Abstract
Sleep disturbances are highly prevalent in women with HIV and few studies examine potential protective factors that may reduce risk for sleep disturbances in this high-risk population. We predicted that HIV-specific social support from various sources (i.e., friends, family, spouses), as well as oxytocin (OT), would explain sleep quality in 71 low income minority women living with HIV. Social support from family members was associated with better sleep quality in women. For women with high OT, support from friends was associated with better sleep quality, while for women with low OT, support from friends was associated with poorer sleep quality. Women with low OT may not effectively interpret and utilize available support resources, which may be associated with sleep disturbances.
Keywords: HIV, Social Support, Oxytocin, Sleep Disturbance, Women
Over 70% of individuals with HIV report sleep related issues including fatigue, difficulties falling asleep, and early morning awakening (Hand, Phillips, Sowell, Rojas, & Becker, 2003; Lee et al., 2012; Rubenstein & Selwyn, 1998). Individuals living with HIV report longer sleep latency (time spent falling asleep), shorter sleep duration (time spent asleep), reduced sleep efficiency (ratio of minutes slept to minutes spent in bed), higher rates of daytime sleepiness, and fatigue (Pence, Barroso, Leserman, Harmon, & Salhuddin, 2008; Salhuddin, Barroso, Leserman, Harmon, & Pence, 2009; Wiegand et al., 1991). Sleep disturbances can have a negative impact on HIV-disease management and progression, and often covary with negative psychological states (Darko, Mitler, & Miller, 1998; Phillips, 1999).
A disproportionate number of women living with HIV are from low income ethnic minority populations, yet they are one of the more understudied groups of individuals with HIV (Publication of revised HIV/AIDS surveillance report, 2005). In general, women have a higher risk of developing chronic sleep problems, which may be due to higher physiological and emotional reactivity to interpersonal stressors (Cross & Madsen, 1997; Troxel, 2010). Women living with HIV, especially those from ethnic minority populations, may be particularly at risk for chronic sleep problems. They often experience high levels of stress and distress, have fewer social resources, and experience HIV-related fatigue and sleep disturbances (Cederfjäll, Langius-Eklöf, Lidman, & Wredling, 2001; Gordillo, Fekete, Platteau, Antoni, Schneiderman, & Nöstlinger, 2009; Lee, Portillo, & Miramontes, 2001). Among women with HIV, poorer sleep quality is associated with greater anxiety and depressive symptoms and poorer health related quality of life (Phillips, Sowell, Boyd, Dudgeon, & Hand, 2005). Experiencing depressive symptoms as a result of sleep disturbance may, in turn, be associated with poorer medication adherence and poorer health outcomes (Phillips et al., 2005; Saberi, Neilands, & Johnson, 2011). Thus, women living with HIV provide a rich context in which to study how internal (OT levels) and external (sources of support received) factors contribute to sleep disturbances.
Just as sleep disturbances can be associated with poorer psychological well-being, increases in stress and distress can manifest themselves as sleep disturbances in individuals living with HIV. Higher distress in women living with HIV was associated with more sleep difficulties and higher fatigue (Marion et al., 2009). Other research links higher distress to greater sleep disturbances in men and women living with HIV, which in turn may be associated with poorer immune function (Cruess et al., 2003). However, few studies have examined how protective psychosocial factors may influence sleep quality in individuals living with HIV.
One psychosocial factor known to be associated with sleep behaviors is social support. An individual’s social functioning and sleep are related to health and well-being through their influence on shared psychological, behavioral, and neurobiological mechanisms (Troxel, 2010). Stress in close social relationships may lead to ruminative thoughts at bedtime, which in turn may contribute to sleep disturbances (Hall et al., 2000; Troxel, 2010). In contrast, having positive social relationships may promote healthier sleep through encouraging positive mood, lowering stress, and preventing social isolation (Cacioppo et al., 2002; Troxel, 2010). In contrast, social stressors are associated with poorer health behaviors, such as excessive drinking or drug use, that are known to have deleterious effects on sleep (Cohen, 2004).
Although the theoretical links between social relationships and sleep are established, research has yet to systematically explore how various aspects of social relationships are associated with sleep quality in individuals with HIV. Theories on social support suggest that the construct of support is multidimensional in nature. Thus, while examining how different types of support (e.g., emotional support, tangible assistance, advice) are associated with health is important, the source of support is an important dimension of social relationships that should not be ignored (Schwarzer, Dunkel-Schetter, & Kemeny, 1994). For men and women living with HIV, support from friends may be qualitatively different than support from family members. Indeed, one study found that the links between support and sleep disturbance in men and women with HIV may be dependent on the source of support (Vosvick et al., 2004). While support from families and partners was not associated with sleep outcomes, support from friends emerged as a predictor of sleep, though in a complex manner. Specifically, emotional support from friends was associated with less sleep disturbance, while instrumental support from friends was associated with greater sleep disturbance (Vosvick et al., 2004). This suggests that there are nuances in the ways in which support from various sources in one’s social network may influence sleep quality in persons living with HIV.
In addition to the links between social support and sleep behaviors in individuals with HIV, there is emerging interest in examining how biological processes may moderate the influence of one’s social affiliations on health outcomes, including sleep (Taylor, 2006). Oxytocin (OT), a neurohormone released during the stress response process, is linked to psychosocial stressors, social support, and anxiety (Taylor, 2006). Increases in OT during times of stress are associated with increased social contact and support seeking behaviors (Taylor et al., 2000; Taylor, 2006). OT may also increase as a function of support and physical contact from close social network members, such as a partner (Grewen et al., 2005; Holt-Lunstad, Birmingham, & Light, 2008).
