Abstract
Objective
This prospective observational postmarketing multicentre study was performed to collect data on the clinical efficacy, safety and tolerability of a licensed herbal combination of myrrh, coffee charcoal and chamomile extracts in patients with symptoms of acute diarrhoea.
Material and methods
Patients aged 12 years and above with symptoms of acute diarrhoea due to acute inflammatory disorders (AID) of the gastrointestinal tract, inflammatory bowel diseases (IBD) or irritable bowel syndrome (IBS) were treated with the herbal preparation either as monotherapy, add-on therapy or with other therapies. The primary outcome parameter was the pre-post change of total mean symptom score. Secondary outcome parameters were changes of score of single symptoms, physician's assessment of the clinical course and efficacy, and patient's satisfaction.
Results
1062 patients (mean age 43.2±17.8 years, range 12–89, 42.3% men) were included. A decrease of the overall mean total symptom score was observed in all treatment groups (monotreatment: 1.33±0.51 to 0.15±0.34, add-on treatment: 1.39±0.41 to 0.30±0.37, other therapy: 1.31±0.43 to 0.24±0.33). No significant differences between three treatment options were observed within AID and IBD groups. However, in the IBS group, monotreatment with the herbal preparation resulted in a significantly better outcome when compared to either add-on treatment (mean difference 0.140; 95% CI 0.036 to 0.245; p=0.009) or other therapy (mean difference 0.217; 95% CI 0.085 to 0.349; p=0.001). Secondary efficacy criteria showed comparable results between different treatment options in the respective disorder groups. Patient satisfaction was generally higher with monotreatment in the AID and IBS groups, while add-on treatment was preferred in the IBD group.
Conclusions
The combination of myrrh, coffee charcoal and chamomile flower extract is effective, well tolerated and safe for use in patients with symptoms of acute diarrhoea. The effects are comparable to conventional therapies used in routine care.
Keywords: DIARRHOEA, INFLAMMATORY BOWEL DISORDERS, IRRITABLE BOWEL SYNDROME
Summary box.
What is already known about this subject
-
▸
Diarrhoea is an important cause of morbidity especially in the young and the elderly.
-
▸
Effective and safe treatment is of particular importance to prevent complications often associated with episodes of acute diarrhoea.
-
▸
A combination of myrrh, coffee charcoal and chamomile has been used for decades for the support of the gastrointestinal function.
-
▸
Positive results have been obtained with the combination in maintaining remission in ulcerative colitis.
What are the new findings
-
▸
A combination of myrrh, coffee charcoal and chamomile is effective in the treatment of acute diarrhoea symptoms in daily practice.
-
▸
Monotreatment with the herbal preparation is as efficacious as add-on treatment and treatment with other substances.
-
▸
Data indicates positive influence on the management of acute diarrhoea due to inflammatory bowel diseases and irritable bowel syndrome.
How might it impact on clinical practice in the foreseeable future?
-
▸
Our findings indicate that symptoms associated with diarrhoea due to acute inflammatory disorders, inflammatory bowel diseases and irritable bowel syndrome can be effectively managed with the herbal preparation
Introduction
Diarrhoea is a frequent symptom caused by different infectious as well as non-infectious aetiologies, reflecting different underlying gastrointestinal disorders, and is one of the most common diagnoses in family medicine.1 2 Here, the term ‘acute’ refers to symptoms lasting no longer than 2 weeks, ‘persistent’ or ‘transient’ refers to symptoms continuing for up to 4 weeks, while the term ‘chronic’ describes symptoms persisting longer than 4 weeks. Symptoms of acute diarrhoea usually refer to acute infective gastrointestinal disorders, but may also be present in other conditions such as inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). Causative infectious agents in acute diarrhoea are bacteria and viruses usually transmitted through contaminated food, water or human contact. According to WHO, there are two billion cases of diarrhoeal disease worldwide every year.3 Children and the elderly are affected the most.1 Although relatively few patients die from diarrhoea in industrialised countries, it is still an important cause of morbidity and is responsible for substantial healthcare costs,3 while significantly contributing to mortality especially of children in developing countries.
