Abstract
Objectives:
We tested the hypothesis that daily vitD3 supplementation increases neuromuscular motor skills, jump power, jump energy, muscular force, and muscular strength.
Methods:
This was a secondary analysis of a randomized controlled trial of 12-months of oral 7,000 IU/day vitD3 supplementation or placebo among 56 persons living with HIV aged 9-25 years. Neuromuscular motor skills were quantified using the Bruininks-Oseretsky Test of Motor Proficiency. Power was quantified using peak jump power, and energy was quantified using peak jump height. Muscular force was quantified using isometric ankle plantar- and dorsiflexion, isokinetic knee flexion and extension. Muscular strength was quantified using isometric handgrip strength.
Results:
After 12-months, serum 25-hydroxyvitamin D [25(OH)D] was higher with supplementation versus placebo (β=12.1 ng/mL; P<0.001). In intention-to-treat analyses, supplementation improved neuromuscular motor skills versus placebo (β=1.14; P=0.041). We observed no effect of supplementation on jump power, jump energy, muscular force, or muscular strength outcomes versus placebo.
Conclusions:
Among HIV-infected children and young adults supplementation with daily high-dose vitD3 increased concentration of serum 25(OH)D and improved neuromuscular motor skills versus placebo.
Keywords: Vitamin D Supplementation, Pediatric HIV, Cholecalciferol, Nutrition, Muscle
Introduction
Due to the availability of combination antiretroviral therapy (cART), the rates of morbidity and mortality attributable to human immunodeficiency virus (HIV) have significantly decreased among children and young adults in the United States[1]. Despite this success, HIV-infected patients experience deficiencies in several domains of health-related fitness. HIV-infected children and young adults may experience deficiencies in neuromuscular motor skills[2], muscular power[3], muscular strength[4], lean body mass[5,6] and cardiorespiratory fitness[7]. Reduced health-related fitness likely contributes to poor physical functioning and low quality of life in this population[8].
Vitamin D (vitD) and its metabolite 25-hydroxyvitamin D [25(OH)D] are required for a variety of physiologic processes including neurologic development, muscular function, immune modulation, and bone growth[9]. In our recent study, over 95% of HIV-infected children and young adults had insufficient (<32 ng/mL), and 64% had deficient (<20 ng/mL) concentrations of serum 25(OH)D[10]. Daily high-dose (7,000 IU/day) supplementation with vitD3 safely and effectively increased concentration of serum 25(OH)D and resulted in improvements in some indicators of immune status among HIV-infected participants[10-12].
It is unknown if supplementation with daily high-dose vitD3 associates with improvements in health-related fitness in this population. We tested the hypothesis that daily vitD3 supplementation increases neuromuscular motor skills, jump power, jump energy, muscular force, and muscular strength among HIV-infected children and young adults. Data were from a prior randomized, double-blind, placebo controlled trial that examined the safety and efficacy of 12-months of daily high-dose vitD3 supplementation among children and young adults with perinatally- and behaviorally-acquired HIV[10-12]. Neuromuscular motor skills, jump power, jump energy, muscular power, and muscular strength were secondary outcomes from this trial.
Materials and methods
Participants
The primary outcome and methods of this trial have been previously reported in detail[11,12]. Briefly, participants were recruited from eight regional HIV centers between July, 2011 and June, 2013. Criteria for participation included age of 5.0-24.9 years for perinatally-acquired HIV or 15.0-24.9 years for behaviorally-acquired HIV and a state of good health two weeks prior to enrollment. Criteria for exclusion included any adverse growth, dietary intake, or nutritional status conditions, pregnancy or lactation, and current supplementation with vitD3. Two participants with cerebral palsy were excluded from this analysis.
Parents/guardians or participants (≥18 years or emancipated minor) gave informed consent, and children seven years and older assented. Study examinations were performed at the Children’s Hospital of Philadelphia (CHOP). The CHOP Institutional Review Board approved the protocol. The trial was monitored prospectively by a data safety monitoring committee and was registered with clinicaltrials.gov as NCT01475890.
Vitamin D supplementation
Participants were stratified by mode of HIV acquisition (perinatal or behavioral) and randomized in parallel (1:1 ratio) to receive 7,000 IU/d of vitD3 or placebo (Life Extension, Ft. Lauderdale, FL). Participants unable to swallow capsules took liquid vitD3 or placebo (J.R. Carlson Laboratories, Inc., Arlington Heights, IL). VitD3 products were independently assessed for potency at the beginning and end of the study. Enrollment was balanced by season. A 3.5-month supply of vitD3 or placebo was provided at baseline and the 3-month visits, and a 7-month supply was provided at the 6-month visit. Participants returned supplement/placebo containers at each visit and the remaining capsules/volumes were recorded. Cumulative 12-month adherence was determined as the percent of capsules or liquid taken. Concentrations of fasting serum 25(OH)D was determined using liquid chromatography tandem mass spectrometry (CHOP Laboratory) at baseline, 3-, 6-, and 12-months. The intra- and inter-assay coefficients of variation of serum 25(OH)D analysis were ≤8%.
Outcome measures
Neuromuscular motor skills
Neuromuscular motor skills were assessed using the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOTMP)[13]. The BOTMP consists of 14 measures that represent eight motor skill domains including: fine motor precision, fine motor integration, manual dexterity, bilateral coordination, balance, speed and agility, upper limb coordination, and strength. Measurement and scoring details of each motor skill domain are described elsewhere[13]. Each motor skill domain can be aggregated to create a composite measure of overall motor proficiency with higher values representing greater neuromuscular motor skill proficiency. The BOTMP has excellent test-retest reliability (r=0.8) and criterion-related validity when compared to other measures of motor performance (r=0.74)[14].
