Abstract
Objective
This study was designed to examine the prospective relations of harsh parenting during preadolescence, anger across adolescence, and a health phenotype at late adolescence among African American youths living in the rural South. A second purpose was to determine whether, for genetic reasons, some youths will be more sensitive than others to a harsh parenting to anger to poor health pathway.
Methods
Participants were 368 youths (age 11.2 at the first assessment) who provided data on receipt of harsh parenting during preadolescence (ages 11 to 13), anger across adolescence (ages 16 to 18), and a health phenotype consisting of C Reactive Protein, depressive symptoms, and health problems at age 19. Youths were genotyped at the 5-HTTLPR at age 16.
Results
The data analysis revealed that (a) harsher parenting was associated positively across time with anger and poor health, (b) anger across adolescence also was associated positively across time with poor health, (c) anger served as a mediator connecting harsh parenting and poor health, and (d) the harsh parenting to anger to poor health pathway was significant only for youths carrying one or two copies of a short allele at the 5-HTTLPR.
Conclusions
These findings are consistent with the hypothesis that harsh parent-child interactions presage health through effects on emotion regulation, particularly anger. This mediational pathway pertained only to youths carrying a gene that confers sensitivity and reactivity to harsh family processes and the negative emotional states they occasion.
A growing body of research has tested the hypothesis that harsh interactions with one’s parents during childhood may contribute to vulnerability to chronic diseases later in life (Repetti, Taylor, & Seeman, 2002; Shonkoff, Boyce, & McEwen, 2009). For example, the Adverse Childhood Experiences Study assessed the medical histories of more than 17,000 adults and found that the rates of cardiovascular disease, autoimmune disorders, and premature death were 1.5 to 2.0 times higher among respondents who were exposed to family violence than among those who were not exposed (Dube et al., 2009). Other studies reveal that adults reared in harsher home environments evince higher blood pressure, worse metabolic profiles, greater inflammatory activity, and higher levels of depressive symptoms than do adults reared in less harsh households (Miller, Chen, & Parker, 2011; Repetti et al., 2002). The present study was designed to advance understanding of the association between harsh parenting practices and health status by testing hypotheses involving prospective pathways among harsh parenting, anger, and health outcomes among a representative sample of rural African American adolescents.
This study was designed specifically to address several conceptual and design issues. Research to date has often confounded the operationalization of harsh parenting with other adversity processes including family conflict, conflict among adult caregivers, family violence, neighborhood violence, and low socioeconomic status (SES). Also, all but one of these studies (Danese et al., 2009) measured harsh parenting using retrospective reports. The present study addressed these issues by assessing exposure to harsh parenting across preadolescence (ages 11–13), anger across adolescence (ages 16–18), and a health phenotype at age 19 to test hypothesized links across time between the receipt of harsh parenting across preadolescence and poor health outcomes.
The health phenotype assessment included three indicators that not only index physical health and emotional well-being at age 19 but also have prognostic significance for health at midlife and beyond. The three indicators included a biomarker of chronic inflammation, C Reactive Protein (CRP); self-reported health problems; and depressive symptoms. CRP was selected because mounting evidence indicates that elevated levels of CRP are associated with heightened risk for age-related diseases, included hypertension, cardiovascular disease (CVD), stroke, diabetes, and cancer in adulthood (Chung et al., 2009; Singh & Newman, 2011). Consequently, CRP is used in clinical settings to evaluate risk of CVD and other chronic diseases of aging (Ridker, 2009; Yeh, 2005). Another emerging body of evidence indicates that stress can increase inflammation; the stimulation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system by stress can increase inflammatory processes as indicated by higher levels of CRP (Miller et al., 2011). Relevant to the present study are recent findings demonstrating that life stress during adolescence is positively associated with contemporaneous CRP levels (Fuligni et al., 2009). The second indicator, self-reported health problems, consistently shows a positive association with life stress (Sutin, Costa, Wethington, & Eaton, 2010) and forecasts mortality over and above current health status (Idler & Benyamini, 1997). Depressive symptoms, the third indicator, have been found to heighten risk for morbidity and mortality from chronic diseases associated with aging, including CVDs, autoimmune conditions, and metabolic disorders (Evans et al., 2005; Hemingway et al., 2003). Growing up in harsh rearing environments has been found to increase vulnerability to the development of depressive symptoms across the lifespan (Heim & Nemeroff, 2001). The following sections focus on the hypothesized prospective contributions that harsh parenting, anger, and genetic sensitivity make to the adolescent health phenotype.