A combination of OT and support appears to be most effective in increasing calmness and decreasing anxiety during stressful social situations (Heinrichs, et al., 2003). However, women with higher levels of OT report reduced contact with various sources of support, lower perceptions of support from partners, and more relationship distress (Taylor et al., 2006; Taylor, Saphire-Bernstein, & Seeman, 2010). OT was also linked to interpersonal distress and other negative interpersonal characteristics such as inappropriate self-disclosures, attention seeking, and having problems being alone (Turner, Altemus, Enos, Cooper, & McGuinness, 1999). One explanation for these inconsistent findings is that OT increases when affiliation is needed or social contact occurs, but it fails to reduce anxiety and distress when social needs are not met (Taylor et al., 2010).
In addition to its anxiety-reducing properties and links with social behaviors, it is likely that OT is associated with sleep behaviors and sleep quality. A main source of OT production is the hypothalamus, a brain structure associated with the sleep-wake process. Animal models suggest that OT may exert its influence on sleep through HPA-axis activity and the regulation of corticotropin releasing hormone (CRH; Neumann, Krömer, et al., 2000; Neumann, Wigger, et al., 2000). Both CRH and HPA-axis activity are linked to sleep-wake behavior in human and animal populations (Opp, 1995). In addition, OT was associated with sleep quality in male rats such that basal levels of OT promoted sleep quality while exogenous injections of OT (mimicking the release of OT in the stress response process) led to wakefulness (Lancel, Krömer, & Neumann, 2003). It is plausible to assume that OT exerts similar effects on sleep behaviors in human populations, particularly in individuals living with health conditions known to be associated with sleep disturbances, such as HIV.
The Present Study
In our prior research, we found that OT may have stress-buffering effects. For low income minority women living with HIV with low levels of OT, perceived stress was associated with poorer immune status. In contrast, for women with high levels of OT, stress perceptions were associated with a better immune status (Fekete et al., 2011). However, we did not examine how social relationships factor into the relationship between OT and HIV-related health behaviors, such as sleep. The current study is a secondary data analysis on a sample of ethnic minority women living with HIV. Our first hypothesis predicted that support from close social network members such as friends, relatives, and spouses/partners would be associated with better global sleep quality in women living with HIV. Our second hypothesis predicted that the associations between support and better sleep quality would be enhanced in women with higher levels of OT. In contrast, we hypothesized that low levels of naturally circulating OT would lessen the benefits of social support on sleep quality.
Methods
Participants and Procedure
Our study utilized baseline data from a larger longitudinal study conducted from 1998 to 2004, which examined associations among psychosocial, behavioral and physiological factors in women living with HIV who were prescribed a highly active antiretroviral therapy (HAART) regimen. After completing a baseline assessment, women were randomized into either a 10-week cognitive behavioral stress management intervention or psychoeducational control condition. To be included in the study, women had to between the ages of 18 and 65 and currently prescribed HAART medication. Exclusionary criteria included being prescribed medications that had immunomodulatory effects, having a history of chemotherapy or whole body irradiation treatment of cancer, or having a history of chronic illness associated with persistent generalized lymphadenopathy or permanent changes in the immune system. Temporary exclusion criteria included any change in antiretroviral regimen during the last month, intravenous drug use within the previous 6 months, acute bodily infection requiring antibiotics within the last 2 weeks, hospitalization for surgery within the past 3 months, and acute bodily infection during the past month. Women provided informed consent prior to participating in the study and completed a screening interview where additional exclusionary criteria were assessed. These criteria included an inability to read at a sixth-grade level, significant cognitive impairment, current psychosis, drug or alcohol dependence, panic disorder, or active suicidality. Women who were eligible for the study completed a battery of psychosocial measures administered via interview and self-report methods and provided morning peripheral venous blood samples.
Of the 322 women initially screened for the study, 134 were eligible and included in the larger study. The most common reasons for exclusion included participants electing not to participate, currently participating in other clinical research trials, having no current prescription for antiretroviral medication, having the presence of a comorbid medical condition known to impact immune functioning, not having the ability to read or write English, having evidence of significant cognitive impairment, having the presence of a psychological condition meeting exclusionary criteria, or testing positive on a urine screen for illicit substances.
Of the 134 women, 85 of these women who identified that they were from an ethnic minority population and provided blood samples for the OT assays were initially included in the current study. We then excluded twelve of these women from the main analyses because they did not have detectable levels of plasma OT (i.e., less than 0.5 pg/ml) and their OT levels could not be evaluated in a systematic or meaningful manner. Two additional cases were excluded from the current analyses because they had extreme OT levels (i.e. greater than 165 pg/ml). Although we extracted OT from our plasma samples, these extreme OT values may reflect immunoreactive molecules that are present in plasma extracts and not true values of OT (Szeto et al., 2011). Thus, the values are difficult to interpret in relation to more normally distributed values of OT, and were determined to be outliers that were skewing the data. The 14 women who were excluded from the current analyses did not differ from the 71 women included in the study on any study variables or sociodemographic characteristics.
The 71 women included in the study had a mean age of 38.4 (SD = 7.3, range 20–49) and 67.1% were currently involved in a relationship. They had been diagnosed with HIV for an average of 7.8 years (SD = 4.4 years), and all participants were taking HAART medications. The majority of participants were Black (86%), and the others were Hispanic (11.3%), Asian/Pacific Islander (1.4%), and American Indian/Alaska Native (1.4%). About half the women completed high school (56.3%), and most of the women were unemployed and receiving disability (74.7%). The average yearly income for the participants was between $5,000 and $10,000.