Jump power and energy
Jump power and jump energy were assessed using a Kistler Quattro Jump Portable Force Plate System (Model 9290AD, Amherst, NY) at baseline, 3-, 6-, and 12-months. Each participant performed five warm-up jumps, followed by five maximal jumps. Each jump started from a static squat position with the knees at 90 degrees flexion, and the hands placed on the hips[15]. Peak jump power has favorable test-retest reproducibility (coefficient of variation 9.8%)[16]. Peak jump power (expressed in watts) was calculated as the product of jump force and jump velocity by integrating ground reaction forces[17], and the highest value recorded was used in the inferential analyses. Peak jump energy (expressed in centimeters) was calculated using velocity (centimeters/second) and time required to complete the jump (seconds), and the highest value recorded was used in the inferential analyses.
Muscular force and strength
Muscular force was assessed using a Biodex Multi-Joint System (System 3 Pro, Shirley, NY) at baseline, 3-, 6-, and 12-months. High intra-rater (0.97-0.99) and inter-rater (0.93-0.96) correlation coefficients[18], and high test-retest values (coefficients of variation <10%) have been reported[19,20]. Prior to testing, each participant completed warm-up consisting of five-minutes of treadmill walking at a comfortable speed at 0% grade. All muscular strength measures were conducted on the left knee and ankle. Peak isometric torque (Newton-meters) was measured in triplicate at each of four angles (-10, 0, 10, and 20 degrees) and the highest value recorded for dorsiflexion and plantarflexion at each angle of the ankle was used in the inferential analysis. Peak isokinetic torque (Newton-meters) was measured in triplicate at 1.05 rad/s (60 degrees/second) and the highest value recorded for extension and flexion of the knee was used in the inferential analysis.
Muscular strength (kilograms) was assessed using an isometric hydraulic hand dynamometer (Takei, Tokyo, Japan) in the dominant hand. The participant stood upright with the shoulder adducted holding the dynamometer, not touching the trunk. The handle was adjusted to the hand size and no extraneous body movement was allowed during testing. Three maximal effort trials lasting four to five seconds with 60-second rests were performed, and the highest value was using in the inferential analysis[15].
Covariates
Covariates including age, race, sex, mode of HIV acquisition, and use of cART were abstracted from the electronic medical record and collected from self-report. Body mass index (BMI) was calculated using height and weight measured following standardized techniques[21]. Leg lean mass (kilograms) was quantified using whole body dual energy x-ray absorptiometry (Hologic Discovery, Hologic Inc., Bedford MA). Tibia length was measured from the distal border of the medial malleolus to the proximal medial border of the tibia plateau[19].
Statistical analysis
Statistical analyses were performed using Stata 13.1 (Stata, College Station, TX). Continuous variables are presented as means ± standard deviation (SD). Least square means (LSMean) from regression or mixed models are presented as means ± standard error (SE). Comparisons between randomized groups were made at baseline using unpaired Student’s t-tests for continuous variables, and Pearson’s chi-square tests or Fisher’s exact tests for categorical variables.
For longitudinal efficacy data, analyses were conducted on an intention-to-treat basis consistent with the primary outcomes report[11]. The difference between randomization groups at each visit time was tested using an unpaired Student’s t-test and change from baseline within each randomization group by paired t-test. Persistence of response over the three study visits was assessed using mixed models with an autoregressive correlation structure that adjusted for baseline values and controlled for visit. Results from the mixed models are the fixed effects of randomization group. The mixed model allows for inclusion of all subjects, including drop outs and those with missing values. To account for multiple testing of the neuromuscular motor skill total score and correlated subscale outcomes we implemented a hierarchical Bonferroni-Holm method[22,23]. The neuromuscular motor skill total score was tested at α=0.05, and the family-wise error rate of the subscales were controlled overall at α=0.05 (i.e., α of 0.05 split over the 14 outcomes, with the threshold for statistical significance ranging from 0.004 to 0.05). Jump power, jump energy, muscular force, and muscular strength outcomes were tested using the procedure by Holm (i.e., α of 0.05 split over the 16 outcomes, with the threshold for statistical significance ranging from 0.003 to 0.05)[22].
Post-hoc exploratory multivariable-adjusted regression models that combined both randomization groups were used to assess the associations between participants with increased concentration of serum 25(OH)D from baseline (i.e., “responders”) versus those who had no change or decreased 25(OH)D (i.e., “non-responders”). A response was defined as a ≥3 ng/mL increase in serum 25(OH)D from baseline at each time point of 3-, 6-, and 12-months. A ≥3 ng/mL change was selected as this was the increase that was sufficiently large enough to rule out a change that was the result of measurement error based upon the known intra- and inter-assay coefficients of variation of the serum 25(OH)D assay. Covariates in the multivariable-adjusted regression models included the baseline value of the dependent variable, age, baseline concentration of 25(OH)D, HIV acquisition route, leg lean muscle mass, tibia length, and total body mass.
Results
Fifty-eight participants aged 9.6 to 24.9 years were enrolled over all seasons and subsequently randomized (Figure 1). The majority of subjects were African American (86%), male (67%), acquired HIV behaviorally (66%), and were on cART (75%; Table 1). BMI was 24.2±6.6 kg/m2 and ranged from 14.0 to 52.1 kg/m2. The BMI-for-age (z score) was 0.11±1.26. Baseline concentrations of serum 25(OH)D ranged from 3.0 to 35.7 ng/dL with a mean concentration of 17.5±8.7 ng/mL; 95% of participants were suboptimal for 25(OH)D (<32 ng/mL), 64% deficient (<20 ng/mL), and 26% severely deficient (<11 ng/mL).