Harsh Parenting, Anger, and Health
The literature on determinants of parenting chronicles the ways in which the strains and multiple demands imposed by socioeconomic stressors tax even the most concerned caregivers and lead them to use harsh parenting practices that, by design, quickly terminate aversive child and adolescent behavior (Belsky, 1984; Brody et al., 2008). These findings generalize across racial and ethnic groups (Conger & Donnellan, 2007). Harsh parenting is often part of a set of parenting practices that also includes high levels of vigilance and control. These practices are used particularly by parents living in dangerous contexts to protect their children against neighborhood dangers, risk behaviors, and the influence of delinquent peers (R. H. Bradley et al., 2000; Brody & Flor, 1998; Brody et al., 2001; J. Taylor, Spencer, & Baldwin, 2000). Generally, however, research suggests that growing up with a large dose of harsh parenting does not lead to positive physical and mental health outcomes (Repetti et al., 2002). Its most prominent legacy concerns problems with emotion regulation, particularly with respect to elevated levels of anger and its expression (Davies, Winter, & Cicchetti, 2006; Simons et al., 2011). Research indicates that youths who receive harsh parenting over time become more likely to maintain a heightened state of vigilance for signs of anger and to respond with anger to incidents in which anger is expressed (Cicchetti & Rogosch, 2009; Dodge, Pettit, & Bates, 1994). We predict that exposure to harsh parenting during preadolescence will be positively associated with anger during adolescence and health problems at age 19.
Episodic and chronic anger states are hypothesized to trigger reactions from the sympathetic-adrenal-medullary (SAM) and HPA axis systems, which results in frequent releases of catecholamines and cortisol (Chorpita & Barlow, 1998; Matthews, Woodall, Kenyon, & Jacob, 1996). Although this chain reaction is adaptive in the short term, continuous exposure to cortisol and catecholamines over time exacts a toll by promoting the development of glucocorticoid resistance, disruptions in the glucocorticoid anti-inflammatory system, and, eventually, elevated levels of systemic inflammation (Black, 2006). The same stress physiology pathways have been hypothesized to contribute to the development of depressive symptoms (McEwen, 1998); recent findings tend to support this hypothesis (Miller & Cole, 2012). Thus, relatively higher levels of anger across adolescence are predicted both to forecast a poor health phenotype and to serve as a mediator connecting harsh parenting with health at age 19.
The Serotonin Transporter Gene as a Moderator
Stressful events have been shown to increase the probability of a wide variety of physical and mental health problems, and yet many individuals exposed to environmental or negative emotional stressors do not become sick or emotionally dysregulated. During the past decade, much research investigated the hypothesis that particular genetic polymorphisms increase an individual's response to stress and enhance the probability of compromised physical and mental health functioning (Way & Taylor, 2010). Much of this research has focused on a genetic marker of differential functioning in the serotonin system, the 5-HTTLPR. This polymorphism has two principal alleles, long (l, 16 repeats) and short (s, 14 repeats). The s allele is associated with reduced serotonergic neurotransmission; low central serotonergic transmission has been linked to robust activity in the amygdala (Heinz et al., 2005), a brain region involved in the processing of verbal and nonverbal threats (Isenberg et al., 1999), and with enhanced reactivity to punishment cues in the environment (Battaglia et al., 2005; Hariri, Drabant, & Weinberger, 2006; Hariri et al., 2002). Recent research has extended these findings by demonstrating that ss and sl allele carriers direct preferential attention toward, and have difficulty in disengaging from, threat-related stimuli; they also engage in more rumination about such stimuli (Beevers, Wells, Ellis, & McGeary, 2009; Osinsky et al., 2008). In addition, researchers have recently investigated the interactions of stressful life events and family characteristics with the 5-HTTLPR genotype (Petersen et al., 2012). Findings demonstrate that stress may affect the likelihood that adolescents with a genotype associated with low serotonin activity will experience symptoms of anxiety or depression. Together, this literature suggests that the effects of the 5-HTTLPR s allele may place youths at greater risk for the negative effects of harsh parenting and anger by increasing vigilance, reactivity, rumination, anxiety, and depressive symptoms.
Method
Participants
The data for this study were drawn from the Strong African American Families Healthy Adult Panel (SHAPE) study (G. Brody, PI). African American primary caregivers and a target youth selected from each family participated in annual data collections; youths' mean age was 11.2 years (SD = 0.34) at the first assessment and 19.2 (SD = 0.61) years at the last assessment. Of the youths in the sample, 53% were female. At the first assessment, 78% of the caregivers had completed high school or earned a general equivalency diploma. The families resided in nine rural counties in Georgia, in small towns and communities in which poverty rates are among the highest in the nation and unemployment rates are above the national average (Proctor & Dalaker, 2003). Although the primary caregivers in the sample worked an average of 39.4 hours per week (SD = 11.51), at the first assessment 46.3% lived below the federal poverty standards with a median family income per month of $1655; at the last assessment, the proportion was 54.8% with a median income of $1169. The increase in the proportion of families living in poverty and the decrease in family income over time may have resulted from the economic recession that was occurring during 2010, when the last wave of data was collected. Overall, the families can be characterized as working poor.
