Abstract
BACKGROUND:
Tobacco smoke exposure (TSE) may increase respiratory morbidities in young children with bronchopulmonary dysplasia (BPD). Rapid respiratory rates, close proximity to a smoking caregiver, and increased dermal absorption of tobacco smoke components can contribute to systemic exposure. In this study, hair nicotine levels were used as a biomarker of chronic TSE in young children with BPD to determine if hair nicotine levels correlate with caregiver self-report of TSE and respiratory morbidities.
METHODS:
From 2012 to 2014, hair nicotine levels were measured from consecutive children seen in a BPD outpatient clinic and compared with caregiver questionnaires on household smoking. The relationship between respiratory morbidities and self-reported TSE or hair nicotine level was assessed.
RESULTS:
The mean hair nicotine level from 117 children was 3.1 ± 13.2 ng/mg. Hair nicotine levels were significantly higher in children from smoking households by caregiver self-report compared with caregivers who reported no smoking (8.2 ± 19.7 ng/mg vs 1.8 ± 10.7; P < .001). In households that reported smoking, hair nicotine levels were higher in children with a primary caregiver who smoked compared with a primary caregiver who did not smoke. Among children with BPD who required respiratory support (n = 50), a significant association was found between higher log hair nicotine levels and increased hospitalizations and limitation of activity.
CONCLUSIONS:
Chronic TSE is common in children with BPD, with hair nicotine levels being more likely to detect TSE than caregiver self-report. Hair nicotine levels were also a better predictor of hospitalization and activity limitation in children with BPD who required respiratory support at outpatient presentation.
Keywords: bronchopulmonary dysplasia, prematurity, secondhand smoke, tobacco smoke exposure, nicotine, respiratory outcomes
What’s Known on This Subject:
Little is known about the impact of tobacco smoke exposure on preterm children with bronchopulmonary dysplasia. It is essential to understand how environmental exposures, such as tobacco smoke, influence respiratory morbidities in this vulnerable population.
What This Study Adds:
Chronic tobacco smoke exposure is common in children with bronchopulmonary dysplasia. In children who required home respiratory support, hair nicotine levels were a better predictor of hospitalization and activity limitation than caregiver self-report.
With advances in neonatal care, larger numbers of extremely-low-birth-weight preterm infants are surviving to hospital discharge.1,2 However, up to 50% of infants with birth weights <1000 g develop bronchopulmonary dysplasia (BPD).3,4 Although the effects of BPD may persist into adulthood,5 infants and toddlers are more severely affected, with ∼50% of infants requiring readmission within the first 2 years of life.6–8 Infants with BPD are frequently prescribed multiple medications and often require home supplemental oxygen and/or cardiorespiratory monitoring. To reduce this significant burden of disease, it is essential to modify or eliminate environmental exposures that may increase respiratory morbidity in this vulnerable population.
Within the general pediatric population, tobacco smoke exposure (TSE) has been causally linked to respiratory morbidities including lower respiratory tract infections, coughing, wheezing, onset of wheezing, decreased lung function, and limitations in lung growth.9 TSE may be particularly detrimental to infants and children with chronic lung disease, since recovery is dependent on adequate postnatal lung growth and avoidance of ongoing lung damage. Studies in neonatal mice have shown that TSE can impair postnatal lung growth and cause structural and functional lung changes in the adult lung.10,11 In preterm infants, wheezing and a higher risk of acute health care visits for respiratory symptoms have been associated with living with a smoker.12–14 Exposure to tobacco smoke may also increase the severity of respiratory viruses in an already vulnerable population of infants and children.15 However, some studies that rely on questionnaire data alone to assess TSE have not found an association with respiratory morbidities.16,17
In this study, we measured hair nicotine levels as a biological marker of TSE in infants and young preschool-aged children with BPD. Hair nicotine levels in children exposed to tobacco smoke have been used to assess TSE in children and have shown to correlate better with caregiver questionnaire responses than urine cotinine levels.18,19 This is likely because hair nicotine levels reflect a more chronic exposure to TSE compared with urine cotinine. We hypothesized that hair nicotine levels would correlate with caregiver self-report of smoking in the household and that hair nicotine levels in young children with BPD would be a better predictor of respiratory morbidities than caregiver self-report of household smoking. We also sought to determine how hair nicotine levels varied by primary caregiver smoking status and number of smokers in the household. Finally, we sought to determine to what extent presence or absence of smoking bans in multi-unit housing were associated with hair nicotine levels in children.