Figure 1.
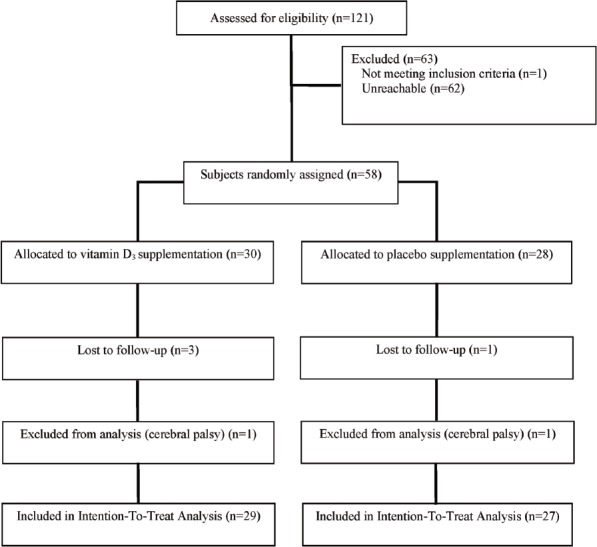
Flow diagram for subjects randomized, drop-outs, and completing the placebo-controlled trial of daily 7000 IU vitamin D3 supplementation in HIV-infected children and young adults.
Table 1.
Characteristics of subjects by randomization group at baseline.
| Variable | Total Sample (n = 56) | Placebo (n = 27) | VitD3 Supplementation (n = 29) |
|---|---|---|---|
| Age, (y) | 20.7±3.8 | 20.0±4.1 | 21.4±3.3 |
| Sex, male, n (%) | 38 (67%) | 18 (67%) | 20 (69%) |
| Racial identification, n (%) | |||
| African-American | 48 (86%) | 23 (86%) | 25 (86%) |
| White | 2 (3%) | 2 (7%) | 0 (0%) |
| Other and mixed | 6 (11%) | 2 (7%) | 4 (14%) |
| Season at enrollment, n (%) | |||
| Summer/Fall | 19 (34%) | 8 (30%) | 11 (38%) |
| Winters/Spring | 37 (66%) | 19 (70%) | 18 (62%) |
| HIV acquisition route, n (%) | |||
| Perinatally | 19 (34%) | 10 (37%) | 9 (31%) |
| Behaviorally | 37 (66%) | 17 (63%) | 20 (69%) |
| cART at baseline, n (%) | 42 (75%) | 20 (74%) | 22 (76%) |
| BMI, (kg/m2) | 24.2±6.6 | 25.1±8.1 | 23.4±5.0 |
| Body mass, (kg) | 68.0±17.8 | 68.7±19.8 | 67.4±16.2 |
| Lean mass of legs, (kg) | 16.2±4.1 | 16.3±4.1 | 16.2±16.1 |
| Tibia length, (cm) | 39.9±3.3 | 39.6±3.1 | 40.1±3.4 |
| Serum 25(OH)D, (ng/ml) | 17.5±8.7 | 17.1±9.3 | 17.9±9.3 |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; VitD3, vitamin D3; 25(OH)D, 25-hydroxyvitamin D; kg, kilogram; cm, centimeter. Data are mean ± SD or number (%). There were no significant differences between the placebo and VitD3 supplementation groups for any of the baseline covariates.
Adherence to supplement/placebo was 92±8% over 12-months, with no differences between groups. The effects of supplementation with daily high-dose vitD3 significantly increased concentrations of serum 25(OH)D compared to the placebo group (β=12.1 ng/mL; P<0.001) after 12-months. Thirty-three percent of participants in the supplementation group achieved a serum 25(OH)D concentration ≥32 ng/mL at 12-months.
The effects of supplementation with daily high-dose vitD3 significantly increased neuromuscular motor skills when compared to the placebo group (β=1.14; P=0.041; Table 2) after 12-months. The effect of vitD3 supplementation on overall neuromuscular motor skill proficiency was moderate (Cohen’s mean difference effect size d=0.57).
Table 2.
Vitamin D3 supplementation vs placebo on Bruininks-Oseretsky test of motor proficiency (BOTMP) outcomes.