At the first assessment, 667 families were selected randomly from lists of fifth-grade students that schools provided (see Brody et al., 2004 for a full description). From a sample of 561 at the age 18 data collection (a retention rate of 84%), 500 families were selected randomly for assessment of variables including CRP levels, measured via analysis of a blood sample, at the age 19 data collection. Of these 500 families, 426 agreed to participate. Analyses indicated that the sample providing blood at age 19 was comparable on indicators of harsh parenting during preadolescence and anger during adolescence to the larger sample who provided data at ages 11 through 18. The 426 families with CRP data reported more cumulative SES risk (described in the Measures section; M = 6.83, SD = 3.99) than did those who did not provide CRP data (M = 5.97, SD = 4.01, t (665) = 2.69, p < .01). Therefore, cumulative SES risk was controlled in all analyses.
Of the 426 participants for whom CRP data had been collected, 368 had provided DNA samples at age 16 and were successfully genotyped at the 5-HTTLPR. Comparisons, using independent t-tests and chi-square tests, of the 368 youths who provided genetic data with the 58 who did not revealed no differences on any of the demographic or study variables. These 368 families constituted the sample for the present study.
Procedure
All data were collected in participants’ homes using a standardized protocol. One home visit that lasted approximately 2 hours was conducted by two African American field researchers at each wave of data collection. Interviews were conducted privately, with no other family members present or able to overhear the conversation. Informed consent was obtained at each data collection wave. Participants were told that the purpose of the study was to identify the predictors of health and well-being among rural African American adolescents. They were compensated $100 at each wave of data collection. Primary caregivers consented to minor youths’ participation in the study, and minor youths assented to their own participation. Youths consented to their own participation at the age 18 and age 19 data collections.
Measures
Control variables
Youth gender and family cumulative socioeconomic risk during preadolescence (ages 11 to 13) were controlled in all analyses. Cumulative risk was defined as the sum of six socioeconomic risk factors measured during each of the three annual preadolescent assessments. This yielded a cumulative risk index that ranged from 0 to 18 (M = 6.83, SD = 3.99). The six risk indicators were family poverty as assessed using United States government criteria (an income-to-needs ratio ≥ 1.5), primary caregiver noncompletion of high school or an equivalent, primary caregiver unemployment, single-parent family structure, family receipt of Temporary Assistance for Needy Families, and income rated by the primary caregiver as adequate to meet all needs.
Preadolescent harsh-inconsistent parenting
Four items that index harsh parenting drawn from the Harsh/Inconsistent Parenting Scale (Brody et al., 2001) assessed caregivers' use of slapping, hitting, and shouting to discipline the youth. Cronbach's alphas ranged from .54 to .66 across the assessments at ages 11, 12, and 13. Low internal consistency is common in the literature for measures of harsh parenting due to low base rates of these disciplinary practices (Brody et al., 2001; Simons & Burt, 2011).
Adolescent anger
Three waves of data were collected when the youths were 16, 17, and 18 years of age. Anger was measured using the 15-item State Anger subscale taken from the State-Trait Anger Expression Inventory developed by Spielberger and associates (Spielberger, Jacobs, Russell, & Crane, 1983). The anger subscale has demonstrated predictive validity with health problems (Johnson, Schork, & Spielberger, 1987). Youths were asked about their feelings over the past three months and to rate discrete emotions (e.g., "I am furious"; "I feel angry") on a scale ranging from 1 (always) to 5 (never). Cronbach's alphas ranged from .91 to .92 across the three waves.
Late adolescent health phenotype
Health at age 19 was measured by three indicators: CRP, a biological marker of systemic inflammation assayed from a blood sample; youths' self-reported depressive symptoms; and their self-reported health problems. After blood was drawn into serum separator tubes by certified phlebotomists, it was frozen and delivered to the Psychiatric Genetics Lab at the University of Iowa for assaying. Phlebotomists went to each participant's home to draw the blood. Serum levels of CRP were determined using a Duo Set Kit (DY1707; R&D Systems, Minneapolis, MN, USA) according to the manufacturer' s directions. A normal concentration of CRP in healthy human serum is usually lower than 10 mg/L. No participants had CRP levels outside the normal range. Because CRP is characterized by a skewed distribution (skewness = 1.90, kurtosis = 2.94), we applied a log transformation to normalize the readings (skewness = 0.91, kurtosis = −0.31 after the transformation).
Self-reports of depressive symptoms at age 19 were obtained using the Center for Epidemiologic Studies Depression scale (CES–D; Radloff, 1977), which is widely used with community samples. Youths rated each of 20 symptoms on a scale of 0 (rarely or none of the time), 1 (some or a little of the time), 2 (occasionally or a moderate amount of the time), or 3 (most or all of the time). The alpha coefficient was .86. Youths reported their health problems at age 19 using the General Health Perceptions subscale from the RAND 36-Item Short-Form Health Survey (Hays, Sherbourne, & Mazel, 1993). The five-item subscale included a single-item rating of overall health ranging from 1 (excellent) to 5 (poor) and four items assessing youths' ratings of their current health status ranging from 1 (definitely false) to 5 (definitely true); e.g., "I am as healthy as anybody I know"; "I seem to get sick a little easier than other people". Some of the items were reversed scored so that higher scores on the subscale indicated more health problems and poorer general health. After reverse scoring, all items were averaged to yield a General Health Problems score with a range of 0 to 100 (α = .72).