Methods
Study Population
All subjects were recruited from the Johns Hopkins Bronchopulmonary Dysplasia Clinic between January 2012 and January 2014. Patients were referred to the clinic by area NICUs or pediatricians on the basis of prematurity and having the diagnosis of BPD. Of 227 patients with a diagnosis of BPD and born at ≤36 weeks of gestation, 145 consented for collection of hair samples. The final study population included 117 subjects from whom hair samples were able to be successfully collected before 3 years of age. This study was approved by the Johns Hopkins Institutional Review Board (protocol # NA_051884).
Demographics
Birth weight percentile was derived from published US norms.20 Race and primary caregiver education level were self-reported. Median household income was derived from 2010 US Census data based on residential zip code. Health insurance coverage (public versus private) was obtained from billing records. The presence or absence of gastrostomy tubes, Nissen fundoplication, home supplemental oxygen, and home mechanical ventilation were ascertained at the first BPD clinic encounter through chart review. Inhaled corticosteroid use was defined as any prescribed outpatient use before 2 years of age.
Questionnaire Data
At the time of recruitment, families completed 2 questionnaires with the aid of a data coordinator as necessary. The first questionnaire used a subset of questions from the 2011 Social Climate Survey of Tobacco Control (www.socialclimate.org) focusing on self-reported exposure to tobacco smoke in the home, including questions on which family members smoke, where they smoke, and household rules regarding smoking inside. Self-reported TSE for a subject was defined as 1 or more smokers residing with the subject regardless of whether smoking took place indoors. The second questionnaire, which has not been validated, assessed the patient’s history of emergency department visits, hospital admissions, systemic steroid use, and antibiotic use for respiratory reasons since the last BPD clinic visit (or since the initial hospital discharge if assessed at the first BPD clinic visit). Chronic symptom morbidities assessed included the presence of cough/wheeze, inhaled rescue β-agonist use, activity limitations owing to respiratory symptoms, and nighttime respiratory symptoms.
Sample Collection and Processing
Hair samples were collected by a trained data coordinator from subjects during the same clinic visit. Samples of hair (30–50 strands) were cut at the hair root near the occiput where hair growth is more uniform.21 Samples were placed in a plastic bag for transport and storage. Hair samples were washed by using dichloromethane and sonication before measurement of nicotine levels to avoid elevated measurements from nicotine that may have been adherent to the surface of the hair. Hair nicotine analysis was performed via gas chromatography and mass spectrometry (GC-17/MS-QP5000; Shimadzu, Kyoto, Japan). Complete details of the measurement process can be found in Wipfli et al.21 The 65 subjects in whom the level of hair nicotine was undetectable were conservatively assigned the analytical limit of detection as a value for analysis to decrease the risk of false-positive results; this included 61 subjects who lived in a household with no smokers and 4 who lived in a household with smokers.
Statistical Methods
Demographic frequencies and log hair nicotine levels were compared using the appropriate parametric tests (χ2 and t tests), and hair nicotine levels were compared by using nonparametric Mann–Whitney tests owing to their nonnormal distribution (Tables 1 and 2). The relationship between respiratory morbidities and self-reported TSE were assessed using logistic regression to generate odds ratios (ORs) adjusted for the age of the subject at the time of questionnaire completion, gestational age (as a marker of prematurity), and socioeconomic factors that differ by self-reported smoke exposure (including both estimated household income and primary caregiver education level) (Table 3). The relationship between respiratory morbidities and log nicotine hair levels (treated as a continuous variable) were assessed using logistic regression with similar adjustments (Table 4). Log nicotine levels were used for the logistic regressions owing to the nonnormal distribution of nicotine levels. The regression analysis was repeated in a similar manner for the subset of the subjects on supplemental oxygen and/or mechanical ventilation at home (n = 50) to assess whether subjects with more severe respiratory disease were at higher risk for respiratory morbidities with exposure (Tables 3 and 4). All analyses were conducted by using Stata IC 11.0 (College Station, TX), and P values <.05 were considered statistically significant.
TABLE 1.