| n | Baseline | n | 3-Month | n | 6-Month | n | 12-Month | Fixed effect of randomization LSMean ± SEa | Pa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | ||||||||||
| - Placebo | 27 | 72.4±0.8 | 26 | 72.7±1.0 | 26 | 71.2±1.2 | 26 | 71.5±1.1 | 71.6±0.4 | 0.041 |
| - Vitamin D | 29 | 71.7±1.1 | 29 | 73.0±1.1 | 29 | 72.8±0.9 | 26 | 72.4±1.0 | 72.8±0.4 | |
| Subscales | ||||||||||
| Fine Motor Precision | ||||||||||
| Line Drawing | ||||||||||
| - Placebo | 27 | 0.0±0.0 | 26 | 0.0±0.0 | 26 | 0.0±0.0 | 26 | 0.0±0.0 | 0.01±0.01 | 0.106 |
| - Vitamin D | 29 | 0.0±0.0 | 29 | 0.0±0.0 | 29 | 0.1±0.0 | 26 | 0.0±0.0 | 0.04±0.01 | |
| Fold paper | ||||||||||
| - Placebo | 27 | 11.4±0.2 | 26 | 11.3±0.4 | 26 | 11.0±0.5 | 26 | 10.2±0.6b | 10.9±0.3 | 0.353 |
| - Vitamin D | 29 | 11.4±0.2 | 29 | 11.3±0.2 | 29 | 11.4±0.3 | 26 | 10.8±0.5 | 11.2±0.3 | |
| Fine Motor Integration | ||||||||||
| Copy Square | ||||||||||
| - Placebo | 27 | 4.9±0.1 | 26 | 4.9±0.1 | 26 | 5.0±0.0 | 26 | 5.0±0.0 | 4.9±0.03 | 0.460 |
| - Vitamin D | 29 | 4.9±0.1 | 29 | 5.0±0.1 | 29 | 4.9±0.1 | 26 | 4.9±0.1 | 4.9±0.03 | |
| Copy Star | ||||||||||
| - Placebo | 27 | 4.6±0.1 | 26 | 4.6±0.1 | 26 | 4.4±0.2 | 26 | 4.5±0.2 | 4.5±0.09 | 0.522 |
| - Vitamin D | 29 | 4.7±0.2 | 29 | 4.7±0.2 | 29 | 4.3±0.3 | 26 | 4.3±0.3 | 4.5±0.09 | |
| Manual Dexterity | ||||||||||
| Penny Transfer | ||||||||||
| - Placebo | 27 | 14.4±0.4 | 26 | 15.1±0.5 | 26 | 14.8±0.5 | 26 | 14.6±0.4 | 14.8±0.2 | 0.068 |
| - Vitamin D | 29 | 14.6±0.6 | 29 | 15.3±0.6 | 29 | 15.8±0.6b | 26 | 16.0±0.5b,c | 15.4±0.2 | |
| Bilateral Coordination | ||||||||||
| Jump in Place | ||||||||||
| - Placebo | 27 | 5.0±0.0 | 26 | 5.0±0.0 | 26 | 5.0±0.0 | 26 | 5.0±0.0 | 5.0±0.04 | 0.418 |
| - Vitamin D | 29 | 4.8±0.2 | 29 | 5.0±0.0 | 29 | 4.8±0.2 | 26 | 5.0±0.0 | 5.0±0.04 | |
| Tap Feet & Fingers | ||||||||||
| - Placebo | 27 | 10.0±0.0 | 26 | 10.0±0.0 | 26 | 10.0±0.0 | 26 | 10.0±0.0 | 10.0±0.0 | 0.999 |
| - Vitamin D | 29 | 10.0±0.0 | 29 | 10.0±0.0 | 29 | 10.0±0.0 | 26 | 10.0±0.0 | 10.0±0.0 | |
| Balance | ||||||||||
| Line Walk | ||||||||||
| - Placebo | 27 | 5.9±0.1 | 26 | 5.9±0.1 | 26 | 6.0±0.0 | 26 | 6.0±0.0 | 6.0±0.01 | 0.176 |
| - Vitamin D | 29 | 6.0±0.0 | 29 | 6.0±0.0 | 29 | 6.0±0.0 | 26 | 6.0±0.0 | 6.0±0.01 | |
| One Leg Balance | ||||||||||
| - Placebo | 27 | 8.4±0.5 | 26 | 9.0±0.4b | 26 | 8.3±0.5 | 26 | 8.0±0.6 | 8.1±0.2 | 0.258 |
| - Vitamin D | 29 | 7.4±0.6 | 29 | 8.7±0.4b | 29 | 8.3±0.4 | 26 | 8.0±0.5 | 8.4±0.2 | |
| Speed and Agility | ||||||||||
| One Leg Hop | ||||||||||
| - Placebo | 27 | 31.5±1.36 | 26 | 31.3±1.0 | 26 | 30.6±1.2 | 26 | 31.3±1.2 | 31.6±0.6 | 0.090 |
| - Vitamin D | 29 | 32.9±0.7 | 29 | 33.5±1.3 | 29 | 33.7±0.9c | 26 | 33.8±1.0 | 33.1±0.6 | |
| Upper Limb Coordination | ||||||||||
| Drop Catch | ||||||||||
| - Placebo | 27 | 4.9±0.1 | 26 | 5.0±0.0 | 26 | 5.0±0.0 | 26 | 5.0±0.0 | 5.0±0.02 | 0.823 |
| - Vitamin D | 29 | 4.9±0.1 | 29 | 5.0±0.0 | 29 | 5.0±0.0 | 26 | 5.0±0.0 | 5.0±0.02 | |
| Dribble Ball | ||||||||||
| - Placebo | 27 | 9.3±0.3 | 26 | 9.2±0.4 | 26 | 9.2±0.3 | 26 | 9.3±0.3 | 9.3±0.1 | 0.137 |
| - Vitamin D | 29 | 9.3±0.4 | 29 | 9.3±0.3 | 29 | 9.7±0.2 | 26 | 9.8±0.2 | 9.5±0.1 | |
| Strength | ||||||||||
| Pushup | ||||||||||
| - Placebo | 27 | 15.5±1.6 | 26 | 15.2±1.8 | 26 | 14.3±2.0 | 26 | 14.8±2.0 | 14.1±0.6 | 0.431 |
| - Vitamin D | 29 | 13.6±1.7 | 29 | 14.3±1.5 | 29 | 14.1±1.3 | 26 | 14.0±1.2 | 14.8±0.6 | |
| Situp | ||||||||||
| - Placebo | 27 | 18.9±1.3 | 26 | 16.8±1.7b | 26 | 16.7±1.5b | 26 | 18.7±1.5 | 16.6±0.5 | 0.088 |
| - Vitamin D | 29 | 16.2±1.5 | 29 | 16.4±1.3 | 29 | 16.6±1.2 | 26 | 16.6±1.5 | 17.7±0.5 | |
Results are the fixed effect of randomization group from multilevel regression models testing for persistence of response at 3-12 months based on untransformed data, also controlling for time of study visit and adjusting for baseline values; data are least-squares mean (LSMean) ± standard error (SE).