Measures
Social Support
The UCLA Social Support Inventory (UCLA-SSI; Schwarzer et al., 1994) measured perceptions of HIV-related social support received from women’s partners, friends, and families. Participants reported how often over the past month they perceived receiving information/advice, aid/assistance, and emotional support from each of the sources of support on a scale ranging from 1(never) to 5 (very often). If participants did not have a particular source of support in their lives, they reported 0 (not applicable). Scores for each of the sources of support were summed across type of support (e.g. emotional, tangible, informational) to create composite scores for each source of support. The mean composite score for support from a spouse/partner was 9.88 (SD = 6.7; α = .94), from friends was 10.25 (SD = 4.9; α = .88), and from family was 11.69 (SD = 5.5; α = .91). Both the potential and the actual range for each of the composite scores of social support was 0 – 20 for each source.
Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) measured sleep quality (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI provides a global measure of sleep ranging from 0 to 21, with higher scores indicating poorer sleep quality. In addition to the global score, seven component scores that measure subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime sleep dysfunction can be assessed. Component scores range from 0 to 3, with higher scores indicating poorer sleep outcomes. On average, women reported that it took them 23.9 minutes to fall asleep each night (SD = 26.7; range = 1 – 120), and they slept for an average of 7.1 hours per night (SD = 1.7; range = 3 – 11). Women’s mean global sleep quality was 6.30 (SD= 3.86; α = .71; range = 1 – 17). 58% of women scored greater than five on the global sleep quality measure, which is an indicator of poor sleep quality (Carpenter & Andrykowsi, 1998). Women’s mean subjective sleep quality was .84 (SD = .76; range = 0 – 3), their mean sleep latency was 1.08 (SD = .96; range = 0 – 3), their mean sleep duration was .98 (SD = .99; range = 0 – 3), their habitual sleep efficiency was .79 (SD = 1.06; range = 0 – 3), their sleep disturbance was 1.17 (SD = .67; range = 0 – 3), their use of sleep medication was .88 (SD = 1.2; range = 0 – 3), and their daytime sleep dysfunction was .66 (SD = .74; range = 0 – 3).
Oxytocin
Blood samples were collected in the morning from participants in ethylenediaminetetraacetic acid (EDTA) tubes (Vacutainer-EDTA, Becton-Dickinson, Rutherford, NJ). Plasma was separated by centrifugation at 4° C, 2000 rpm, for 30 minutes and stored at −80° C. Our lab recently addressed important issues in the measurement of plasma OT using commercially available methods (Szeto et al., 2011), and we have suggested that it is essential to extract plasma samples to eliminate compounds that artificially increase the apparent plasma OT levels. Additionally we identified several immunoreactive molecules present in plasma extracts that together account for OT-like immunoreactivity. These molecules likely represent OT precursors and/or degradation products and together account for plasma OT levels. Therefore, OT levels were measured after solid phase extraction of 1 ml of plasma (resulting in a 10-fold concentration) and measured by a commercially available enzyme immunoassay (Assay Design, Ann Arbor, MI; Szeto et al., 2011). The limit of detection was 1.2 pg/well and intra- and inter-assay variability were 9% and 15% as reported by the manufacturers. The women in our study had a mean plasma OT level of 21.0 pg/mL (SD = 22.4; median = 13.92).
Covariates
Potential covariates included sociodemographic (i.e., age, ethnicity, employment, income), social (i.e., marital status, living arrangements), health (i.e., months post HIV diagnosis, HAART medication adherence, menstrual cycle), and mood (i.e. depression, stress, anxiety) characteristics. None of these factors were significantly associated with OT, the global PSQI score, or any component scores, thus we did not control for these factors in our analysis. However, prior research links perceived stress, depression, anxiety, and disease status to both OT and sleep quality (Cruess et al., 2003; Fekete et al., 2011; Marion et al., 2009; Nokes & Kendrew, 2001; Phillips et al., 2005; Scantamburlo et al., 2007; Wibbeler, Reichelt, Husstedt, & Evers, 2012). Therefore in order to hold stress and mood levels constant our study controlled for perceived stress, anxiety, and depression. Because these affective variables can be highly correlated, we examined correlation coefficients among the three variables before including them as covariates. No correlations exceeded .60, suggesting that multicollinearity was not an issue among these three covariates (Tabachnik & Fidell, 2012). Bivariate correlation analyses revealed that both depression and anxiety were associated with the PSQI total score (r = .37, p < .01 for depression; r = .32, p < .01 for anxiety), but perceived stress was not correlated with the PSQI total score. Finally, we controlled for medication adherence and number of months post-HIV diagnosis in all analyses, as some evidence suggests that disease severity and HAART medications may contribute to sleep disturbance in individuals with HIV (Phillips et al., 2005; Reid & Dwyer, 2005).
Perceived stress was measured using the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983), which measured how often women appraised situations in their lives as being stressful in the past month. Depression was measured using the Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977), which measures both cognitive and somatic aspects of depression. Anxiety was measured using the tension subscale of the Profile of Mood States, which measures the extent to which participants report being anxious, nervous, and tense (POMS; McNair, Lorr, & Droppleman, 1971). Number of months post-HIV diagnosis were measured through self-report, and medication adherence was also measured via self-report using The Adult AIDS Clinical Trial Group Adherence to Combination Therapy Guide (ACTG; Chesney et al., 2000). Women’s mean PSS score was 24.76 (SD = 7.4), their mean CES-D score was 18.04 (SD = 10.1), and their mean POMS-tension score was 3.72 (SD = 2.81).