Study Population Demographics
| Characteristic | Entire Study Population | No Smokers Living in the Home | Smokers Living in the Home | P |
|---|---|---|---|---|
| n | 117 | 93 | 24 | |
| Demographics | ||||
| Gender, % male | 64.1 | 63.4 | 66.7 | .77 |
| Age, y | .14 | |||
| Mean ± SD | 1.23 ± 0.71 | 1.27 ± 0.73 | 1.03 ± 0.56 | |
| Median (IQR) | 1.05 (0.70–1.53) | 1.07 (0.76–1.69) | 0.92 (0.58–1.18) | |
| Range | 0.12–2.92 | 0.12–2.92 | 0.50–2.52 | |
| Race, % nonwhite | 64.4 | 62.4 | 70.8 | .44 |
| Gestation, wks | ||||
| Mean ± SD | 27.2 ± 3.1 | 27.3 ± 3.1 | 27.1 ± 3.2 | .88 |
| Median (IQR) | 26.9 (24.9–28.1) | 26.9 (25.0–28.1) | 26.9 (24.5–28.8) | |
| Range | 22.9–35.0 | 23.0–35.0 | 22.9–35.0 | |
| Birth weight, g | ||||
| n | 115 | 92 | 23 | |
| Mean ± SD | 1017 ± 479 | 1016 ± 468 | 1018 ± 530 | .99 |
| Median (IQR) | 879 (690–1220) | 855 (709–1220) | 880 (680–1100) | |
| Range | 390–2650 | 390–2650 | 415–2610 | |
| Birth weight, percentile | ||||
| n | 115 | 92 | 23 | |
| Mean ± SD | 42.4 ± 25.0 | 42.2 ± 25.5 | 43.4 ± 23.2 | .83 |
| Median (IQR) | 43 (22–60) | 42.5 (22–61.5) | 45 (28–55) | |
| Range | 1–90 | 1–90 | 1–89 | |
| Socioeconomic status | ||||
| Median household income ($, thousands) | ||||
| Mean ± SD | 66.2 ± 23.4 | 68.8 ± 23.2 | 57.2 ± 22.3 | .031 |
| Median (IQR) | 62.6 (50.9–80.4) | 67.4 (53.0–81.7) | 52.4 (39.3–80.0) | |
| Range | 25.2–156.6 | 25.2–156.6 | 25.2–100.6 | |
| Public insurance, % | 60.7 | 57.0 | 75.0 | .11 |
| High school education or less, % | 25.4a | 20.0b | 45.8 | .010 |
| Clinical characteristics | ||||
| Age at discharge from NICU, mo | ||||
| Mean ± SD | 4.2 ± 3.1 | 4.3 ± 3.4 | 3.8 ± 1.6 | .52 |
| Median (IQR) | 3.6 (2.5–4.8) | 3.5 (2.4–4.9) | 3.6 (2.9–4.6) | |
| Range | 0.2–24.4 | 0.4–24.4 | 0.4–8.7 | |
| Home supplemental oxygen, % | 41.0 | 44.1 | 29.2 | .19 |
| Home ventilator, % | 5.1 | 5.4 | 4.2 | .81 |
| Gastrostomy tube, % | 29.1 | 31.2 | 20.8 | .32 |
| Nissen, % | 17.1 | 19.3 | 8.3 | .20 |
| Inhaled corticosteroid use before age 2 y, % | 85.5 | 83.9 | 91.7 | .33 |
| Technology dependent,a % | 53.0 | 55.9 | 41.7 | .21 |
IQR, interquartile range.
n = 114.
n = 90.
Presence of any of the following: home supplemental oxygen, home ventilator, gastrostomy tube, or Nissen fundoplication.
TABLE 2.
Hair Nicotine Data
| Characteristic | Entire Study Population | No Smokers Living in the Home | Smokers Living in the Home | P |
|---|---|---|---|---|
| n | 117 | 93 | 24 | |
| Age at time of sampling, y | ||||
| Mean ± SD | 1.23 ± 0.71 | 1.27 ± 0.73 | 1.03 ± 0.56 | .14 |
| Median (IQR) | 1.05 (0.70–1.53) | 1.07 (0.76–1.69) | 0.92 (0.58–1.18) | |
| Range | 0.12–2.92 | 0.12–2.92 | 0.50–2.52 | |
| Weight of hair sample, mg | ||||
| Mean ± SD | 15.0 ± 9.7 | 15.4 ± 9.9 | 13.7 ± 8.9 | .44 |
| Median (IQR) | 13 (7–24) | 14 (7–25) | 10.5 (7–22) | |
| Range | 1–33 | 1–33 | 3–30 | |
| Hair nicotine, ng/mg | ||||
| Mean ± SD | 3.1 ± 13.2 | 1.8 ± 10.7 | 8.2 ± 19.7 | <.001 |
| Median (IQR) | 0.4 (0.2–0.80) | 0.3 (0.1–0.6) | 1.7 (0.5–3.7) | |
| Range | 0.05–102.9 | 0.05–102.9 | 0.09–89.4 | |
| Log of hair nicotine, log ng/mg | ||||
| Mean ± SD | −0.31 ± 0.64 | −0.45 ± 0.53 | 0.21 ± 0.77 | <.001 |
| Median (IQR) | −0.40 (−0.76 to −0.10) | −0.47 (−0.84 to −0.24) | 0.24 (−0.28 to 0.57) | |
| Range | −1.33 to 2.01 | −1.33 to 2.01 | −1.00 to 1.95 |
IQR, interquartile range.