Acute diarrhoea is defined as the presence of three or more loose stools per day, a water content of more than 75% or a daily stool weight of more than 250 g. It is often accompanied by other symptoms such as vomiting, nausea or pain. Treatment starts with oral rehydration therapy to prevent or correct dehydration and, if necessary, is supplemented with antidiarrhoeal drugs.3 4
In Germany, a combination of myrrh, coffee charcoal and chamomile is well-known and has been used for more than 50 years for the treatment and support of gastrointestinal function.5 Myrrh is the aromatic resin from the Commiphora myrrha tree, which is valued for its astringent and antiseptic properties.6 7 Coffee charcoal has a high adsorptive capacity and is often utilised for treatment of acute diarrhoea.8 Chamomile flower extract possesses anti-inflammatory, antispasmodic and wound-healing effects and is used internally for symptomatic relief of gastrointestinal complaints.9 However, despite its long-term use and well-known safety profile, clinical data on the combination are scant. Recently, a randomised controlled trial demonstrated non-inferiority of the herbal preparation to the gold standard mesalazine in maintenance of remission in ulcerative colitis.10 The aim of this study was to verify the clinical efficacy and safety of the herbal preparation in the treatment of patients with symptoms associated with acute diarrhoea in daily practice.
Methods
Study design
This open prospective multicentre observational postmarketing study was conducted in 131 practices of family medicine and respiratory internal medicine in Germany between March 2012 and December 2013. The postmarketing study conforms to the current guidelines and was notified to the federal authority and the relevant institutions. According to German Drug Law, no approval by an institutional review board is required for observational studies. The duration of the observational period was defined according to the respective underlying disorder and was 7–14 days for patients with acute inflammatory disorders (AID) and 24–28 days for patients with IBD or IBS. At maximum, three visits were conducted: visit 1 (day 0 or baseline), visit 2 (day 7–14=last visit for patients with AID) and visit 3 (day 24–28=last visit for IBD and patients with IBS). The herbal medicinal product (Myrrhinil-Intest) used in this study is a combination of 100 mg myrrh powder, 50 mg coffee charcoal powder and 70 mg chamomile flower dry extract per coated tablet. No specifications were made regarding dosing, albeit most patients received the herbal preparation according to the Summary of Product Characteristics.5
Participants
Participants aged at least 12 years with symptoms of acute diarrhoea due to AID of the gastrointestinal tract (enteritis, enterocolitis, gastroenteritis), IBD (ulcerative colitis, Crohn's disease) or IBS were included in this study after the appropriate therapy was chosen by the physician. Patients with IBS were not selected according to a specific IBS subtype. There were no preferences or limits regarding the choice of therapy. Prior to inclusion, informed patient consent was obtained.
Assessment
The primary outcome parameter was the pre-post change of symptoms, which was quantified as the change of the total mean score between beginning and study end. The total mean symptom score comprises the mean scores of the eight single symptoms (general well-being, stool frequency, stool consistency, blood or mucous in stool, flatulence, pain intensity, pain persistency, nausea and/or vomiting). This score was calculated separately for each subgroup (AID, IBD, IBS), each treatment option and, additionally, as an overall mean total symptom score which included all subgroups. Symptoms were recorded at each visit and assessed on 4-point Likert scales for the respective scores (table 1).
Table 1.
4-point Likert scales used to assess symptoms of acute diarrhoea
| Symptom score | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| General well-being | Good | Impaired | Bad | Very bad |
| Stool frequency | ≤2 | 3–5 | 6–8 | ≥9 |
| Stool consistency | Hard or normal | Somewhat loose | Runny | Watery |
| Blood or mucous in stool | None | Some | Moderate | Many |
| Flatulence | Absent | Mild | Moderate | Severe |
| Pain intensity | Absent | Mild | Moderate | Severe |
| Pain persistency | Absent | Sporadic | Prolonged with relief after defecation | Persistent |
| Nausea and/or vomiting | Absent | Mild | Moderate | Severe |
Secondary efficacy objectives involved changes in score of single symptoms, duration of symptoms (according to diaries), physician's assessment of the clinical course and efficacy (5-point Likert scale from 0=worse to 4=complete resolution), and patient's satisfaction regarding the efficacy of the therapy (unsatisfactory, satisfactory, good, very good). Safety analysis included documentation of the frequency and severity of adverse events at each visit as well as daily in the patient's diary. Data on concomitant disorders, medication and therapy were collected at each visit. The physician-rated tolerability (poor, moderate, good, very good) was noted at each visit. Compliance and tolerability (good, not good) were checked via the patient's diary.