Genotyping
Participants’ DNA was obtained at age 16 using Oragene DNA kits (DNA Genotek; Kanata, Ontario, Canada). Participants rinsed their mouths with tap water, then deposited 4 ml of saliva in the Oragene sample vial. The vial was sealed, inverted, and shipped via courier to a central laboratory in Iowa City, where samples were prepared according to the manufacturer’s specifications. Genotype at the 5-HTTLPR was determined for each participant as described previously (S. L. Bradley, Dodelzon, Sandhu, & Philibert, 2005) using the primers F-GGCGTTGCCGCTCTGAATGC and R-GAGGGACTGAGCTGGACAACCAC, standard Taq polymerase and buffer, standard dNTPs with the addition of 100 μM 7-deaza GTP, and 10% DMSO. The resulting polymerase chain reaction products were electrophoresed on a 6% nondenaturing polyacrylamide gel and products visualized using silver staining. Genotype was then called by two individuals blind to the study hypotheses and other information about the participants. Of the sample, 5.7% were homozygous for the s allele, 35.1% were heterozygous, and 59.2% were homozygous for the l allele. None of the alleles deviated from Hardy-Weinberg equilibrium. Consistent with prior research (Brody et al., 2011; Hariri et al., 2005), genotyping results were used to form two groups of participants: those homozygous for the l allele (coded as 0, n = 218, 59.2%) and those with either one or two copies of the s allele (coded as 1, n = 150, 40.8%).
Results
Plan of Analysis
The hypothesized mediation model was tested with structural equation modeling (SEM) executed using Mplus 6.11 (Muthén & Muthén, 1998–2010) using full information maximum likelihood estimation on the data obtained from age 11 to age 19. This estimation does not delete cases that are missing on endogenous variables, nor does it delete cases that are missing a variable within a wave of data collection. This method thus avoids problems, such as biased parameter estimates, that are more likely to occur if pairwise or listwise procedures are used (Acock, 2005). This analysis tested the hypotheses that (a) receipt of harsh parenting across preadolescence (ages 11 to 13) would be positively associated with anger across adolescence (ages 16 to 18) and health indicators (CRP levels, depressive symptoms, and general health problems) at age 19, (b) anger across adolescence would be positively associated with health problem indicators at age 19, and (c) adolescent anger would serve as a mediator connecting harsh parenting across preadolescence with health problem indicators at age 19. The significance of the indirect effect was tested using the delta method to compute the standard error (Muthén & Muthén, 1998–2010). In addition, nonparametric bootstrapping, which has been found to be sensitive in mediational analyses, was used to estimate standard errors of the indirect effects for significance testing. In each model, standard errors were standard deviations for a pseudo-population based upon 1000 samples drawn with replacement from the data. The conceptual model was then estimated separately for participants carrying an s allele or two l alleles at the 5-HTTLPR to test for hypothesized genetic moderation effects. Multigroup comparison procedures were then used to determine whether the estimated parameters differed significantly between groups. The Wald test (Wald, 1947) was used to confirm the hypothesized group differences on the structural path coefficients.
Adolescent Anger Mediates the Longitudinal Association Between Receipt of Harsh Parenting Across Preadolescence and Health at Age 19
The structural equation model was specified with harsh parenting across preadolescence as the exogenous construct, not predicted by any prior variable in the model. Anger across adolescence and the health phenotype in late adolescence were specified as endogenous constructs. Table 1 presents the means and standard deviations separately by genotype group for the SEM variables; t test comparisons revealed no significant differences on any of the study variables. Table 2 presents the correlation matrix. The measurement model fit the data fairly well: χ2(24) = 43.25, p < .01; CFI = .98; RMSEA = .04 (.02, .06). All indicators loaded on their respective constructs significantly and in the expected direction. The goodness-of-fit indices indicate an acceptable fit between the proposed model and the data: χ2(36) = 90.59, p < .001; CFI = .95; RMSEA = .06 (.04, .08). Figure 1 presents the results of the tests of the hypothesized model. As expected, receipt of harsh parenting during preadolescence forecast anger across adolescence and health phenotype in late adolescence. The significant positive coefficients indicate that harsher parenting was associated with more anger and poorer health. Also consistent with the hypothesis, the results indicated that higher levels of anger forecast poorer health outcomes. These results emerged with cumulative SES risk and gender controlled. Finally, Figure 1 also shows the results of the mediational analysis. As hypothesized, the presence of the mediator, anger across adolescence, reduced the direct effect of harsh parenting during preadolescence on health during late adolescence from β = .18, p < .02 to β = .08, ns. The indirect effect of preadolescent exposure to harsh parenting on young adult health problems via adolescent anger was significant (estimates = .128, SE = .042, p < .001 with 1000 bootstrapping). An additional multigroup SEM model was executed to determine whether any path in the mediational model differed by gender. No such differences emerged for either the main effect or any mediational effects, Wald χ2(3) = .523, p = ns.