TABLE 3.
Self-Reported Smoker Residing in the Home as a Predictor of Selected Respiratory Outcomes
| Outcome | All Subjects (n = 114) | P | Subjects on Home Respiratory Supporta | P |
|---|---|---|---|---|
| n | 114 | 50 | ||
| Emergency department visit | 0.54 (0.16–1.86) | .33 | .022 (0.02–3.30) | .28 |
| Inpatient hospitalization | 0.68 (0.17–2.73) | .58 | 0.25 (0.01–5.10) | .37 |
| Systemic steroid use | 0.38 (0.07–1.91) | .24 | 0.92 (0.08–11.21) | .95 |
| Antibiotic use | 0.95 (0.27–3.38) | .94 | 0.35 (0.02–6.31) | .48 |
| Cough or wheeze | 1.93 (0.72–5.13) | .19 | 0.56 (0.10–3.01) | .50 |
| Rescue β-agonist use | 1.48 (0.51–4.26)b | .47 | 5.19 (0.77–34.86)c | .09 |
| Activity limitations | 1.25 (0.29–5.36)b | .76 | 0.69 (0.10–4.59)c | .70 |
| Nighttime symptoms | 1.03 (0.36–2.97)b | .96 | 0.83 (0.12–5.75)c | .85 |
Values are presented as the adjusted OR (95% confidence interval). ORs were generated through logistic regression by using self-reported smoker residing in the home (yes/no) and adjusted for gestational age, log of estimated household income, primary caregiver education level (high school graduate or less versus some college or more), and age at the time of questionnaire completion.
Of the 50 subjects in this study on home respiratory support, 44 were on supplemental oxygen via nasal cannula, 4 were on home ventilators with supplemental oxygen entrainment, and 2 were on home ventilators without supplemental oxygen. Of these 50 subjects, 8 (16%) were self-reported as residing with a smoker.
n = 113.
n = 49.
TABLE 4.
Continuous Log of Hair Nicotine Values as a Predictor of Selected Adjusted ORs of Selected Respiratory Outcomes
| Outcome | All Subjects (n = 114) | P | Subjects on Home Respiratory Supporta (n = 50) | P |
|---|---|---|---|---|
| Emergency department visit | 1.75 (0.83–3.67) | .14 | 2.06 (0.53–7.99) | .29 |
| Inpatient hospitalization | 1.82 (0.78–4.23) | .16 | 6.42 (1.35–30.62) | .020 |
| Systemic steroid use | 1.09 (0.46–2.59) | .84 | 0.54 (0.11–2.78) | .46 |
| Antibiotic use | 0.43 (0.16–1.16) | .10 | 0.32 (0.06–1.78) | .19 |
| Cough or wheeze | 1.57 (0.81–3.02) | .18 | 0.77 (0.24–2.46) | .66 |
| Rescue β-agonist use | 1.00 (0.50–2.03)b | .99 | 2.89 (0.71–11.88)c | .14 |
| Activity limitations | 1.33 (0.50–3.50)b | .57 | 7.52 (1.59–35.60)c | .011 |
| Nighttime symptoms | 0.80 (0.38–1.66)b | .55 | 1.36 (0.33–5.51)c | .67 |
Values are presented as the adjusted OR (95% confidence interval). ORs were generated through logistic regression by using log of hair nicotine values as a continuous variable and adjusted for gestational age, log of estimated household income, primary caregiver education level (high school graduate or less versus some college or more), and age at the time of questionnaire completion.
Of the 50 subjects in this study on home respiratory support, 44 were on supplemental oxygen via nasal cannula, 4 were on home ventilators with supplemental oxygen entrainment, and 2 were on home ventilators without supplemental oxygen.
n = 113.
n = 49.
Results
Demographics
A total of 227 subjects were approached for the collection of hair for nicotine levels, and 145 of those consented. Children who consented for hair samples had a higher birth weight percentile (P = .031), but not birth weight (P = .32), and were more likely to require home ventilator support (P = .039), but were not overall more likely to be technology dependent (P = .84) (Supplemental Table 5). Of the 117 subjects in this study who had hair samples collected before 3 years of age, 24 (20.5%) lived in a household with at least 1 smoker by caregiver self-report. Maternal smoking during pregnancy was reported for 3 subjects (2.6%), of whom 1 no longer resided with a smoker and 2 did. There was no difference by gender, age at the time of hair collection, race, gestational age, or birth weight percentile between children living with a smoker or not (Table 1). Those living with a smoker had a lower mean estimated household income than those not living with a smoker ($57 283 vs $68 815; P = .031). The primary caregivers in smoking households were also more likely to have less education (high school degree or less) compared with primary caregivers in nonsmoking households (45.8% vs 20.0%; P = .020). However, there was no difference in the proportion of subjects on public versus private health insurance by smoking household status (P = .11). There were no differences in selected markers of disease by smoking household status, including age of initial discharge from the NICU, home oxygen or ventilator use, gastrostomy tube placement, presence of Nissen fundoplication, inhaled corticosteroid use before 2 years of age, or technology dependence in the home (presence of any of the following in the home: supplemental oxygen, ventilator, or gastrostomy tube).