Statistical analyses
Estimation of the sample size was based on the assumption that rare events (incidence of 1%) are detected with a probability of 95% within a population. Accordingly, a sample size of at least 600 patients was estimated to be required. An exact sample size calculation was not performed. It was planned to include approximately 1200 patients.
For statistical analysis, three subgroups were formed according to the cause of acute diarrhoea (AID, IBD, IBS). Within each subgroup, patients were then divided into three groups according to the prescribed treatment. The herbal preparation was either prescribed as monotherapy or as add-on therapy (treatment group). A third group received treatments other than the herbal preparation (control group).
Descriptive statistics were used for the analyses of all data. Adjusted analyses were conducted since the treatment according to physician's judgment might result in biased data. Thus, regression analyses were performed. The primary outcome parameter was rated using analysis of covariance. The duration of the disease was examined using Kaplan-Meier-method and Log-rank test.11 Change in the score of single symptoms was analysed using analysis of variance and F-test. The patients’ and physicians’ assessments (efficacy, tolerability) and other secondary efficacy parameters were calculated using contingency tables.
Results
During the observation period, data of 1062 patients were collected; 804 (75.5%) patients experienced acute diarrhoea due to AID, 53 (5%) patients due to IBD and 205 (19.3%) patients due to IBS. For subsequent visits, data were documented for 1045 patients at visit 2 and 255 patients at visit 3. Anthropometric data are presented in table 2.
Table 2.
Demographic data of the study population (n=number of patients)
| Demographic data | AID (n=804) | IBD (n=53) | IBS (n=205) | Total (n=1062) |
|---|---|---|---|---|
| Age, years (mean±SD) | 43.1±18.3 | 46.9±16.5 | 42.5±15.9 | 43.2±17.8 |
| Female, n (%) | 441 (55.0) | 31 (58.5) | 140 (68.3) | 612 (57.7) |
| Body weight, kg (mean±SD) | 74.4±15.8 | 72.9±12.8 | 71.4±15.5 | 73.8±15.7 |
| Body temperature, °C (mean±SD) | 36.9±0.6 | 36.8±0.6 | 36.8±0.5 | 36.9±0.6 |
| Risk factors for acute diarrhoea | ||||
| Alcohol, n (%) | 90 (11.3) | 8 (15.1) | 38 (18.7) | 136 (12.9) |
| Travelling, n (%) | 72 (9.1) | 5 (9.4) | 32 (15.7) | 109 (10.4) |
| Hormonal imbalance, n (%) | 46 (5.8) | 4 (7.5) | 21 (10.3) | 71 (6.7) |
| Drug intake, n (%) | 57 (7.2) | 11 (20.8) | 33 (16.3) | 101 (9.6) |
| Food intolerance, n (%) | 101 (12.7) | 14 (26.4) | 109 (53.7) | 224 (21.3) |
| Smoking, n (%) | 206 (25.9) | 9 (17.0) | 56 (27.5) | 271 (25.8) |
| Metabolic disorder, n (%) | 88 (11.1) | 5 (9.4) | 10 (4.9) | 103 (9.8) |
| Stress, n (%) | 282 (35.3) | 35 (66.0) | 155 (76.0) | 472 (44.7) |
| Concomitant illness, n (%) | 232 (54.8) | 36 (8.5) | 155 (36.6) | 423 (39.8) |
| Concomitant medication, n (%) | 240 (52.3) | 50 (10.9) | 169 (36.8) | 459 (43.2) |
| Treatment chosen by physician | ||||
| Monotreatment, n (%)* | 524 (65.2) | 21 (39.6) | 86 (42.0) | 631 (59.4) |
| Add-on treatment, n (%)* | 144 (17.9) | 28 (52.8) | 82 (40.0) | 254 (23.9) |
| Other therapy | 136 (16.9) | 4 (7.5) | 37 (18.0) | 177 (16.7) |
*Treatment with the herbal preparation.
AID, acute inflammatory disorders; IBD, inflammatory bowel diseases; IBS, irritable bowel syndrome.
Age ranged from 12 to 89 years: 35 patients were younger than 18 years (26 women, 19 men) and 22 patients were older than 80 years (14 women, 2 men). Most patients were 18–59 years old. Within the AID subgroup, gastroenteritis was the most frequent disease (table 3).
Table 3.
