMed] [Google Scholar]
- Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. Expression of a mouse brain cDNA encoding novel γ-aminobutyric acid transporter. J Biol Chem. 1992;267:17491–17493. [PubMed] [Google Scholar]
- Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Marshall J, Voaden M. An investigation of the cells incorporating [3H]GABA and [3H]glycine in the isolated retina of the rat. Exp Eye Res. 1974;18:367–370. doi: 10.1016/0014-4835(74)90113-4. [DOI] [PubMed] [Google Scholar]
- Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Conti F. GAT-3, high affinity GABA plasma membrane transporter, is localized exclusively to astrocytic processes in the cerebral cortex. J Neurosci. 1996 doi: 10.1523/JNEUROSCI.16-19-06255.1996. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res. 1986;42:631–644. doi: 10.1016/0014-4835(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, Araki M, McGeer EB. GABA-T-intensive neurons in the rat brain. In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of Chemical Neuroanatomy, Volume 3: Classical Transmitters and Transmitter Receptors in the CNS, Part 11. New York: Elsevier; 1984. pp. 247–272. [Google Scholar]
- Neal MJ. The uptake and release of γ-aminobutyric acid (GABA) by the retina. In: Levi G, Battistin L, Lajtha A, editors. Transport Phenomena in the Nervous System: Physiological and Pathological Aspects. London: Plenum Press; 1976. pp. 211–220. [Google Scholar]
- Neal MJ, Bowery NG. Cis-3-aminocyclohexanecarboxylic acid: a substrate for the neuronal GABA transport system. Brain Res. 1977;138:169–174. doi: 10.1016/0006-8993(77)90793-4. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Iversen LL. Autoradiographic localization of 3H-GABA in rat retina. Nature New Biology. 1972;235:217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Cunningham JR, Shah MA, Yazulla S. Immunocytocbemical evidence that vigabatrin in rats causes GABA accumulation in glial cells of the retina. Neurosci Lett. 1989;98:39–32. doi: 10.1016/0304-3940(89)90368-6. [DOI] [PubMed] [Google Scholar]
- Nelson H, Mandiyan S, Nelson N. Cloning of the human brain GABA transporter. FEBS Lett. 1990;269:181–184. doi: 10.1016/0014-5793(90)81149-i. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Berger B, Vigny A, Alvarez C. Presence of interplexiform dopaminergic neurons in the rat retina. Brain Res Bull. 1982;9:379–381. doi: 10.1016/0361-9230(82)90148-4. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radian R, Ottersen OP, Storm-Mathisen J, Castel M, Kanner BI. Immunocytochemical localization of the GABA transporter in rat brain. J Neurosci. 1990;10:1319–1330. doi: 10.1523/JNEUROSCI.10-04-01319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray M, Priestly JV. Differential expression of GABA transporter-1 messenger RNA in subpopulations of GABA neurones. Nerosci Lett. 1993;156:163–166. doi: 10.1016/0304-3940(93)90463-u. [DOI] [PubMed] [Google Scholar]
- Redburn DA. Uptake and release of [14C]GABA from rabbit retina synaptosomes. Exp Eye Res. 1977;25:265–275. doi: 10.1016/0014-4835(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Redburn DA, Madtes P., Jr Postnatal development of 3H-GABA accumulating cells in the rabbit retina. J Comp Neurol. 1986;243:41–57. doi: 10.1002/cne.902430105. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WMY, Brecha NC. The GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Egal H, Sarthy V, Qian X, Sarkar HK. Cloning, expression, and localization of a mouse retinal γ-aminobutyric acid transporter. Invest Opthalmol Vis Sci. 1994;35:4039–4048. [PubMed] [Google Scholar]
- Schloss P, Püschel AW, Betz H. Neurotransmitter transporters: new members of known families. Curr Opin Cell Biol. 1994;6:595–599. doi: 10.1016/0955-0674(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Distribution and immunoreactivity of glia in the retina of the rabbit. J Comp Neurol. 1985;240:128–142. doi: 10.1002/cne.902400203. [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984;4:2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons and glial cells. Int Rev Neurobiol. 1981;22:l-45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Calcium-independent release of GABA from isolated cells of the toad retina. J Physiol (Lond) 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Sivakami S, Ganapathy V, Leibach FH, Miyamoto Y. The γ-aminobutyric acid transporter and its interaction with taurine in the apical membrane of the bovine retinal pigment epithelium. Biochem J. 1992;283:391–397. doi: 10.1042/bj2830391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr MS, Voaden MJ. The uptake of [14C]-γ-aminobutyric acid by the isolated retina of the rat. Vis Res. 1972;12:549–557. doi: 10.1016/0042-6989(72)90150-2. [DOI] [PubMed] [Google Scholar]
- Sternini C, Brecha N. Immunocytochemical identification of islet cells and nerve fibers containing calcitonin gene-related peptide-like immunoreactivity in the rat pancreas. Gastroenterology. 1986;90:1155–1163. doi: 10.1016/0016-5085(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Swan M, Najlerahim A, Watson REB, Bennett JP. Distribution of mRNA for the GABA transporter GAT-1 in the rat brain: evidence that GABA uptake is not limited to presynaptic neurons. J Anat. 1994;185:315–323. [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Umbach JA, Gundersen CB. Glia of the cholinergic electromotor nucleus of Torpedo are the source of the cDNA encoding a GAT-1-like GABA Transporter. J Neurochem. 1994;63:1–12. doi: 10.1046/j.1471-4159.1994.63010001.x. [DOI] [PubMed] [Google Scholar]
- Tian Y, Kapatos G, Granneman JG, Bannon MJ. Dopamine and γ-aminohutyric acid transporters: differential regulation by agents that promote phosphorylation. Neurosci Letts. 1994;173:143–146. doi: 10.1016/0304-3940(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Vardi N, Kaufman DL, Sterling P. Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Visual Neurosci. 1994;11:135–142. doi: 10.1017/s0952523800011172. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Famiglietti EV, Jr, Barber RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol. 1981;197:113–127. doi: 10.1002/cne.901970109. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Pochet R, Nguyen-Legros J. Immunohistochemical localization of GABA-containing neurons during postnatal development of the rat retina. Invest Opthalmol Vis Sci. 1989;30:652–659. [PubMed] [Google Scholar]
- Wood JD, Sidhu HS. A comparative study and partial characterization of multi-uptake systems for γ-aminobutyric acid. J Neurochem. 1987;49:1202–1208. doi: 10.1111/j.1471-4159.1987.tb10011.x. [DOI] [PubMed] [Google Scholar]
- Wulle I, Wagner HJ. GABA and tyrosine hydroxylase immunocytochemistry reveal different patterns of colocalization in retinal neurons of various vertebrates. J Comp Neurol. 1990;296:173–178. doi: 10.1002/cne.902960111. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Uchida S, Moo Kwon H, Preston AS, Robey RB, Garcia-Perez A, Burg MB, Handler JS. Cloning of a Na+-and Cl−-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992;267:649–652. [PubMed] [Google Scholar]
- Yazulla S. GABAergic mechanisms in the retina. In: Osborne NN, Chader GJ, editors. Progress in Retinal Research. Vol. 5. Oxford: Pergamon Press; 1986. pp. 1–52. [Google Scholar]
- Yazulla S, Kleinschmidt J. Carrier mediated release of GABA from retinal horizontal cells. Brain Res. 1983;263:63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]