Table 1.
Descriptive Statistics among Study Variables for 5-HTTLPR Genotype Groups
| 5-HTTLPR Genotype Groups
|
|||||
|---|---|---|---|---|---|
| Long Allele Group
|
Short Allele Group
|
||||
| Variables at pretest | M | SD | M | SD | T |
| 1. Gender (male) | 0.44 | 0.50 | 0.47 | 0.50 | −.71 |
| 2. SES-related risk (11–13 yrs.) | 6.81 | 3.85 | 6.93 | 3.88 | −.29 |
| Harsh parenting | |||||
| 3. 11 yrs. | 7.32 | 2.04 | 8.59 | 2.10 | −1.22 |
| 4. 12 yrs. | 6.90 | 1.96 | 7.04 | 2.02 | −0.63 |
| 5. 13 yrs. | 6.51 | 2.06 | 6.66 | 2.02 | −0.70 |
| Youth anger | |||||
| 6. 16 yrs. | 32.45 | 9.55 | 32.43 | 10.86 | 0.02 |
| 7. 17 yrs. | 33.35 | 10.39 | 34.58 | 13.60 | −0.89 |
| 8. 18 yrs. | 33.84 | 10.72 | 33.18 | 10.98 | 0.57 |
| 9. Ln (CRP) (19 yrs.) | 0.65 | 0.66 | 0.73 | 0.72 | −1.16 |
| 10. Health (19 yrs.) | 24.60 | 17.43 | 26.38 | 18.82 | −0.92 |
| 11. Depression (19 yrs.) | 13.45 | 9.27 | 13.68 | 9.30 | −0.22 |
Note. SES = socioeconomic status. Ln = log transformation. CRP = C Reactive Protein.
Table 2.
Descriptive Statistics and Correlations among Harsh Parenting, Anger, Adolescent Health Indicators, and Control Variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender, male | -- | ||||||||||
| 2. SES-related risk, 11–13 yrs. | −.028 | -- | |||||||||
| Harsh parenting | |||||||||||
| 3. 11 yrs. | .002 | −.038 | -- | ||||||||
| 4. 12 yrs. | −.004 | .004 | .446*** | -- | |||||||
| 5. 13 yrs. | −.003 | .035 | .369*** | .530*** | -- | ||||||
| Youth anger | |||||||||||
| 6. 16 yrs. | −.043 | .041 | .063 | .212*** | .141* | -- | |||||
| 7. 17 yrs. | −.044 | −.029 | .121* | .179** | .099 | .593*** | -- | ||||
| 8. 18 yrs. | −.131** | .007 | .096* | .174*** | .086 | .564*** | .653*** | -- | |||
| 9. Ln (CRP) 19 yrs. | −.323*** | .065 | .147** | .095 | .132** | .079 | .071 | .130** | -- | ||
| 10. Health, 19 yrs. | −.243*** | .155** | .077 | .085 | .041 | .165** | .202*** | .273*** | .223*** | -- | |
| 11. Depression, 19 yrs. | −.110* | .056 | .098* | .109* | .048 | .332*** | .374*** | .336*** | .073 | .406*** | -- |
|
| |||||||||||
| Mean | 0.45 | 6.83 | 7.39 | 6.94 | 6.54 | 32.24 | 33.89 | 33.38 | 0.68 | 25.71 | 13.43 |
| SD | 0.50 | 3.99 | 2.05 | 1.96 | 2.02 | 10.37 | 11.76 | 10.90 | 0.68 | 18.02 | 9.15 |
Note. SES = socioeconomic status. Ln = log transformation. CRP = C Reactive Protein.
p < .05.
p < .01.
p < .001.
Figure 1.
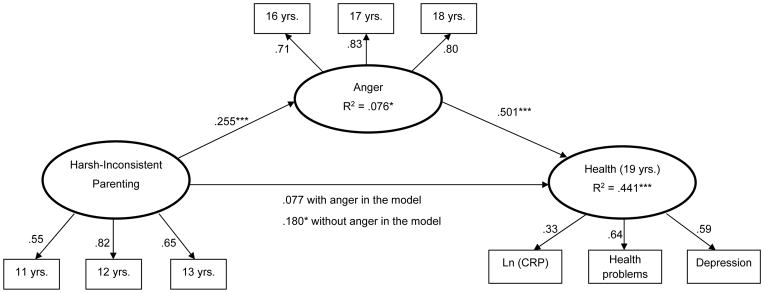
A mediation model of preadolescent harsh parenting, adolescent anger, and late adolescent health with cumulative socioeconomic risk and gender controlled. Standardized coefficients are presented. Ln = log transformation. CRP = C Reactive Protein.