Specific medical history data of AID and IBD. No subdivision was possible for irritable bowel syndrome due to diverse symptomatology of unknown cause
| Monotreatment | Add-on treatment | Other therapy | Total | |
|---|---|---|---|---|
| AID | ||||
| Enteritis, n (%) | 175 (21.8) | 19 (2.4) | 15 (1.9) | 209 (26.1) |
| Enterocolitis, n (%) | 35 (4.4) | 19 (2.4) | 5 (0.6) | 59 (7.4) |
| Gastroenteritis, n (%) | 312 (38.9) | 106 (13.2) | 116 (14.5) | 534 (66.6) |
| IBD | ||||
| Ulcerative colitis, n (%) | 10 (18.9) | 14 (26.4) | 3 (5.7) | 27 (50.9) |
| Acute, n (%) | 8 (15.1) | 7 (13.2) | 3 (5.7) | 18 (34.0) |
| In remission, n (%) | 2 (3.8) | 7 (13.2) | – | 9 (17.0) |
| Crohn's disease, n (%) | 11 (20.8) | 14 (26.4) | 1 (1.9) | 26 (49.1) |
| Acute, n (%) | 7 (13.2) | 9 (17.0) | – | 16 (30.2) |
| In remission, n (%) | 4 (7.5) | 5 (9.4) | 1 (1.9) | 10 (18.9) |
AID, acute inflammatory disorders; IBD, inflammatory bowel diseases.
Predominant aetiology for enteritis, enterocolitis and gastroenteritis was an assumed viral infection. Self-attributed common risk factors for an episode of acute diarrhoea were ‘stress’ (44.7%), ‘smoking’ (25.8%) and ‘food intolerance’ (21.3%). Concomitant diseases were documented in 233 patients; among these, the most common were metabolic disorders (113 patients, 26.7%) and cardiovascular disorders (103 patients, 24.3%). Most frequent concomitant medications were antihypertensive drugs (71 patients, 15.5%).
The majority of patients received a dose of four tablets of the herbal preparation thrice daily. The most common preparations in the add-on herbal preparation group were gastrointestinal drugs in the AID (30.1%) and IBD (5.3%) groups. Patients with IBS applied spasmolytic/anticholinergic medication (7.9%) and probiotics (11%) more often. Other therapy consisted mainly of gastrointestinal preparations (AID: 44.8%, IBD: 2.0%, IBS: 12.3%) and homoeopathic medicine (AID: 15.8%) (see online supplementary data).
Efficacy
A reduction of the total mean symptom score was observed in all groups with all treatment options. The score decreased from 1.37±0.48 to 0.13±0.31, 1.54±0.50 to 0.40±0.43 and 1.21±0.42 to 0.43±0.35 in the AID, IBD and IBS groups, respectively. The pre-post change of the overall mean total symptom score was 1.34±0.48 to 0.20±0.35 (mono: 1.33±0.51 to 0.15±0.34, add-on: 1.39±0.41 to 0.30±0.37, other therapy: 1.31±0.43 to ±0.24±0.33). Covariance analysis of the primary efficacy criterion revealed a significant influence of the mean symptom score at baseline on the total mean symptom score. Thus, the change of clinical symptoms was adjusted to the same baseline criteria resulting in significant differences between disorder groups (AID, IBD, IBS; p=0.000) and therapy treatment (mono, add-on, other; p=0.023). Further analyses were conducted in the respective disorder groups, demonstrating no significant difference between treatment options in the AID (p=0.320) and IBD (p=0.554) groups. The pre-post change of the total mean symptom scores ranged from 1.21±0.03 to 1.25±0.01 in the AID group (mean difference: 0.000–0.035). In the IBD group, the change of the respective scores ranged from 1.08±0.08 to 1.27±0.22 (mean difference: 0.073–0.187). For IBS, significant differences (p=0.002) were observed between monotreatment and add-on treatment (mean difference: 0.140; 95% CI 0.036 to 0.245; p=0.009) and monotreatment and other therapy (mean difference: 0.217; 95% CI 0.085 to 0.349; p=0.001). Monotreatment of IBS with the herbal preparation resulted in a significantly higher total mean symptom score change compared to other treatment groups.