Analysis Plan
To minimize type 1 error due to multiple comparisons, women’s global sleep quality score was treated as an omnibus test, and the component scores were only examined if an omnibus test was significant. To examine our first hypothesis we conducted hierarchical regression analyses. Covariates were entered into the first step of the regression followed by all sources of HIV-specific support in the second step. For our second hypothesis, we conducted moderated regression analyses. Covariates were again entered into the first step followed by the predictor variable (source of support). The moderator variable (OT) was entered into the third step, and an interaction term between the centered predictor variable and centered moderator variable (OT X support) was entered into the final step of the model. Predictor and moderator variables were centered to protect against multicollinearity and significant interaction terms were decomposed and graphed with one standard deviation above and below the centered mean representing high and low levels of the moderator variable (Jaccard & Turrisi, 2003).
Results
Characteristics of Women with High and Low Levels of Oxytocin
Because women with high OT may differ from women with low levels of OT (Fekete et al., 2011), we first divided women into high, middle and low tertiles based on their OT levels. Women in the low group had OT values ranging from 1.3 – 9.1 pg/mL, women in the medium group had OT values ranging from 9.2 – 22.7 pg/mL, and women in the high group had OT values ranging from 22.8 – 115.1 pg/mL. We conducted a series of independent t-tests and chi-square analyses to determine whether women in the high (n = 23) versus low (n = 23) tertile differed on any of the study variables (sociodemographic and health characteristics, HIV-specific support measures, sleep measures, neuroendocrine measures, and affective measures). No significant differences were found between the OT groups in age, education, time since diagnosis, income, adherence to HAART medication, time since HIV diagnosis, depression, anxiety, stress, sources of social support, or PSQI total score.
Direct Associations Between HIV-Specific Social Support and Sleep
Hierarchical regression analyses were first conducted to examine the direct effects of all sources of social support on women’s total PSQI score (top portion of Table 1). After controlling for depressive symptoms, stress, anxiety, medication adherence, and months since HIV-diagnosis, HIV-specific social support from families was significantly associated with a lower overall PSQI score. Because the support from families was significantly associated with better overall sleep quality, we examined the relationships between HIV-specific family support and the PSQI component scores. These analyses revealed that women with more support from relatives reported longer sleep duration (β = −.28 SE = .13, p < .05) and less use of sleep medication (β = −.28 SE = .13, p < .05).
Table 1.
Direct and Interactive Effects of Social Support on Sleep Quality in Low-Income Ethnic Minority Women Living with HIV (n=71).
| UCLA-SSI Sources of Social Support1 | PSQI Total Score |
|---|---|
| β (S.E.) | |
| Partner/Spouse Support | .08(.12) |
| Friend Support | .15(.12) |
| Family Support | −.29(.13)* |
| UCLA-SSI Sources of Social Support × Oxytocin (OT)1 |
|
|---|---|
| β (S.E.) | |
| Partner/Spouse Support × OT | .12(.12) |
| Friend Support × OT | −.28(.12)* |
| Family Support × OT | −.08(.12) |
p < .05
Models control for medication adherence, months since HIV diagnosis, depression, anxiety, and perceived stress.
Interactive Effects of Social Support and Oxytocin on Sleep
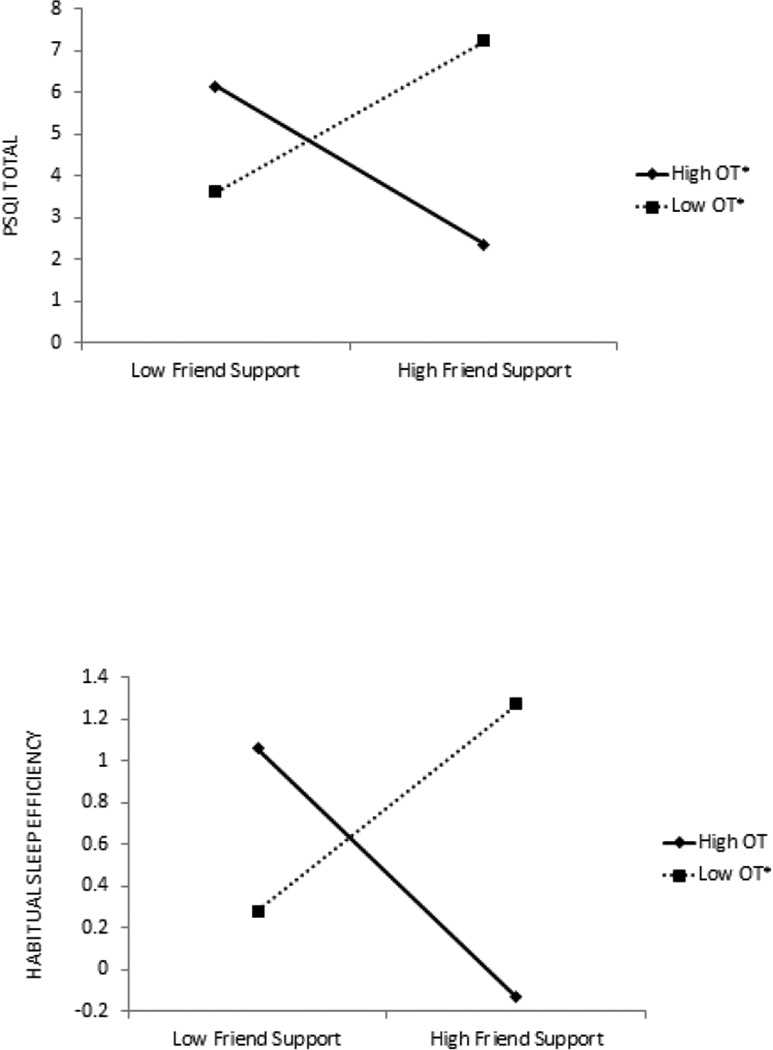
Next, we examined the interactive effects of HIV-specific social support and OT on women’s sleep. As shown in the bottom portion of Table 1, moderated hierarchical regression analyses revealed a significant interaction between HIV-specific social support from friends and OT in explaining women’s total PSQI score. No significant interactions emerged between the other sources of support and OT in explaining women’s total PSQI score. Decomposed interaction effects (Figure 1) reveal that for participants with high levels of OT, HIV-specific support from friends was associated with higher overall sleep quality (t67 = −2.08, p < .05). In contrast, at low levels of OT, support from friends was associated with lower levels of overall sleep quality (t67 = 1.99, p < .05).