Hair Nicotine Levels
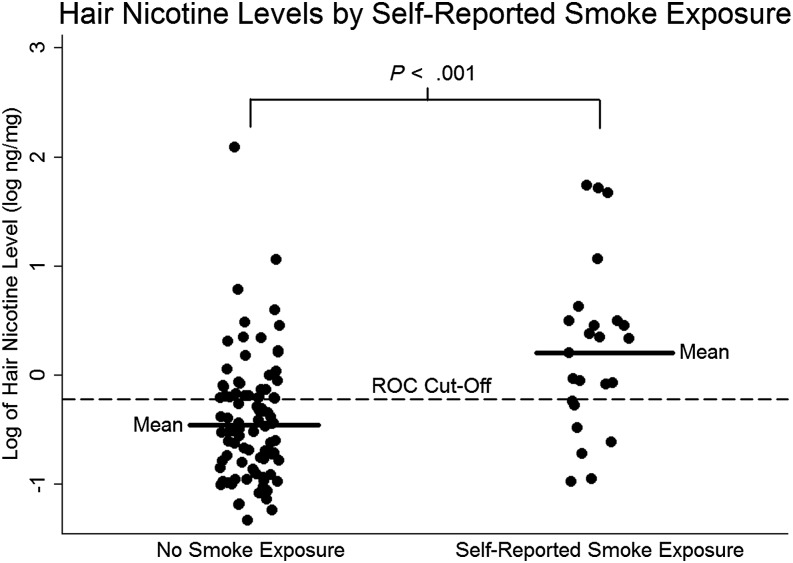
The mean hair nicotine level for our study population was 3.1 ± 13.2 ng/mg (Table 2). The hair nicotine levels of children from smoking households as reported by caregiver questionnaire were significantly higher than in children whose caregivers reported no smoking (nicotine 8.2 ± 19.7 vs 1.8 ± 10.7, P < .001; log nicotine 0.21 ± 0.77 log vs −0.45 ± 0.53, P < .001). The area under the receiver operator characteristic (ROC) curve equaled 0.7762 when caregiver self-report of smoking in the household and hair nicotine levels of subjects were compared. The ROC cutoff for hair nicotine was 0.6 or −0.22 log ng/mg between those subjects not exposed to tobacco smoke and those exposed (Fig 1). We found that 21 of the 93 subjects who reported not residing with a smoker had a nicotine value greater than the ROC cutoff, which suggests that questionnaires in a clinic-based setting may miss a substantial number of infants exposed to tobacco smoke.
FIGURE 1.
Hair nicotine levels of 117 subjects with BPD divided into no TSE and TSE by caregiver self-report. The dashed reference line depicts a ROC cutoff of −0.221 log ng/mg.
Households With Smokers
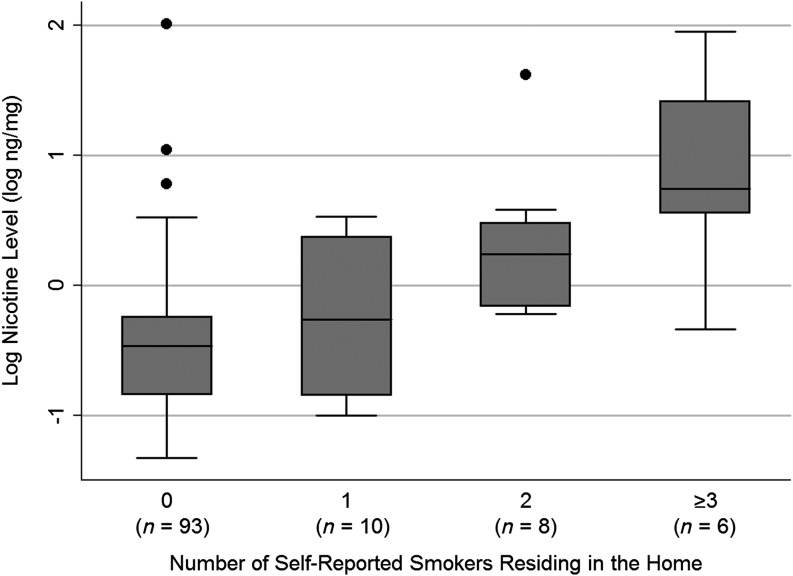
Of the 24 households that reported the presence of a smoker, 58% reported more than 1 smoker residing in the home. There was a positive association between the number of smokers residing in a household and higher log nicotine level by unadjusted linear regression (Fig 2) (P < .001 for all 117 subjects and P = .002 for the 24 households reporting smokers in the home). In the households that reported the presence of a smoker, the primary caregiver was the only smoker 13% of the time, the primary caregiver and someone else smoked in the household 50% of the time, and someone other than the primary caregiver smoked in the household 38% of the time. In households that reported the presence of a smoker, the mean nicotine level was higher in children who had a primary caregiver that smoked (n = 15) compared with households in which the primary caregiver did not smoke (n = 9) (nicotine 12.2 ± 24.3 vs 1.4 ± 1.4, P = .046; log nicotine 0.46 ± 0.73 vs −0.21 ± 0.66, P = .035).