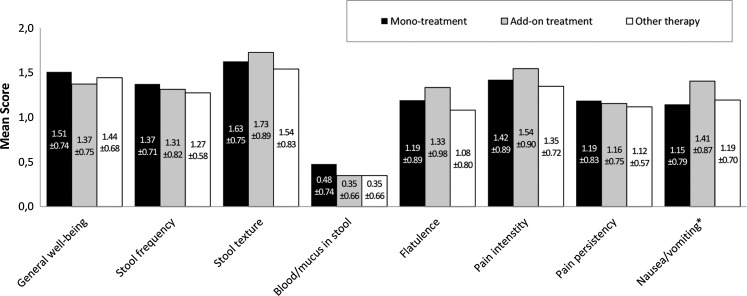
Considering single symptoms, significant difference between groups was observed for the symptom ‘nausea/vomiting’ (p=0.003) in the AID group resulting in a better improvement with herbal preparation add-on treatment compared with monotreatment and other therapy (figure 1). At last visit, 94% of patients receiving monotreatment, 89.2% of patients receiving add-on treatment and 97% of patients receiving other therapy were free of symptoms. Median time until symptom resolution was 4 days for all groups. Paired comparison demonstrated a significantly smaller probability to be free of symptoms for patients applying the herbal preparation as add-on compared to monotreatment (p=0.019) or other therapy (p=0.005). No difference was observed between monotreatment and other therapy groups (p=0.138).
Figure 1.
Change of the score of each single symptom in the acute inflammatory disorders group (visit 1—last visit). Positive score difference values indicate an improvement.
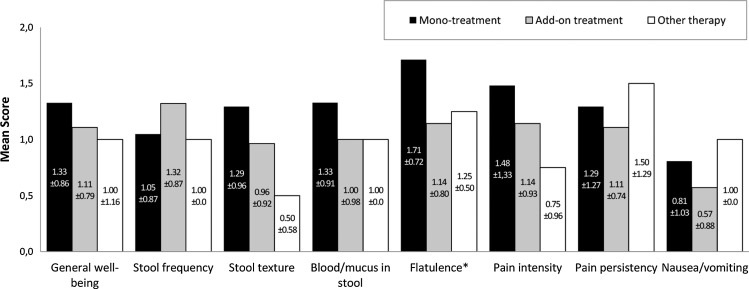
In the IBD group, stool frequency decreased significantly in the add-on treatment subgroup (p=0.026) compared to monotreatment and other therapy. At last visit, significant differences were observed for the symptom ‘flatulence’ (figure 2). Monotreatment resulted in significantly higher reduction of flatulence compared to add-on treatment or other therapy (p=0.038). The proportion of patients who were free of symptoms at the last visit was 80%, 64.3%, and 50% receiving monotreatment, add-on treatment or other therapy, respectively. Median time until symptom resolution was 16, 25 and 26 days for monotreatment, add-on treatment and other therapy, respectively. No difference was observed between groups regarding the probability to be free of symptoms.
Figure 2.
Change of the score of each single symptom in the inflammatory bowel diseases group (visit 1—last visit). Positive score difference values indicate an improvement.
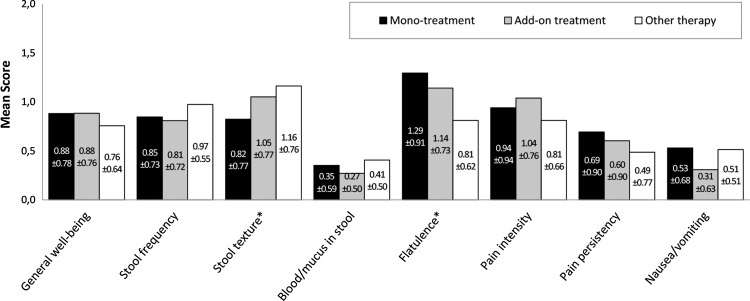
In the IBS group, improvement of stool frequency, flatulence and pain intensity was noted during the observation period. Stool frequency improved significantly with add-on treatment and other therapy compared with monotreatment (p=0.023). Pain intensity was lower with add-on treatment compared with monotreatment and other treatment options (p=0.018). Flatulence improved with monotreatment during the observation (p=0.001) and at last visit (p=0.010). A further significantly improved symptom at study end was ‘stool texture’ with add-on treatment and other therapy (p=0.047; figure 3). At last visit, 54.2% of patients receiving monotreatment, 38.5% receiving add-on treatment and 35.1% receiving other therapy were free of symptoms. Median time until symptoms resolution was shortest for monotreatment (27 days). No difference was observed between groups regarding the probability to be free of symptoms.