Significantly different from baseline mean within randomization group by paired t-test at P<0.05.
Significantly different from placebo mean within time of study visit by Student’s t-test at P<0.05.
The effects of supplementation with daily high-dose vitD3 did not significantly increase peak jump power or energy when compared to the placebo group (Table 3). Within-group comparisons revealed that peak jump energy was higher at 12-months compared to baseline in the supplementation group (P<0.05), but not in the placebo group.
Table 3.
Vitamin D3 supplementation vs. placebo on muscle function outcomes.
| n | Baseline | n | 3-Month | n | 6-Month | n | 12-Month | Fixed effect of randomization LSMean ± SEa | Pa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Force Plate | ||||||||||
| Peak Jump Power (watts) | ||||||||||
| - Placebo | 27 | 2596±148 | 25 | 2734±176 | 25 | 2732±178 | 26 | 2735±160b | 2697±59 | 0.369 |
| - Vitamin D | 29 | 2671±171 | 28 | 2823±187 | 28 | 2910±197b | 24 | 2665±187 | 2770±57 | |
| Peak Jump Energy (cm) | ||||||||||
| - Placebo | 27 | 32.8±2.0 | 25 | 35.5±1.8 | 25 | 33.7±2.0 | 26 | 34.5±2.1 | 34.6±0.7 | 0.142 |
| - Vitamin D | 29 | 33.0±2.0 | 28 | 35.1±1.8 | 28 | 36.4±1.9 | 24 | 35.9±2.0b | 36.1±0.8 | |
| Biodex Ankle (Newton-meters) | ||||||||||
| Isometric Plantar Extension Peak Torque | ||||||||||
| -10° | ||||||||||
| - Placebo | 24 | 70.0±4.8 | 22 | 73.7±4.8 | 22 | 80.7±5.3 | 18 | 75.6±6.3 | 72.5±2.7 | 0.698 |
| - Vitamin D | 26 | 68.6±6.8 | 25 | 79.2±6.3 | 22 | 78.6±6.5 | 22 | 76.0±7.4 | 74.0±2.6 | |
| 0° | ||||||||||
| - Placebo | 27 | 64.3±5.6 | 26 | 61.6±3.6 | 26 | 71.9±4.8 | 26 | 67.7±4.1 | 64.9±2.3 | 0.429 |
| - Vitamin D | 29 | 62.7±5.3 | 29 | 67.1±4.5 | 29 | 70.4±5.1 | 26 | 64.2±5.9 | 67.4±2.2 | |
| 10° | ||||||||||
| - Placebo | 27 | 54.1±4.8 | 26 | 50.9±3.8 | 26 | 58.1±4.7 | 26 | 55.7±3.8 | 53.5±1.9 | 0.944 |
| - Vitamin D | 29 | 52.7±4.1 | 29 | 53.2±3.6 | 29 | 52.5±4.3 | 26 | 50.3±4.7 | 53.2±1.8 | |
| 20° | ||||||||||
| - Placebo | 27 | 42.0±3.9 | 26 | 37.7±3.3 | 26 | 44.7±4.0 | 26 | 41.9±3.1 | 40.0±1.5 | 0.696 |
| - Vitamin D | 29 | 39.6±3.0 | 29 | 36.3±2.8 | 29 | 38.2±3.6 | 26 | 37.7±3.5 | 39.2±1.5 | |
| Dorsiflexion Peak Torque | ||||||||||
| -10° | ||||||||||
| - Placebo | 24 | 16.4±1.2 | 22 | 17.0±1.4 | 22 | 16.6±1.5 | 18 | 15.1±1.3 | 16.2±0.6 | 0.954 |
| - Vitamin D | 26 | 16.8±1.4 | 25 | 17.1±1.4 | 22 | 17.4±1.6 | 22 | 15.1±1.5 | 16.1±0.6 | |
| 0° | ||||||||||
| - Placebo | 27 | 22.0±1.4 | 26 | 21.2±1.5 | 26 | 21.4±1.5 | 26 | 20.1±1.2 | 21.2±0.6 | 0.452 |
| - Vitamin D | 29 | 22.1±1.4 | 29 | 22.0±1.5 | 29 | 23.0±1.3 | 26 | 19.8±1.4 | 21.7±0.6 | |
| 10° | ||||||||||
| - Placebo | 27 | 26.6±1.7 | 26 | 23.9±1.6b | 26 | 26.1±1.7 | 26 | 24.0±1.4 | 24.9±0.6 | 0.522 |
| - Vitamin D | 29 | 25.8±1.4 | 29 | 25.9±1.6 | 29 | 25.3±1.5 | 26 | 23.4±1.4 | 25.4±0.6 | |
| 20° | ||||||||||
| - Placebo | 27 | 27.5±1.7 | 26 | 25.1±1.7 | 26 | 27.8±2.0 | 26 | 25.7±1.6 | 26.1±0.7 | 0.456 |
| - Vitamin D | 29 | 26.6±1.4 | 29 | 26.6±1.5 | 29 | 26.7±1.6 | 26 | 26.0±1.5 | 26.8±0.7 | |
| Biodex Knee (Newton-meters) | ||||||||||
| Extension Peak Torque | ||||||||||
| 60°/sec | ||||||||||
| - Placebo | 27 | 78.8±4.9 | 26 | 73.6±4.3 | 26 | 76.0±5.8 | 26 | 65.2±4.2b | 76.8±2.1 | 0.927 |
| - Vitamin D | 29 | 88.1±6.2 | 29 | 84.3±6.5 | 29 | 76.7±4.9b | 26 | 68.2±5.2b | 76.5±2.0 | |
| Flexion Peak Torque | ||||||||||
| 60°/sec | ||||||||||
| - Placebo | 27 | 36.7±2.2 | 26 | 36.6±2.3 | 26 | 37.7±2.8 | 26 | 31.7±2.1b | 35.8±1.3 | 0.902 |
| - Vitamin D | 29 | 37.4±2.4 | 29 | 38.6±2.8 | 29 | 38.3±3.1 | 26 | 30.2±2.4b | 36.1±1.3 | |
| Handgrip Strength (kg) | ||||||||||
| - Placebo | 27 | 32.3±2.1 | 26 | 33.9±2.1 | 26 | 33.3±2.1 | 25 | 33.7±2.3 | 34.7±0.5 | 0.901 |
| - Vitamin D | 29 | 35.3±1.9 | 29 | 36.6±2.1 | 29 | 36.0±1.9 | 26 | 35.7±2.1c | 34.6±0.5 | |
Results are the fixed effect of randomization group from multilevel regression models testing for persistence of response at 3-12 months based on untransformed data, also controlling for time of study visit and adjusting for baseline values; data are least-squares mean (LSMean) ± standard error (SE).