FIGURE 2.
Lower log hair nicotine levels were found in BPD children of caregivers who reported no smoking in the household compared with caregivers that reported 1, 2, or ≥ 3 smokers in the household. There was a positive association between the number of smokers residing in a household and higher log nicotine level by unadjusted linear regression (P < .001 for all 117 subjects and P = .002 for the 24 households reporting smokers in the home).
Housing Characteristics
Of the 117 subjects in the study population, 49 (42%) reported living in a home detached from any other home, and 64 (58%) reported living in a home attached to other homes or in a multi-unit building (apartments or condominiums). Of the 64 living in attached or multi-unit housing, 26 (41%) reported that smoking was permitted, 25 (39%) reported that smoking was not permitted anywhere on the property, and 13 (20%) were unsure of their property’s rules with regard to smoking. For nonsmoking households, the mean nicotine for subjects was lower for families in attached or multi-unit properties where smoking was not permitted (n = 22) compared with families in attached or multi-unit properties where smoking was permitted (n = 19), but this difference did not reach statistical significance (hair nicotine 0.34 ± 0.36 vs 6.08 ± 23.47, P = .17; log nicotine: −0.61 ± 0.35 vs −0.31 ± 0.74, P = .09). Using the value of zero for analysis for subjects whose hair nicotine level was less than the level of detection did not change the nonsignificance of this finding.
Respiratory Morbidities
Using logistic regression models adjusted for age, gestational age, highest completed primary caregiver education level, and log value of the estimated household income, we tested for an association between respiratory morbidities and TSE by caregiver questionnaire. Because severity of BPD was variable in our patient population, we also tested for an association between respiratory morbidities and the presence of household smoking in subjects who required respiratory support at the initial outpatient visit. Respiratory support was defined as a need for supplemental oxygen and/or mechanical ventilation in the home setting (n = 50). By caregiver self-report, no association was found between the presence of a smoker in the household and increased respiratory morbidities in all BPD subjects or in the subgroup of subjects who required respiratory support (Table 3).
Using hair nicotine levels as a biomarker for TSE in the household, we then tested for an association between respiratory morbidities and log hair nicotine levels in subjects with BPD. We did not find an association between respiratory morbidities and log hair nicotine levels in the total BPD population. However, in BPD subjects who required respiratory support at their initial outpatient visit, we found a significant association between higher log hair nicotine levels and increased hospitalizations and limitation of activity. In this group of infants and children with moderate to severe BPD, the presence of a smoker at home increased the risks for inpatient hospitalization (adjusted OR 6.42; P = .020) and activity limitations (adjusted OR 7.52; P = .011) with every 10-fold increase in hair nicotine level (Table 4).
Discussion
Underlying lung injury and decreased pulmonary reserve increase the risk for hospitalization with respiratory viruses and chronic respiratory symptoms in young children with BPD. However, little is known regarding the impact of TSE on the respiratory health of young children with BPD. In this study, we found that 20% of caregivers of children with BPD reported smoking in the household. We assessed hair nicotine levels as a biomarker for TSE in young children with BPD to determine the predictive value of these levels for reported exposure and respiratory outcomes. We found that higher levels of hair nicotine were predictive of a caregiver reporting smoking in the household. Hair nicotine levels were also found to be higher in children of caregivers who smoked and higher in children who lived in households that reported more than 1 smoker. Interestingly, higher hair nicotine levels were found to be predictive of a greater likelihood of hospitalization and increased activity limitation in children with BPD who required supplemental oxygen and/or ventilator support at the initial outpatient visit. We also estimate that in our population up to 46% of children with BPD exposed to TSE by hair nicotine levels may be missed using caregiver questionnaire.