Figure 3.
Change of the score of each single symptom in the irritable bowel syndrome group (visit 1—last visit). Positive score difference values indicate an improvement.
At study end, physicians predominantly assessed the clinical course as considerable improvement to complete resolution in all groups. The difference between respective treatment options was statistically not significant. At least 70% of the physicians rated the efficacy as good to very good. No differences regarding efficacy were observed in the IBD group. In the AID and IBS groups, good to very good efficacy occurred significantly often in the monotreatment group (p<0.001). Patient's satisfaction regarding efficacy was significantly higher with monotreatment in the AID and IBS groups (p<0.001) and with add-on treatment in IBD group (p=0.043). Tolerability was rated predominantly good to very good in all groups. Treatment with the herbal preparation was generally better tolerated (AID, IBS: p<0.001; IBD: p=0.027).
Safety
Only two side effects were reported in relation to the herbal preparation. No serious adverse reactions in relation to the study medication were reported. In one case ‘vomiting’ was reported in a patient with IBD, who received 3×2 tablets daily, and ‘itching’ in another patient with IBS receiving 4×3 tablets daily. Both patients applied the herbal preparation as add-on therapy. No dose adjustment or additional treatment was necessary and both patients finished the study regularly.
Discussion
The aim of this observational study was to collect data regarding the efficacy, safety and tolerability of an herbal preparation containing a combination of myrrh, coffee charcoal and chamomile in the treatment of gastrointestinal disorders associated with symptoms of acute diarrhoea.
Analysis of the change of the mean total symptom score indicates that monotreatment with the herbal preparation is as effective as add-on treatment or other therapy in the management of acute diarrhoea due to AID and IBD. However, the results obtained for the IBD subgroup must be interpreted with caution due to a small number of patients. Thus the trend observed in the IBD subgroup needs to be verified in a larger population. Regarding IBS, a significantly higher reduction of symptoms was observed with the monotreatment indicating better management with the herbal preparation. Further, the results of the secondary efficacy criteria suggest that monotreatment with 3×4 tablets herbal preparation per day is at least as effective as add-on and other therapy.
The efficacy of the herbal preparation in the management of gastrointestinal symptoms is based on a proposed synergistic effect of the three active ingredients. Owing to a diverse spectrum of constituents, plants often possess various pharmacological effects. Myrrh was recently shown to reduce intestinal muscle tone and acetylcholine-induced contraction of inflamed rat ileum/jejunum preparations, thus contributing to reduction of intestinal motility and spasmolytic effects.12 Similar results were obtained for chamomile extract and its flavonoids in guinea pig ileum.13 14 Several constituents of the chamomile flower possess anti-inflammatory potential, for example, chamomile essential oil reduced TNBS-induced colitis in mice and mouse paw oedema.15 Chamazulen was reported to prevent leucotriene formation and chemical peroxidation of arachidonic acid, the latter likely through inhibition of COX-2.16 17 Chamomile extract also produced a 41% inhibition of rat paw oedema, and chamomile and myrrh showed a gastroprotective effect in rats.18 19 Anti-inflammatory and analgesic activity of myrrh extracts was recently demonstrated utilising the paw oedema mice model.20 Sesquiterpenes seem to be the main constituents responsible for the pharmacological effect of myrrh. These substances possess antibacterial and antifungal activity, an effect that might act supportive in infectious diarrhoea. Additionally, sesquiterpenes exert local anaesthetic activity by blocking the inward sodium current of excitable mammalian membranes.21 Coffee charcoal contributes its high adsorptive capacity and astringent activity of chlorogenic acid to the spectrum of activity of the herbal preparation.22
Of special interest are the positive results obtained in patients with IBD such as ulcerative colitis and Crohn's disease and in patients with IBS since those subgroups frequently require treatment of acute diarrhoea episodes due to the underlying disease. The nature of IBD is complex and involves chronic, uncontrolled inflammation of the intestinal mucosa with the inability to downregulate this activity. Factors contributing to the development of mucosal inflammation range from environmental to genetic influences. IBD is also characterised by intestinal barrier defects that are associated with increased permeability of the epithelial surface.23 The mechanisms responsible for IBS are not yet clear, but disturbances of the intestinal epithelial barrier seem to play a major role in development of symptoms. Patients with diarrhoea-predominant IBS frequently display abnormal small intestinal permeability.24 Epithelial permeability is determined by tight junctions (TJ), a complex of molecules that regulate the paracellular transport of ions and act as a barrier. Altered regulation of TJ in the form of upregulation of the poreforming claudin-2 and downregulation of the sealing proteins claudin-5 and -8 in the sigmoid colon lead to barrier dysfunction in active Crohn's disease, while downregulation of the sealing proteins occludin, claudin-1 and -4 and upregulation of claudin-2 were observed in ulcerative colitis.25 26 Proinflammatory cytokines also play an important role in epithelial damage by affecting TJ regulation, for example, tumour necrosis factor α (TNFα) causes upregulation of claudin-2.27 Myrrh, a component of the herbal preparation, and the combination itself were shown to inhibit TNFα-induced decrease in epithelial resistance through inhibition of claudin-2 expression. Further, a redistribution of claudin-1 into subapical compartments was suppressed. Similarly, chamomile extract induced an increased expression of claudin-7.28 Thus, regulation of TJs might constitute a possible mechanism for the improvement observed in these subgroups in the study.