*p < .05. ***p < .001.
Testing the 5-HTTLPR Moderation Hypothesis
To determine whether the links in the mediation model would differ by 5-HTTLPR status, the model was next estimated for youths carrying an s allele or two l alleles. Using multigroup comparison procedures, a two-group invariance model was first estimated. Equality constraints were imposed on every coefficient estimate. One equality constraint at a time was then relaxed for the specific coefficient under investigation, allowing the coefficient to differ across groups, and the model was re-estimated. Before this multigroup SEM model was executed, however, the measurement equivalence across the two groups was examined. This was done by comparing a model that constrained factor loadings and intercepts of the observed indicators to be equal across groups with a model in which the factor loadings and intercepts were allowed to vary across groups. The fit of the constrained model was not significantly different from the fit of the unconstrained model, Δχ2(14) = 17.45 p = ns, indicating the measurement model was equivalent across the two genotype groups.
Next, the multigroup SEM model was executed; the measurement model was constrained to be equal across groups and the structural paths were freely estimated for each group. The residual factor variances of CRP levels and depressive symptoms were correlated because evidence indicates that CRP levels and depressive symptoms covary (see Miller & Cole, 2012). The goodness-of-fit indices indicate an acceptable model fit: χ2(82) = 116.59, p < .01; CFI = .96; RMSEA = .05 (.03, .07); see Figure 2. The 5-HTTLPR moderation hypothesis was partially supported. Contrary to our predictions, the contribution of harsh parenting to anger across adolescence did not vary as a function of 5-HTTLPR status. Consistent with the moderation hypothesis, however, was the finding that the association between anger across adolescence and health at age 19 was stronger for youths carrying an s allele at the 5-HTTLPR (β = .61, p < .001) than for carriers of two l alleles (β = .28, p < .01; Wald χ2(1) = 4.17, p < .05). The indirect effect of harsh parenting on late adolescent health via anger was also examined. The indirect effect was significant for carriers of an s allele at the 5-HTTLPR (estimate = .161, SE = .080, p < .05 with 1000 bootstrapping) but was not significant for carriers of two l alleles (estimate = .05, SE = .036, p = .165 with 1000 bootstrapping). A review of Figure 2 reveals that the moderated-mediation model for carriers of the s allele accounted for an impressive 53% of the variance in the late adolescent health phenotype. Conversely, the same model for carriers of two l alleles accounted for 29% of the variance in the phenotype.
Figure 2.
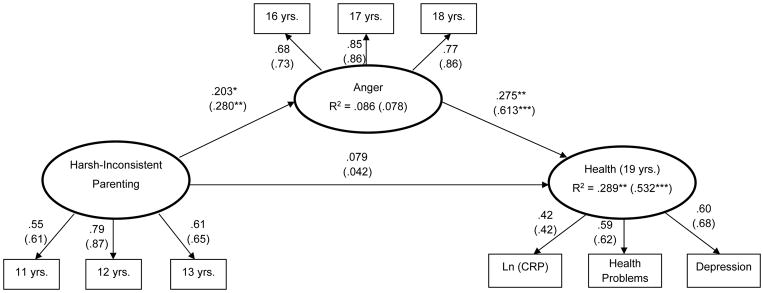
A moderated mediation model of preadolescent harsh parenting, adolescent anger, and late adolescent health by genotype at the 5-HTTLPR with cumulative socioeconomic risk and gender controlled. Numbers in parentheses are standardized coefficients for the 5-HTTLPR short allele genotype group (n = 218; n = 150 for the homozygous long allele group). Ln = log transformation. CRP = C Reactive Protein.
*p < .05. **p < .01. ***p < .001.
Discussion
A longitudinal research design was used to test hypotheses through which receipt of harsh parenting across preadolescence is linked to poorer health among African American adolescents at age 19. Exposure to harsh parenting was linked to higher levels of anger across adolescence that in turn forecast a health phenotype composed of CRP, depressive symptoms, and self-reported health problems. As predicted, elevated levels of anger across adolescence served as a mediator that was responsible for the prospective association between harsh parenting and poorer health. This mediation model was refined further, as adolescent anger forecast the health phenotype only for carriers of the s allele at the 5-HTTLPR. Consistent with the study hypotheses, not all youths who experienced harsh parenting and anger evinced poorer physical and mental health outcomes.
The finding that carrying two copies of the l allele at the 5-HTTLPR buffered youths from compromised health is pertinent to research on youth resilience. This literature has addressed the reasons why some youths who experience many discrete and chronic stressors do not succumb to their negative effects (Rutter, 2012). Typically the locus of these resilience effects is identified through contextual processes at different levels of analysis (family, peer, school, or neighborhood) that alter several types of pathways, including the reduction of risk factors. The present results reinforce recent suggestions that genetic status also contributes to resilience, including resilience to poor health outcomes (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Rutter, 2012).