Figure 1.
Oxytocin as a moderator of the association between social support from friends and sleep quality. Note. Intercept scores reflect centered scores as opposed to raw scores.
Because the interaction between support from friends and OT was significantly associated with the overall PSQI scale score, we examined the interactive effects of support from friends and OT on the PSQI component scores. Analyses revealed a significant interaction between HIV-specific support from friends and OT in predicting habitual sleep efficiency (β = −.28 SE = .13, p < .05). Figure 1 reveals that for participants with high levels of OT, HIV-specific support from friends was marginally associated with better habitual sleep efficiency (t67= −1.89, p = .06). In contrast, for participants with low levels of OT, support from friends was associated with poorer habitual sleep efficiency (t67 = 2.10, p < .05). No other interactions emerged between support from friends and OT in explaining the PSQI component scores.
Interactions Between Oxytocin and Type of Support from Friends
We conducted post-hoc analyses to further probe the significant interaction effects between OT and type support from friends (e.g., informational support, tangible assistance, and emotional support) on overall sleep quality and habitual sleep efficiency. Women’s mean informational support from friends was 2.33 (SD = 1.4), mean assistance support from friends was 2.55 (SD = 1.4), and mean emotional support from friends was 5.37 (SD = 2.7). A significant interaction emerged between informational support from friends and OT in explaining women’s global sleep quality (β = −.25 SE = .12, p < .05). Consistent with the results from our main analyses, women with high OT who reported receiving more informational support had better overall sleep quality (t67 = −2.60, p < .05) whereas women with low OT who reported receiving more informational support had poorer overall sleep quality (t67 = 5.08, p < .05).
Analyses Including Women With Undetectable Levels of OT
We conducted all study analyses including the 12 women with undetectable OT values (n = 83 women). Adding the 12 undetectable OT cases into the analyses did not have a substantive impact the main study findings. Specifically, in regards to the direct effects of various sources of support on sleep quality, support from relatives marginally predicted the global PSQI total score (n = 83; β = −.21 SE = .08, p = .07). In addition, neither spouse/partner support nor friend support predicted the total PSQI score in the expanded data. Support from relatives marginally predicted the use of sleep medication in the expanded data (n = 83; β = −.23 SE = .03, p = .06). However, while the direction of the association did not change, the relationship between support from relatives and sleep duration was reduced to non-significance (n = 83: β = −.12, SE = .02, p = .30). Family support did not predict any other PSQI subscales.
In regards to the interactive effects of sources of social support and OT on sleep quality, the OT and friend support interaction remained significant in predicting PSQI total score (n = 83; β = −.25 SE = .01, p < .05). In addition, no other interactions (OT x spouse/partner support, OT x relative support) predicted PSQI total score. The interaction between OT and friend support marginally predicted habitual sleep efficacy (n = 83; β = −.21 SE = .01, p = .08). In addition, the OT x friend support interaction did not predict any other PSQI subscale.
Discussion
Findings from our study suggest that while support from families may be directly associated with better sleep quality, longer sleep duration, and less use of sleep medications, the associations between support from friends and sleep quality are not as straightforward. Neurohormones associated with social support processes may play a role in the extent to which HIV-specific support from friends is associated with sleep quality. In some cases, OT enhanced the benefits of support from friends on sleep in women living with HIV, while deficiencies in OT may have prevented women from deriving health-related benefits from their friendships.
HIV-specific support from friends was related to poorer sleep quality and poorer sleep efficiency in women living with HIV who had low levels of OT. In contrast, women with high OT who received support from friends experienced better overall sleep quality and marginally better sleep efficiency. These results are among the first to suggest that qualitative aspects of social relationships and their associations with stress-related symptoms such as sleep quality may vary as a function of OT level. It is possible that OT facilitated the associations between interactions with friends and sleep quality. Increases in stressful life events are typically associated with increased levels of OT and social support (Taylor, 2006). OT and support, in turn, are thought to be associated with reductions in stress, distress, and anxiety (Heinrichs et al., 2003; Taylor, 2006). Therefore, women who had a combination of high OT and high support from friends may have experienced fewer factors associated with sleep disturbance, such as ruminative thoughts and distress. In contrast, women who had support from friends but had lower levels of OT did not yield the same health benefits.
It is also possible that the type of HIV-specific social support women received from friends did not match the type of support that was desired. Post-hoc analyses revealed that women with higher levels of OT who received informational support from friends reported better overall sleep quality, while women with lower levels of OT reported poorer sleep quality with the receipt of informational support from friends. Informational support has been linked to lower distress in individuals coping with chronic illnesses (Helgeson, 1993). However, if women received information from their friends that elicited distress or caused ruminative thoughts, deficits in OT may have made women more susceptible to sleep problems.