Significantly different from baseline mean within randomization group by paired t-test at P<0.05.
Significantly different from placebo mean within time of study visit by Student’s t-test at P<0.05.
The effects of supplementation with daily high-dose vitD3 did not significantly increase muscular force or muscular strength when compared to the placebo group (Table 3). Within-group comparisons revealed that isokinetic knee extension and flexion peak torque force decreased at 12-months compared to baseline in both the supplementation and placebo groups (P<0.05). Within-time comparisons revealed that maximal isometric handgrip strength was higher in the supplementation group compared to the placebo group at 12-months (P<0.05).
In exploratory analyses, supplementation with daily high-dose vitD3 associated with more responders at 3-months (86% vs 37%; P<0.001) and 12-months (62% vs 22%; P=0.003), but not at 6-months (66% vs 41%; P=0.063) versus placebo, respectively. Responders had higher overall neuromuscular motor skills (β=0.92; P=0.014), higher jump power (β=165.1 watts; P=0.001) and jump energy (β=2.7 cm; P=0.030) than non-responders. Responder status was not associated with muscular force or muscular strength outcomes (results not shown).
Discussion
In this randomized, double-blind, placebo-controlled trial of predominately African-American HIV-infected children and young adults, we observed a significant effect of vitD3 supplementation on neuromuscular motor skills versus placebo (β=1.14; P=0.041). There were no significant effects of vitD3 supplementation on jump power, jump energy, muscular force or muscular strength outcomes versus placebo after 12-months using intention-to-treat analyses. Post-hoc exploratory responder analyses suggest that participants who increased concentrations of serum 25(OH)D improved neuromuscular motor skills, jump power and jump energy.
The novel finding of this study is that supplementation with vitD3 may have a significant effect on neuromuscular motor skill proficiency. It has been reported that neuromuscular motor function abnormalities among HIV-infected children may predict progression of HIV[2]. We have previously reported that vitD3 supplementation may improve immune markers of HIV[11,12]. Collectively these data are consistent with the hypothesis that vitD3 supplementation positively impacts clinical outcomes among HIV-infected children and young adults by improving neuromuscular motor skills and immune parameters. The benefits of vitD3 supplementation on clinical outcomes in this population warrant further investigation.
A variety of cellular mechanism have characterized the relationship between vitD or 25(OH)D and neuromuscular motor skills. VitD receptors have been reported in muscle cells[24], and have been implicated in muscle development[25]. VitD receptor knockout (VDRKO) mice have impaired neuromuscular motor performance when compared to that of wild-type mice. VDRKO mice have smaller type I and II muscle fibers[25], impaired forced swimming capacity, and worse dynamic balance compared to wild-type mice[26]. Induced vitD deficiency in rats has also been found to alter neuromuscular function compared to rats with adequate vitD[27]. Collectively, these data provide mechanistic preclinical data to support our findings and support the hypothesis that vitD may have an important role in neuromuscular function[28].
Our findings are consistent with prior trials of vitD2 or vitD3 supplementation on muscle function outcomes. Among girls aged 12-14 years, oral supplementation with 150,000 IU vitD2 administered every 3-months did not increase jump power or jump energy over 12-months[29]. Similarly, among 250 adults aged 18-50 years, daily oral supplementation with 1,000 IU vitD3 did not increase jump energy, handgrip strength, or lower-extremity strength[30]. A recent systematic review of 30 randomized controlled trials that included 5,615 participants concluded vitD2 or vitD3 supplementation may produce a small increase in muscular strength (P=0.02), but no increase in muscular power (P=0.66)[31]. The benefits of vitD2 or vitD3 supplementation on muscular strength were most pronounced among those with 25(OH)D concentrations <12 ng/mL, and among people ≥65 years[31].