Treatment with the herbal preparation was generally better tolerated and was assessed as superior. Only two already known adverse reactions were described in 1062 patients resulting in a very low incidence of 0.2%. Both side effects were of short duration and both patients recovered. Allergic reactions to myrrh or composite plants including chamomile are not unlikely and might result in the symptom ‘itching’.29 30 ‘Vomiting’ occurred in a patient suffering from food intolerances and might also be connected to the underlying disorder.
Limitations generally encountered in non-interventional studies are lack of randomisation, blinding or standardised treatment protocol. Since the allocation to treatment was conducted by the physician in accordance with the patient, no balance between groups regarding baseline score or treatment data was expected. Thus, regression to the mean was performed to reduce potential bias resulting from different treatment procedures. Further, the number of patients with IBD treated with other therapy was smaller compared to monotreatment and add-on treatment. Thus, only a tendency regarding comparability to other therapies can be made for the IBD group. However, in contrast to data derived from clinical trials in a restricted population, the large body of data provides a more accurate description on efficacy and safety of the herbal preparation in the management of acute diarrhoea in a heterogeneous population encountered in daily practice.
Conclusion
The combination of myrrh, coffee charcoal and chamomile flower extract is effective, well-tolerated and safe for use in patients with symptoms of acute diarrhoea. Its efficacy is comparable to other therapies used in routine care and apparently more effective in patients with IBS.
Supplementary Material
Footnotes
Contributors: UA was involved in planning, conducting and reporting of the study. BS was the responsible statistician and performed the statistical analyses. RS and VM drafted the article. All authors contributed to subsequent and final drafts. UA is the guarantor.
Funding: This research was funded by Repha GmbH and the company was also the sponsor of the study.
Competing interests: BS received remuneration from Repha GmbH for performing statistical analyses, RS for his consulting service and UA was compensated for the coordination of the study. VM has no competing interests.
Patient consent: Obtained.
Ethics approval
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Gerlach K. Durchfall als Beratungsanalss in der allgemeinmedizinischen Sprechstunde. Dresden: Technische Universität Dresden, 2011. [Google Scholar]
- 2.Laux G, Kühlein T, Gutscher A, et al. . Versorgungsforschung in der Hausarztpraxis—Ergebnisse aus dem CONTETN-Projekt 2006–2009. München: Springer Medizin Urban und Vogel GmbH, 2010. [Google Scholar]
- 3.Farthing M, Salam M, Lindberg G, et al. ; Acute diarrhea in adults and children: a global perspective. World Gastroenterology Organisation, 2012. [DOI] [PubMed]
- 4.Sander M, Gerlach K. [DEGAM Guideline. Acute diarrhea]. AWMF-Registernr. 053/030, Klasse 1. Sep 2013.
- 5.Summary of Product Characteristics. Myrrhinil-Intest®. Repha GmbH. Feb 2013.
- 6.El Ashry ESH, Rashed N, Salama OM, et al. . Components, therapeutic value and uses of myrrh. Pharmazie 2003;58:163–8. [PubMed] [Google Scholar]
- 7.ESCOP Monographs. Myrrha—Myrrh. Thieme; 2003 Nov, 2nd edition, 340–343.