However, friends may have also engaged in problematic support efforts such as minimizing women’s HIV-related problems or forcing women to be optimistic about their illness. This type of support, though benevolent in intention, is typically interpreted by support recipients as being unhelpful and is often associated with increased stress and distress (Stephens & Clark, 1997). While higher OT may have buffered the negative effects of these types of social interactions, deficits in OT may have been associated with higher stress manifested as disturbances in sleep. Unfortunately, our measure of HIV-specific social support did not assess negative or problematic aspects of social interactions, or women’s interpretations of support received from friends. Future research should examine the links between OT and more complex aspects of social relationships on sleep outcomes in individuals with HIV.
It is interesting that OT did not play a role in the relationship between HIV-specific support from family members and sleep quality. Compared to men, women tend to draw support from a broader number of sources (Antonucci, 1994). However, for women living with HIV from ethnic minority populations, families are often the first place women turn to as a primary source of emotional and tangible support in helping them cope with their illness (Owens, 2003). In addition, research examining links between the stressful process of disclosing one’s HIV positive serostatus and stress/distress in ethnic minority women living with HIV finds that support from close family members can sometimes act as a buffer of the negative effects of stress on physical and psychological health (Fekete et al., 2009).
The direct associations between family support and better sleep quality may have reflected long-standing patterns or expectations of support. Support from close family members and relatives may be more emotionally salient to women living with HIV, and more strongly associated with their well-being, than support from friends (Serovich et al., 2001). It may be that support from family members was more accessible to the women in our study, whereas support from friends was solicited on an as needed basis. Therefore, OT may have been more critical for women living with HIV when it was necessary to seek support from sources beyond their immediate social network.
No significant direct or interaction effects emerged between OT, support from spouses, and sleep quality. A recent review of the literature on sleep outcomes suggests that sleep is a shared health behavior for individuals who are married or partnered, and that while sleeping with a partner yields psychological benefits it may also negatively impact sleep quality (Troxel, 2010). Our study was not powered to examine differences in sleep behaviors between partnered and unpartnered women. Future research should examine whether the patterns of associations between OT, support, and sleep quality differs between women who are partnered or married versus unpartnered women who receive support from other sources. In addition, prior research from our lab suggests that OT may buffer the negative effects of stress on immune function (Fekete et al., 2011) and that sleep disturbances are associated with poorer immune function (Cruess et al., 2003) in individuals with HIV. Future research may examine more complex growth curve models examining longitudinal associations between OT, support interactions, and both sleep quality and immune function in individuals with HIV.
There are several limitations to our study. Our data are cross-sectional and causal relationships cannot be established. Although research suggests that social support and OT levels precede psychosocial health outcomes, it is possible that women with more sleep disturbances were more socially isolated due to feeling fatigued. It is also possible that OT and sleep are bi-directionally related. While research suggests that OT may regulate the sleep-wake process in individuals through its effect on HPA-axis activity, chronic sleep disturbances or chronic insomnia may be associated with dysregulation of HPA-axis activity (Rodenbeck & Hajak, 2001) and other hormonal processes, possibly affecting OT levels. Secondly, our study controlled for factors associated with sleep disturbances, but it is possible that other factors such as BMI, pain, and presence of sleep apnea may have caused women’s sleep disturbance. Future research may consider the role that factors beyond stress and psychological well-being play in the sleep disturbances of women living with HIV. Third, our sample consisted of ethnic minority women living with HIV, and it is unclear how our pattern of findings would extend to other populations of individuals such as ethnic minority men, gay men, or older adults with HIV. Finally, there are emerging insights on the limits of the assays currently available for capturing differences in plasma OT levels. Although we did perform extraction to remove interfering compounds and to concentrate samples before assaying OT (Szeto et al., 2011), this method may still allow for the inclusion of the chemical degradation products associated with OT, in addition to intact OT. The OT assay does not detect very low levels of OT, and 12 women were deleted from our initial sample due to undetectable levels of OT. It is unclear how the pattern of results may have differed if we had variability in the levels of OT in the 12 women. However, including them in the analyses did not produce substantive changes in the results.
Despite its limitations, our study provides important evidence linking social relationships, biological factors, and sleep behaviors in a sample of low income minority women living with HIV. A significant proportion of individuals with HIV experience sleep problems (Hand et al., 2003; Lee et al., 2012; Rubenstein & Selwyn, 1998), which may be linked to a less efficient immune response or impaired immune function (Gómez-González et al., 2012). Understanding how various psychosocial and biological factors are related to sleep disturbances in individuals living with HIV may help to prevent or delay disease progression. The findings from our study may also help to guide psychosocial and behavioral interventions conducted with individuals living with HIV. Support interventions may focus on helping individuals understand the benefits of soliciting both different types of support as well as support from different sources. These interventions can focus on helping individuals understand the importance of healthy sleep behaviors. Targeting women who have naturally low levels of OT and utilizing cognitive behavioral stress management techniques that teach women various coping skills including support seeking behaviors (e.g., Antoni, Schneiderman & Ironson, 2007) may help to alleviate stress and distress that may be associated with sleep disturbance.
Acknowledgments
This research was supported by National Institute of Mental Health grants P01 MH49548 and T32 MH19817-17 and University of Miami Developmental Center for Aids Research (DCFAR) Grant SB04 1P30AI073961-02. The NIMH and DCFAR had no further role in study design, collection, analysis and interpretation of data, writing of the manuscript, or in the decision to submit the paper for publication. The authors would like to thank Corina Lopez, M.A. and Amanda Sussex, MPH for their help in collecting data for this project, and Hilary Duckworth, M.A. for her help in preparing, proofreading and formatting the manuscript.
Contributor Information
Erin M. Fekete, School of Psychological Sciences, University of Indianapolis
Julia Seay, Department of Psychology, University of Miami.
Michael H. Antoni, Department of Psychology, University of Miami
Armando J. Mendez, Department of Medicine and The Diabetes Research Institute, University of Miami Miller School of Medicine
Mary Ann Fletcher, Department of Medicine, University of Miami Miller School of Medicine.