BPD is one of the most costly diseases of infancy and can lead to significant direct and indirect costs to family and society.22,23 Our study is the first to show that higher hair nicotine levels in children with more severe BPD (those who required respiratory support) predict increased hospitalizations and activity limitation in the first 3 years of life. TSE is a modifiable risk factor, and interventions that lower TSE in children with moderate to severe BPD could decrease hospitalizations, improve activity levels, and optimize long-term respiratory outcomes. Minimizing the number and severity of respiratory illnesses in children with BPD through the elimination of TSE could potentially prevent further lung damage, enhance repair of existing lung injury, and allow for “catch-up” lung growth during the critical first years of life.24 Longitudinal studies are needed in children with BPD to determine the impact of early postnatal TSE on subsequent lung function and respiratory disease severity in later life.
We anticipated that subjects with BPD would have minimal TSE because caregivers of infants and children attending the BPD outpatient clinic are educated against smoking in the household. Nevertheless, we found that ∼20% of young children with BPD were exposed to tobacco smoke by self-reported caregiver questionnaires regardless of technology use in their children. Hair nicotine levels from children with BPD were also found to correlate with caregiver self-report of household smoking status. The mean hair nicotine level in all children with BPD was 3.1 ± 13.2 ng/mg, with significant differences between children of caregivers that reported smoking in the household (8.2 ± 19.7 ng/mL) and children of caregivers who reported no smoking in the household (1.8 ± 10.7). Higher hair nicotine levels were found in children who lived with a primary caregiver who smoked. This finding was similar to that found in a study published by Al-Delaimy and colleagues.19
Chronic TSE as detected by hair nicotine levels were found in some children of caregivers who reported no smoking in the household. The reason for this may be multifactorial. Detectable hair nicotine in subjects exposed to minimal tobacco smoke may occur because of increased systemic absorption through multiple routes in the young child.25 Nicotine absorption may be greater in children with higher respiratory rates, as found in children with BPD. Increased nicotine absorption through dermal and oral routes by thirdhand smoke exposure may also contribute to higher hair nicotine levels in children with BPD.26 In addition, caregiver recall bias or reluctance to admit to smoking may account for discrepancies between caregiver self-report and hair nicotine levels in young children. Self-report may not correlate with hair nicotine levels perfectly, as we did not assess whether the subject was exposed to tobacco smokers other than those living in the primary residence or whether they were exposed to sources of nicotine not generated through smoking, such as secondhand electronic cigarette emissions. Furthermore, living in multi-unit housing where smoking is permitted in common areas may be another factor that may increase hair nicotine levels in our study. We found a trend toward higher hair nicotine in nonsmoking households of children who lived in multi-unit housing that permitted smoking, but this did not reach significance. A larger study examining hair nicotine levels in children living in multi-unit housing that permits or regulates smoking in common areas may be warranted.
A limitation to our study was that hair was collected at only 1 time point. Although this study was cross-sectional, hair nicotine levels are more likely to reflect chronic or intermittent exposures to tobacco smoke in contrast to urine, saliva, and serum levels, which are more likely to reflect a more acute exposure to tobacco smoke.27 Furthermore, using hair nicotine as a biomarker for systemic nicotine levels and TSE in a young child can be advantageous. In our study, hair was easily obtainable, removal of hair did not cause pain or anxiety to the child, and measurement of hair nicotine levels allowed for the assessment of chronic exposure to tobacco smoke in the home environment. Although urine, saliva, and blood cotinine and nicotine levels can be easily measured in many laboratories, these specimens can be challenging to obtain in the infant or young child. A limitation to using hair nicotine as a predictive biomarker for TSE in young children with BPD is that many children had too little hair to cut and measure. Our study may have also underestimated the effect of TSE on our population, since for analysis purposes we conservatively assigned the level of detection value for samples with hair nicotine levels below the level of detection. Additionally, respiratory morbidities were assessed by an unvalidated instrument, which may have the potential to misclassify outcomes (eg, reported activity limitations could have been mistaken for neurodevelopmental delay). Finally, other limitations include recruitment of patients from an urban setting and a selection bias of preterm infants/children at high risk for respiratory morbidity. Because our patients were recruited from an urban medical center, results from our study may not be generalizable to other geographic areas; however, poor urban areas have a high incidence of preterm births and our results may be applicable to this high-risk population.