The majority of participants in our trial had low concentrations of serum 25(OH)D at baseline; 95% of participants were suboptimal for 25(OH)D (<32 ng/mL), 64% deficient (<20 ng/mL), and 26% severely deficient (<11 ng/mL). This observation is similar to other reports that HIV-infected children and young adults are likely to have low concentrations of serum 25(OH)D[32,33], particularly African Americans[34]. Adherence to vitD3 supplementation in our study was 92%, and supplementation significantly increased concentrations of serum 25(OH)D over 12-months by 12.1 ng/mL (P<0.001) compared to placebo. However only 33% of participants in the supplementation group achieved a serum 25(OH)D concentration ≥32 ng/mL at 12-months. These data indicate that the study participants were at-risk for vitD deficiency and were able to significantly increase concentration of serum 25(OH)D through vitD3 supplementation, but the range and duration of the increases in 25(OH)D may have been insufficient to positively impact muscle function in this sample of participants.
Using post hoc exploratory multivariable-adjusted regression models we characterized the relationship between serum 25(OH)D responders and non-responders. Responder status was associated with increases in jump power and jump energy. Prior cross-sectional studies have suggest a positive association between concentration of serum 25(OH)D and jump outcomes among adolescent girls[35]. Serum 25(OH)D was associated with jump power (P=0.003), jump velocity (P=0.002), and jump height (P=0.005)[35]. A randomized controlled trial of vitD supplementation among postmenarchal females suggested that girls with the lowest baseline 25(OH)D concentrations experienced the greatest improvements in jump velocity[29]. Our post hoc analyses provide evidence that suggest concentration of serum 25(OH)D may favorably impact jump outcomes among HIV-infected children and young adults. The results from these multivariable-adjusted regression analyses should be interpreted exploratory and as hypothesis-generating.
HIV-infected children and young adults experience deficiencies in neuromuscular motor skills[2], muscular power[3], muscular strength[4], lean body mass[5,6] and cardiorespiratory fitness[7]. These deficiencies likely contribute to poor physical functioning and reduced quality of life in this population[8]. Identifying safe and effective interventions to improve components of health and fitness may translate to improvements in quality of life. For example, a 24-session exercise training program increased lower extremity muscular strength by 25.9% (P<0.001) and lean body muscle mass by 4.5% (P<0.001) among 17 HIV-infected children and young adults aged 6.0 to 22.6 years[36]. Physical activity or exercise is positively associated with a variety of outcomes among HIV-infected adults[37,38] including quality of life[39]; however less is known about the safety and efficacy of physical activity or exercise among HIV-infected children and young adults[36]. Given the benefits of vitD3 supplementation on neuromuscular motor skills and immune parameters in HIV-infected children and young adults[11,12], future studies may wish to examine the interactive effects of vitD3 supplementation with physical activity or exercise on components of health, fitness, and quality of life in this population.
There are several limitations to this study. This was a secondary analysis of muscle health outcomes from a randomized trial[11]. This trial was powered to detect a significant change in the primary study outcome, concentration of serum 25(OH)D[11]. Perhaps due in part to the modest sample size we did not identify statistically significant differences in muscular power and muscular strength outcomes between randomized groups (i.e., using an intention-to-treat analysis). An additional limitation was that we did not measure levels of physical activity.
There are several strengths to this trial. Our outcome using the BOTMP provides a unique insight to the potential efficacy of daily high-dose vitD3 supplementation on neuromuscular motor skills. We examined the effects of daily high-dose vitD3 supplementation (7,000 IU), on long-term (12-month) outcomes with high adherence to the supplement/placebo. Our study sample included predominately African-American participants, many of whom (95%) had insufficient concentrations of serum 25(OH)D at baseline.
In summary, daily supplementation with high-dose (7,000 IU) vitD3 for 12-months among HIV-infected children and young adults may positively impact neuromuscular motor skill proficiency. These hypothesis-generating findings require further study in larger prospective trials.
Footnotes
Edited by: F. Rauch
References
- 1.Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345(21):1522–8. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 2.Pearson DA, McGrath NM, Nozyce M, et al. Predicting HIV disease progression in children using measures of neuropsychological and neurological functioning. Pediatric AIDS clinical trials 152 study team. Pediatrics. 2000;106(6):E76. doi: 10.1542/peds.106.6.e76. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Guttierrez-Teissoonniere S, Conde JG, Baez-Cordova JA, Guzman-Villar B, Lopategui-Corsino E, Frontera WR. Anaerobic power and muscle strength in human immunodeficiency virus-positive preadolescents. PM&R. 2012;4(3):171–5. doi: 10.1016/j.pmrj.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphries C, Potterton J, Mudzi W. A pilot study to investigate the muscle strength of children infected with HIV. International Journal of Therapy and Rehabilitation. 2014;21(1):19–24. [Google Scholar]
- 5.Macdonald HM, Chu J, Nettlefold L, et al. Bone geometry and strength are adapted to muscle force in children and adolescents perinatally infected with HIV. J Musculoskelet Neuronal Interact. 2013;13(1):53–65. [PubMed] [Google Scholar]
- 6.Miller TL, Evans SJ, Orav EJ, Morris V, McIntosh K, Winter HS. Growth and body composition in children infected with the human immunodeficiency virus-1. Am J Clin Nutr. 1993;57(4):588–92. doi: 10.1093/ajcn/57.4.588. [DOI] [PubMed] [Google Scholar]