Angela Szeto, Department of Medicine and The Diabetes Research Institute, University of Miami Miller School of Medicine.
Neil Schneiderman, Department of Psychology, University of Miami.
References
- Antoni MH, Ironson G, Schneiderman N. Stress Management for Persons with HIV Infection. Oxford, New York: 2007. [Google Scholar]
- Antonucci TC. A life-span view of women's social relations. In: Turner B, Troll LE, editors. Women growing older: Psychological perspectives. Thousand Oaks, CA US: Sage Publications, Inc; 1994. pp. 239–269. [Google Scholar]
- Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Hawkley L, Crawford L, Ernst J, Burleson M, Kowalewski R, Berntson G. Loneliness and health: potential mechanisms. Psychosomatic Medicine. 2002;64(3):407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal Of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Cederfjäll C, Langius-Eklöf A, Lidman K, Wredling R. Gender differences in perceived health-related quality of life among patients with HIV infection. AIDS Patient Care & Stds. 2001;15(1):31–39. doi: 10.1089/108729101460083. [DOI] [PubMed] [Google Scholar]
- Chesney M, Ickovics J, Chambers D, Gifford A, Neidig J, Zwickl B, Wu A. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG Adherence InstrumentsAIDS Impact: 4th International Conference on the Biopsychosocial Aspects of HIV Infection, 15–18 July1999,pOttawa, Canada. AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social Relationships and Health. American Psychologist. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal Of Health & Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cross SE, Madson L. Models of the self: Self-construals and gender. Psychological Bulletin. 1997;122(1):5–37. doi: 10.1037/0033-2909.122.1.5. [DOI] [PubMed] [Google Scholar]
- Cruess DG, Antoni MH, Gonzalez J, Fletcher M, Klimas N, Duran R, Schneiderman N. Sleep disturbance mediates the association between psychological distress and immune status among HIV-positive men and women on combination antiretroviral therapy. Journal Of Psychosomatic Research. 2003;54(3):185. doi: 10.1016/s0022-3999(02)00501-9. [DOI] [PubMed] [Google Scholar]
- Darko D, Mitler M, Miller J. Growth hormone, fatigue, poor sleep, and disability in HIV infection. Neuroendocrinology. 1998;67(5):317–324. doi: 10.1159/000054329. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Antoni MH, Lopez C, Mendez AJ, Szeto A, Fletcher M, Schneiderman N. Stress buffering effects of oxytocin on HIV status in low-income ethnic minority women. Psychoneuroendocrinology. 2011;36(6):881–890. doi: 10.1016/j.psyneuen.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete E, Antoni M, Durán R, Stoelb B, Kumar M, Schneiderman N. Disclosing HIV serostatus to family members: Effects on psychological and physiological health in minority women living with HIV. International Journal Of Behavioral Medicine. 2009;16(4):367–376. doi: 10.1007/s12529-009-9041-9. [DOI] [PubMed] [Google Scholar]
- Gómez-González B, Domínguez-Salazar E, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano J, Velázquez-Moctezuma J. Role of sleep in the regulation of the immune system and the pituitary hormones. Annals Of The New York Academy Of Sciences. 2012;1261(1):97–106. doi: 10.1111/j.1749-6632.2012.06616.x. [DOI] [PubMed] [Google Scholar]
- Gordillo V, Fekete EM, Platteau T, Antoni MH, Schneiderman N, Nöstlinger C. Emotional support and gender in people living with HIV: effects on psychological well-being. Journal Of Behavioral Medicine. 2009;32(6):523–531. doi: 10.1007/s10865-009-9222-7. [DOI] [PubMed] [Google Scholar]
- Grewen K, Girdler S, Amico J, Light K. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse D, Nowell P, Nofzinger E, Houck P, Reynolds C, Kupfer D. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosomatic Medicine. 2000;62(2):227–230. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Hand GA, Phillips KD, Sowell RL, Rojas M, Becker J. Prevalence of poor sleep quality in a HIV+ population of Americans. The Journal of the South Carolina Medical Association. 2003;99:201–205. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Helgeson VS. Two important distinctions in social support: Kind of support and perceived vs. received. Journal of Applied Social Psychology. 1993;23:825–845. [Google Scholar]
- Holt-Lunstad J, Birmingham W, Light K. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70(9):976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Jaccard J, Turrisi R. Interaction effects in multiple regression. 2nd ed. CA: Sage; 2003. [DOI] [PubMed] [Google Scholar]
- Lancel M, Krömer S, Neumann I. Intracerebral oxytocin modulates sleep-wake behaviour in male rats. Regulatory Peptides. 2003;114(2–3):145–152. doi: 10.1016/s0167-0115(03)00118-6. [DOI] [PubMed] [Google Scholar]
- Lee K, Gay C, Portillo C, Coggins T, Davis H, Pullinger C, Aouizerat B. Types of Sleep Problems in Adults Living with HIV/AIDS. Journal Of Clinical Sleep Medicine. 2012;8(1):67–75. doi: 10.5664/jcsm.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Portillo C, Miramontes H. The influence of sleep and activity patterns on fatigue in women with HIV/AIDS. JANAC: Journal Of The Association Of Nurses In AIDS Care. 2001;12:19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- Marion I, Antoni M, Pereira D, Wohlgemuth W, Fletcher M, Simon T, O'Sullivan M. Distress, Sleep Difficulty, and Fatigue in Women Co-Infected With HIV and HPV. Behavioral Sleep Medicine. 2009;7(3):180–193. doi: 10.1080/15402000902976721. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Neumann I, Krömer S, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory Peptides. 2000;96(1–2):31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Neumann, Wigger, Torner, Holsboer, Landgraf, Neumann ID. Brain Oxytocin Inhibits Basal and Stress-Induced Activity of the Hypothalamo-Pituitary-Adrenal Axis in Male and Female Rats: Partial Action Within the Paraventricular Nucleus. Journal Of Neuroendocrinology. 2000;12(3):235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nokes K, Kendrew J. Correlates of sleep quality in persons with HIV disease. JANAC: Journal Of The Association Of Nurses In AIDS Care. 2001;12(1):17–22. doi: 10.1016/S1055-3290(06)60167-2. [DOI] [PubMed] [Google Scholar]