Conclusions
In this study, we found that higher hair nicotine levels were predictive of caregiver self-report of smoking in the household. Hair nicotine levels were a better predictor of hospitalization and activity limitation in young children with moderate to severe BPD compared with caregiver self-report of TSE. Hair nicotine levels also detected children exposed to TSE that would have been missed by caregiver questionnaire. This study indicates that young children with BPD are commonly exposed to tobacco smoke. Hair nicotine levels may be useful in the clinical setting to predict which children may be at increased risk for respiratory morbidities during the first 3 years of life and to identify children for appropriate TSE interventions.
Supplementary Material
Acknowledgments
The authors thank the families who participated in this study.
Footnotes
Dr Collaco conceptualized and designed the study, carried out the initial analyses, and drafted the initial manuscript; Ms Aherrera designed the data collection instruments, coordinated data collection, and critically reviewed the manuscript; Dr Breysse coordinated and supervised the processing of biological samples and critically reviewed and revised the manuscript; Drs Winickoff and Klein assisted with interpretation of analyses and critically reviewed and revised the manuscript; Dr McGrath-Morrow conceptualized and designed the study, supervised data collection, and revised the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by a Center of Excellence grant to the American Academy of Pediatrics from the Flight Attendant Medical Research Institute and a Johns Hopkins Center of Excellence grant from the Flight Attendant Medical Research Institute.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131(3):548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemons JA, Bauer CR, Oh W, et al. NICHD Neonatal Research Network . Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. Pediatrics. 2001;107(1). Available at: www.pediatrics.org/cgi/content/full/107/1/e1 [DOI] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Walsh MC, Vohr BR, et al. National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Walsh M, Bobashev G, et al. NICHD Neonatal Research Network . Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenough A. Long-term pulmonary outcome in the preterm infant. Neonatology. 2008;93(4):324–327 [DOI] [PubMed] [Google Scholar]
- 6.Smith VC, Zupancic JA, McCormick MC, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144(6):799–803 [DOI] [PubMed] [Google Scholar]
- 7.Furman L, Baley J, Borawski-Clark E, Aucott S, Hack M. Hospitalization as a measure of morbidity among very low birth weight infants with chronic lung disease. J Pediatr. 1996;128(4):447–452 [DOI] [PubMed] [Google Scholar]
- 8.Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health. 1995;31(2):105–111 [DOI] [PubMed] [Google Scholar]
- 9.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32(6):542–543 [DOI] [PubMed] [Google Scholar]
- 10.McGrath-Morrow S, Rangasamy T, Cho C, et al. Impaired lung homeostasis in neonatal mice exposed to cigarette smoke. Am J Respir Cell Mol Biol. 2008;38(4):393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath-Morrow SA, Lauer T, Collaco JM, et al. Neonatal hyperoxia contributes additively to cigarette smoke-induced chronic obstructive pulmonary disease changes in adult mice. Am J Respir Cell Mol Biol. 2011;45(3):610–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halterman JS, Lynch KA, Conn KM, Hernandez TE, Perry TT, Stevens TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Arch Dis Child. 2009;94(1):28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elder DE, Hagan R, Evans SF, Benninger HR, French NP. Recurrent wheezing in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74(3):F165–F171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palta M, Sadek-Badawi M, Sheehy M, et al. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol. 2001;154(6):521–529 [DOI] [PubMed] [Google Scholar]
- 15.DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC Pediatr. 2012;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collaco JM, Aherrera AD, Ryan T, McGrath-Morrow SA. Secondhand smoke exposure in preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49(2):173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks JL, Holditch-Davis D, Landerman LR, Miles MS, Engelke SC. Exploring modifiable risk factors for wheezing in African American premature infants. J Obstet Gynecol Neonatal Nurs. 2011;40(3):302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Wipfli H, Navas-Acien A, et al. FAMRI Homes Study Investigators . Determinants of hair nicotine concentrations in nonsmoking women and children: a multicountry study of secondhand smoke exposure in homes. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3407–3414 [DOI] [PubMed] [Google Scholar]
- 19.Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wipfli H, Avila-Tang E, Navas-Acien A, et al. Famri Homes Study Investigators . Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98(4):672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blencowe H, Cousens S, Chou D, et al. Born Too Soon Preterm Birth Action Group . Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annu Rev Public Health. 2011;32:367–379 [DOI] [PubMed] [Google Scholar]
- 24.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37(8):564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valcke M, Krishnan K. Evaluation of the impact of the exposure route on the human kinetic adjustment factor. Regul Toxicol Pharmacol. 2011;59(2):258–269 [DOI] [PubMed] [Google Scholar]
- 26.Matt GE, Quintana PJ, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control. 2014;23(3):264–272 [DOI] [PubMed] [Google Scholar]
- 27.Al-Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tob Control. 2002;11(3):176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.