The mechanisms underlying the stronger associations in the harsh parenting to anger to health phenotype pathway among carriers of the s allele at the 5-HTTLPR are not well understood. One possible explanation is that recipients of harsh parenting are more likely to have difficulty regulating their thoughts and emotions in response to various events and are more prone to rumination, particularly if they carry an s allele at the 5-HTTLPR (Beevers et al., 2009; Brosschot, Gerin, & Thayer, 2006). Ruminative processes may occasion more frequent allostatic activations of the SAM and HPA axis systems, which over time take a toll on the body as evidenced by higher levels of systemic inflammation, depressive symptoms, and health problems (Cicchetti & Toth, 2005; Miller et al., 2011).
These findings are consistent with propositions that poor health during adulthood is tied to experiences earlier in life, particularly for persons growing up with the stressors associated with low SES (Shonkoff et al., 2009). Studies have found that adults growing up in environments with higher levels of adversity present higher levels of inflammatory activity and depressive symptoms (Danese et al., 2009; Miller & Chen, 2010; S. E. Taylor, Lehman, Kiefe, & Seeman, 2006). This study extends previous research by using a large representative sample of African Americans using an 8-year prospective research design and a health phenotype that included two risk factors for subsequent development of chronic disease, CRP and depressive symptoms, as an outcome. The results complement those reported by Miller and colleagues (Miller & Chen, 2010; Miller & Cole, 2012), who assessed psychological stress and inflammatory activity over 1.5 years among a sample of female adolescents who were at risk for depression. To the extent that these youths were reared in harsh families, they evinced an inflammatory phenotype at follow-up analyses. Even though this at-risk sample of female adolescents and the current sample differed on many dimensions, including race/ethnicity, SES, and geographical region, both studies demonstrated that harsh family environments prospectively contribute to health profiles that have been found to contribute to aging-related conditions such as metabolic syndrome, autoimmune disorders, and cardiovascular disease (Nathan, 2002). These studies at least suggest that CRP levels should be measured throughout adolescence at yearly physical examinations . Elevated levels not only would have prognostic value for the early onset of chronic disease but also could serve as a marker of exposure to high levels of life stress.
Limitations of the present study should be noted. Future replications of this prospective study with younger rural African American children are indicated. Understanding the complex relationships among early family environments, genes that confer sensitivity, and health phenotypes is highly relevant to public health. It is not known whether the results of this study generalize to European American or Latino families living in either rural or urban communities. A second limitation is that the prospective assessments were obtained only from the youths and the validity of these reports was not confirmed through collateral sources. The self-reports appear to have value, however, because they have been shown to forecast prospectively long-term health and psychiatric outcomes (Brody & Sigel, 1990; Cicchetti & Blender, 2006). In the present study, reports concerning the receipt of harsh parenting forecast levels of the biomarker CRP 6 years later; this also supports the likelihood that the obtained results were not merely a product of method variance. Finally, implications for prevention should be considered. The present results suggest that youths who carry a sensitivity gene and are exposed to harsh parenting that occasions angry emotional states are at high risk for dysregulation across biological regulatory systems and compromised physical health and psychological functioning. Research on the design of preventive interventions for these youths may yield approaches that are effective in deterring the development of chronic physical and psychiatric disease.
Acknowledgments
This research was supported by Award Number R01HD030588 from the National Institute on Child Health and Human Development and Award Number P30DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, or the National Institutes of Health.
Contributor Information
Gene H. Brody, University of Georgia
Tianyi Yu, University of Georgia.
Steven R. H. Beach, University of Georgia
Steven M. Kogan, University of Georgia
Michael Windle, Emory University.