- Opp M. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Advances In Neuroimmunology. 1995;5(2):127–143. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- Owens S. African American Women Living with HIV/AIDS: Families as Sources of Support and of Stress. Social Work. 2003;48(2):163–171. doi: 10.1093/sw/48.2.163. [DOI] [PubMed] [Google Scholar]
- Pence B, Barroso J, Leserman J, Harmon J, Salahuddin N. Measuring fatigue in people living with HIV/AIDS: psychometric characteristics of the HIV-Related Fatigue Scale. AIDS Care. 2008;20(7):829–837. doi: 10.1080/09540120701694063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K. Physiological and pharmacological factors of insomnia in HIV disease. The Journal Of The Association Of Nurses In AIDS Care: JANAC. 1999;10(5):93–97. doi: 10.1016/S1055-3290(06)60346-4. [DOI] [PubMed] [Google Scholar]
- Phillips KD, Sowell RL, Boyd M, Dudgeon WD, Hand GA. Sleep quality and health-related quality of life in HIV-infected African-American women of childbearing age. Quality Of Life Research. 2005;14(4):959–970. doi: 10.1007/s11136-004-2574-0. [DOI] [PubMed] [Google Scholar]
- Publication of revised HIV/AIDS surveillance report, 2005. MMWR: Morbidity & Mortality Weekly Report. 2007;56(25):634. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosomatic Medicine. 2005;67(2):260–269. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- Rodenbeck A, Hajak G. Neuroendocrine dysregulation in primary insomnia. Revue Neurologique. 2001;157(11 Pt 2):S57–S61. [PubMed] [Google Scholar]
- Rubinstein M, Selwyn P. High prevalence of insomnia in an outpatient population with HIV infection. Journal Of Acquired Immune Deficiency Syndromes And Human Retrovirology: Official Publication Of The International Retrovirology Association. 1998;19(3):260–265. doi: 10.1097/00042560-199811010-00008. [DOI] [PubMed] [Google Scholar]
- Saberi P, Neilands TB, Johnson MO. Quality of Sleep: Associations with Antiretroviral Nonadherence. AIDS Patient Care & Stds. 2011;25(9):517–524. doi: 10.1089/apc.2010.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin N, Barroso J, Leserman J, Harmon J, Pence B. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. The Journal Of The Association Of Nurses In AIDS Care: JANAC. 2009;20(1):6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantamburlo GG, Hansenne MM, Fuchs SS, Pitchot WW, Maréchal PP, Pequeux CC, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Dunkel-Schetter C, Kemeny M. The multidimensional nature of received social support in gay men at risk of HIV infection and AIDS. American Journal Of Community Psychology. 1994;22(2):319. doi: 10.1007/BF02506869. [DOI] [PubMed] [Google Scholar]
- Serovich J, Kimberly J, Mosack K, Lewis T. The role of family and friend social support in reducing emotional distress among HIV-positive women. AIDS Care. 2001;13(3):335–341. doi: 10.1080/09540120120043982. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe P, Nation D, Tabak B, Rossetti M, McCullough M, Mendez A. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73(5):393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Clark SL. Reciprocity in the expression of emotional support among later-life couples coping with stroke. In: Gottlieb BH, editor. Coping with chronic stress. New York, NY US: Plenum Press; 1997. pp. 221–242. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. United States: Pearson; 2012. [Google Scholar]
- Taylor SE, Klein L, Lewis BP, Gruenewald TL, Gurung RR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and Befriend: Biobehavioral Bases of Affiliation Under Stress. Current Directions In Psychological Science. 2006;15(6):273–277. [Google Scholar]
- Taylor SE, Gonzaga G, Klein LC, Hu P, Greendale GA, Seeman SE. Relation of oxytocin to psychological and biological stress responses in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are Plasma Oxytocin in Women and Plasma Vasopressin in Men Biomarkers of Distressed Pair-Bond Relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Troxel W. It's More than Sex: Exploring the Dyadic Nature of Sleep and Implications for Health. Psychosomatic Medicine. 2010;72(6):578–586. doi: 10.1097/PSY.0b013e3181de7ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62(2):97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Vosvick M, Gore-Felton C, Ashton E, Koopman C, Fluery T, Israelski D, Spiegel D. Sleep disturbances among HIV-positive adults: The role of pain, stress, and social support. Journal Of Psychosomatic Research. 2004;57(5):459–463. doi: 10.1016/j.jpsychores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wibbeler T, Reichelt D, Husstedt IW, Evers S. Sleepiness and sleep quality in patients with HIV infection. Journal Of Psychosomatic Research. 2012;72(6):439–442. doi: 10.1016/j.jpsychores.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Möller A, Schreiber W, Krieg J, Fuchs D, Wachter H, Holsboer F. Nocturnal sleep EEG in patients with HIV infection. European Archives Of Psychiatry And Clinical Neuroscience. 1991;240(3):153–158. doi: 10.1007/BF02190756. [DOI] [PubMed] [Google Scholar]