- 7.Keyser RE, Peralta L, Cade WT, Miller S, Anixt J. Functional aerobic impairment in adolescents seropositive for HIV: a quasiexperimental analysis. Arch Phys Med Rehabil. 2000;81(11):1479–84. doi: 10.1053/apmr.2000.17810. [DOI] [PubMed] [Google Scholar]
- 8.Lee GM, Gortmaker SL, McIntosh K, Hughes MD, Oleske JM Pediatric AIDS Clinical, Trials Group Protocol 219 C Team. Quality of life for children and adolescents: impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117(2):273–83. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty KA, Schall JI, Zemel BS, Tuluc F, Hou X, Rutstein RM, Stallings VA. Safety and efficacy of high-dose daily vitamin D3 supplementation in children and young adults infected with human immunodeficiency virus. J Pediatr Infect Dis Soc. 2014 doi: 10.1093/jpids/piu012. doi: 10.1093/jpids/piu012. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stallings VA, Schall JI, Hediger ML, et al. High-dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014 Jul 1; doi: 10.1097/INF.0000000000000483. doi:10.1097/INF.0000000000000483. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun RF, Liu NQ, Lee T, et al. Vitamin D supplementation and antibacterial immune responses in adolescents and young adults with HIV/AIDS. J Steroid Biochem Mol Biol. 2014 Aug 1; doi: 10.1016/j.jsbmb.2014.07.013. doi:10.1016/j.jsbmb.2014.07.013. [Epub ahead of print] PMID: 25092518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruininks R. Bruininks-Oseretsky Test of Motor Proficiency. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 14.Deitz JC, Kartin D, Kopp K. Review of the Bruininks-Oseretsky test of motor proficiency, (BOT-2) Phys Occup Ther Pediatr. 2007;27(4):87–102. [PubMed] [Google Scholar]
- 15.Dougherty KA, Schall JI, Rovner AJ, Stallings VA, Zemel BS. Attenuated maximal muscle strength and peak power in children with sickle cell disease. J Pediatr Hematol Oncol. 2011;33(2):93–7. doi: 10.1097/MPH.0b013e318200ef49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veilleux L, Rauch F. Reproducibility of jumping mechanography in healthy children and adults. J Musculoskelet Neuronal Interact. 2010;10(4):256–66. [PubMed] [Google Scholar]
- 17.Cavagna GA. Force platforms as ergometers. J Appl Physiol. 1975;39(1):174–9. doi: 10.1152/jappl.1975.39.1.174. [DOI] [PubMed] [Google Scholar]
- 18.Leggin BG, Neuman RM, Iannotti JP, Williams GR, Thompson EC. Intrarater and interrater reliability of three isometric dynamometers in assessing shoulder strength. J Shoulder Elbow Surg. 1996;5(1):18–24. doi: 10.1016/s1058-2746(96)80026-7. [DOI] [PubMed] [Google Scholar]
- 19.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48(5):1103–8. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deighan M, De Ste Croix M, Armstrong N. Reliability of isokinetic concentric and eccentric knee and elbow extension and flexion in 9/10 year old boys. Isokinetics Exerc Sci. 2003;11(2):109–15. [Google Scholar]
- 21.Groleau V, Herold RA, Schall JI, et al. Blood lead concentration is not altered by high-dose vitamin D supplementation in children and young adults with HIV. J Pediatr Gastroenterol Nutr. 2013;56(3):316–9. doi: 10.1097/MPG.0b013e3182758c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979:65–70. [Google Scholar]
- 23.Schüler S, Mucha A, Doherty P, Kieser M, Rauch G. Easily applicable multiple testing procedures to improve the interpretation of clinical trials with composite endpoints. Int J Cardiol. 2014;175(1):126–32. doi: 10.1016/j.ijcard.2014.04.267. [DOI] [PubMed] [Google Scholar]
- 24.Buitrago CG, Arango NS, Boland RL. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J Cell Biochem. 2012;113(4):1170–81. doi: 10.1002/jcb.23444. [DOI] [PubMed] [Google Scholar]
- 25.Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144(12):5138–44. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 26.Burne TH, Johnston AN, McGrath JJ, Mackay-Sim A. Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull. 2006;69(1):74–8. doi: 10.1016/j.brainresbull.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Rodman JS, Baker T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int. 1978;13(3):189–93. doi: 10.1038/ki.1978.28. [DOI] [PubMed] [Google Scholar]
- 28.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2012;34(1):33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 29.Ward K, Das G, Roberts S, Berry J, Adams J, Rawer R, Mughal M. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95(10):4643–51. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 30.Knutsen KV, Madar AA, Lagerløv P, Brekke M, Raastad T, Stene LC, Meyer HE. Does vitamin D improve muscle strength in adults? A randomized, double-blind, placebo-controlled trial among ethnic minorities in Norway. J Clin Endocrinol Metab. 2014;99(1):194–202. doi: 10.1210/jc.2013-2647. [DOI] [PubMed] [Google Scholar]
- 31.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 32.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clin Nutr. 2011;30(5):624–8. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Eckard AR, Judd SE, Ziegler TR, et al. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antivir Ther. 2012;17(6):1069–78. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial US adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123(3):797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 35.Ward KA, Das G, Berry JL, Roberts SA, Rawer R, Adams JE, Mughal Z. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94(2):559–63. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 36.Miller TL, Somarriba G, Kinnamon DD, Weinberg GA, Friedman LB, Scott GB. The effect of a structured exercise program on nutrition and fitness outcomes in human immunodeficiency virus-infected children. AIDS Res Hum Retroviruses. 2010;26(3):313–9. doi: 10.1089/aid.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;8(8):CD001796. doi: 10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O Brien K, Nixon S, Tynan A, Glazier RH. Effectiveness of aerobic exercise in adults living with HIV/AIDS: systematic review. Med Sci Sports Exerc. 2004;36:1659–66. doi: 10.1249/01.mss.0000142404.28165.9b. [DOI] [PubMed] [Google Scholar]
- 39.Clingerman E. Physical activity, social support, and health-related quality of life among persons with HIV disease. J Community Health Nurs. 2004;21(3):179–97. doi: 10.1207/s15327655jchn2103_5. [DOI] [PubMed] [Google Scholar]