Robert A. Philibert, University of Iowa
References
- Acock AC. Working with missing values. Journal of Marriage and Family. 2005;67:1012–1028. [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, Marino C. Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Archives of General Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. The determinants of parenting: A process model. Child Development. 1984;55:83–96. doi: 10.2307/1129836. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Medical Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Caldwell BM, Whiteside-Mansell L, Wasserman GA, Mink IT. Measuring the home environments of children in early adolescence. Journal of Research on Adolescence. 2000;10:247–288. doi: 10.1207/SJRA1003_1. [DOI] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Chen Y-f, Obasi E, Philibert RA, Kogan SM, Simons RL. Perceived discrimination, serotonin transporter linked polymorphic region status, and the development of conduct problems. Development and Psychopathology. 2011;23:617–627. doi: 10.1017/S0954579411000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Kogan SM, Murry VM, Logan P, Luo Z. Linking perceived discrimination to longitudinal changes in African American mothers’ parenting practices. Journal of Marriage and Family. 2008;70:319–331. doi: 10.1111/j.1741-3737.2008.00484.x. [DOI] [Google Scholar]
- Brody GH, Flor DL. Maternal resources, parenting practices, and child competence in rural, single-parent African American families. Child Development. 1998;69:803–816. doi: 10.2307/1132205. [DOI] [PubMed] [Google Scholar]
- Brody GH, Ge X, Conger RD, Gibbons FX, Murry VM, Gerrard M, Simons RL. The influence of neighborhood disadvantage, collective socialization, and parenting on African American children's affiliation with deviant peers. Child Development. 2001;72:1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, Neubaum-Carlan E. The Strong African American Families program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Sigel IE. Methods of family research: Biographies of research projects: Vol. 2. Clinical populations. Hillsdale, NJ: Erlbaum; 1990. [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: The role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Chung HW, Kim JW, Lee J-h, Song SY, Chung JB, Kwon OH, Lim JB. Comparison of the validity of three biomarkers for gastric cancer screening: Carcinoembryonic antigen, pepsinogens, and high sensitive C-reactive protein. Journal of Clinical Gastroenterology. 2009;43:19–26. doi: 10.1097/MCG.0b013e318135427c. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Adaptive coping under conditions of extreme stress: Multilevel influences on the determinants of resilience in maltreated children. In: Skinner EA, Zimmer-Gembeck MJ, editors. New directions for child and adolescent development: No. 124. Coping and the development of regulation. San Francisco, CA: Jossey-Bass; 2009. pp. 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Winter MA, Cicchetti D. The implications of emotional security theory for understanding and treating childhood psychopathology. Development and Psychopathology. 2006;18:707–735. doi: 10.1017/S0954579406060354. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Development. 1994;65:649–665. doi: 10.1111/j.1467-8624.1994.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Valvo WJ. Mood disorders in the medically ill: Scientific review and recommendations. Biological Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower JE, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin America and European backgrounds. Psychosomatic Medicine. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. Retrieved from http://archpsyc.ama-assn.org/cgi/content/abstract/62/2/146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: Perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana BS, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002 Jul 19;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Economics. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Büchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Mullen MJ, Kumari M, Brunner EJ, Taylor M, Marmot M. Social and psychosocial influences on inflammatory markers and vascular function in civil servants (the Whitehall II study) American Journal of Cardiology. 2003;92:984–987. doi: 10.1016/S0002-9149(03)00985-8. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. Retrived from http://www.jstor.org/stable/2955359. [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Stern E. Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences of the USA. 1999;19:10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EH, Schork NJ, Spielberger CD. Emotional and familial determinants of elevated blood pressure in Black and White adolescent females. Journal of Psychosomatic Research. 1987;31:731–741. doi: 10.1016/0022-3999(87)90022-5. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, Kenyon K, Jacob T. Negative family environment as a predictor of boy's future status on measures of hostile attitudes, interview behavior, and anger expression. Health Psychology. 1996;15:30–37. doi: 10.1037/0278-6133.15.1.30. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 6. Los Angeles, CA: Authors; 1998–2010. [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002 Dec 19;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Osinsky R, Reuter M, Küpper Y, Schmitz A, Kozyra E, Alexander N, Hennig J. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Lansford JE, Pettit GS, Dick DM. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents' trajectories of anxious/depressed symptoms. Developmental Psychology. 2012;48:1463–1475. doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor BD, Dalaker J. Poverty in the United States: 2002. Washington, DC: U.S. Bureau of the Census; 2003. (Current Population Reports, P60-222) Retrieved from http://www.dlc.org/documents/Census_2002_Poverty.pdf. [Google Scholar]
- Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–336. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-reactive protein: Eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clinical Chemistry. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience as a dynamic concept. Development and Psychopathology. 2012;24:335–344. doi: 10.1017/S0954579412000028. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009 Jun 3;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simons RL, Burt CH. Learning to be bad: Adverse social conditions, social schemas, and crime. Criminology. 2011;49:553–598. doi: 10.1111/j.1745-9125.2011.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social environment, genes, and aggression: Evidence supporting the differential susceptibility perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Research Reviews. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Jacobs G, Russell S, Crane RS. Assessment of anger: The State-Trait Anger Scale. In: Butcher JN, Spielberger CD, editors. Advances in personality assessment. Hillsdale, NJ: Erlbaum; 1983. pp. 159–187. [Google Scholar]
- Sutin AR, Costa PT, Jr, Wethington E, Eaton WW. Perceptions of stressful life events as turning points are associated with self-rated health and psychological distress. Anxiety, Stress, & Coping. 2010;23:479–492. doi: 10.1080/10615800903552015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Spencer N, Baldwin N. Social, economic, and political context of parenting. Archives of Disease in Childhood. 2000;82:113–120. doi: 10.1136/adc.82.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wald A. Sequential analysis. Oxford, UK: Wiley; 1947. [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ETH. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular disease. Clinical Cardiology. 2005;28:408–412. doi: 10.1002/clc.4960280905. [DOI] [PMC free article] [PubMed] [Google Scholar]