- 8.BGA Monograph. [Coffeae carbo, coffee charcoal]. 27. Erg.-Lieferung ZRvA, Apr 1990. [Google Scholar]
- 9.ESCOP Monographs. Matricariae flos—Matricaria flower. Thieme; 2003 Nov, 2nd edition, 312–319. [Google Scholar]
- 10.Langhorst J, Varnhagen I, Schneider B, et al. . Randomised clinical trial: a herbal preparation of myrrh, chamomile and coffee charcoal compared with mesalazine in maintaining remission in ulcerative colitis—a double-blind, double-dummy study. Aliment Pharmacol Ther 2013;38:490–500. [DOI] [PubMed] [Google Scholar]
- 11.Zwiener I, Blettner M, Hommel G. [Survival analysis—part 15 of a series on evaluation of scientific publications]. Dtsch Arztebl Int 2011;108:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vissiennon C, Goos K, Goos O, et al. . Calcium antagonistic effects of ethanolic myrrh extract in inflamed intestinal smooth muscle preparations. Planta Med 2013;79:SL70. [DOI] [PubMed] [Google Scholar]
- 13.Forster HB, Niklas H, Lutz S. Antispasmodic effects of some medicinal plants. Planta Med 1980;40:309–19. [DOI] [PubMed] [Google Scholar]
- 14.Achterrath-Tuckermann U, Kunde R, Flaskamp E, et al. . [Pharmacological investigations with compounds of chamomile. V. Investigations on the spasmolytic effect of compounds of chamomile and Kamillosan on the isolated guinea pig ileum]. Planta Med 1980;39:38–50. [DOI] [PubMed] [Google Scholar]
- 15.Fabian D, Juhás Š, Bukovská A, et al. . Anti-inflammatory effects of chamomile essential oil in mice. Slovak J Anim Sci 2011;44: 111–6. [Google Scholar]
- 16.Safayhi H, Sabieraj J, Sailer ER, et al. . Chamazulene: an antioxidant-type inhibitor of leukotriene B4 formation. Planta Med 1994;60:410–13. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci 2009;85:663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hindawi MK, Al-Deen IH, Nabi MH, et al. . Anti-inflammatory activity of some Iraqi plants using intact rats. J Ethnopharmacol 1989;26:163–8. [DOI] [PubMed] [Google Scholar]
- 19.Al-Harbi MM, Qureshi S, Raza M, et al. . Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol 1997;55:141–50. [DOI] [PubMed] [Google Scholar]
- 20.Su S, Wang T, Duan JA, et al. . Anti-inflammatory and analgesic activity of different extracts of Commiphora myrrha. J Ethnopharmacol 2011;134:251–8. [DOI] [PubMed] [Google Scholar]
- 21.Dolara P, Corte B, Ghelardini C, et al. . Local anaesthetic, antibacterial and antifugal properties of sesquiterpenes from myrrh. Planta Med 2000;66:356–8. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn A, Schäfer G. Experimentelle Beiträge zur Chemie der Heislerschen “Kaffeekohle”. Süddeutsche Apotheker-Zeitung 1939;79:434–42. [Google Scholar]
- 23.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006;12(Suppl 1):S3–9. [DOI] [PubMed] [Google Scholar]
- 24.Dunlop SP, Hebden J, Campbell E, et al. . Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastronenterol 2006;101:1288–94. [DOI] [PubMed] [Google Scholar]
- 25.Zeissig S, Bürgel N, Günzel D, et al. . Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 2007; 56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller F, Florian P, Bojarski C, et al. . Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129:550–64. [DOI] [PubMed] [Google Scholar]
- 27.Mankertz J, Amasheh M, Krug SM, et al. . TNFalpha up-regulated claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 2009;336:67–77. [DOI] [PubMed] [Google Scholar]
- 28.Schulzke JD, Rosenthal R. [Barrier-effects of Myrrhinil-Intest® in a cell culture model] 2013. Unpublished raw data.
- 29.Andres C, Chen WC, Ollert M, et al. . Anaphylactic reaction to chamomile tea. Allergol Int 2009;58:135–6. [DOI] [PubMed] [Google Scholar]
- 30.Sheir Z, Nasr AA, Massoud A, et al. . A safe, effective, herbal antischistosomal therapy derived from myrrh. Am J Trop Med Hyg 2001;65:700–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.