Abstract
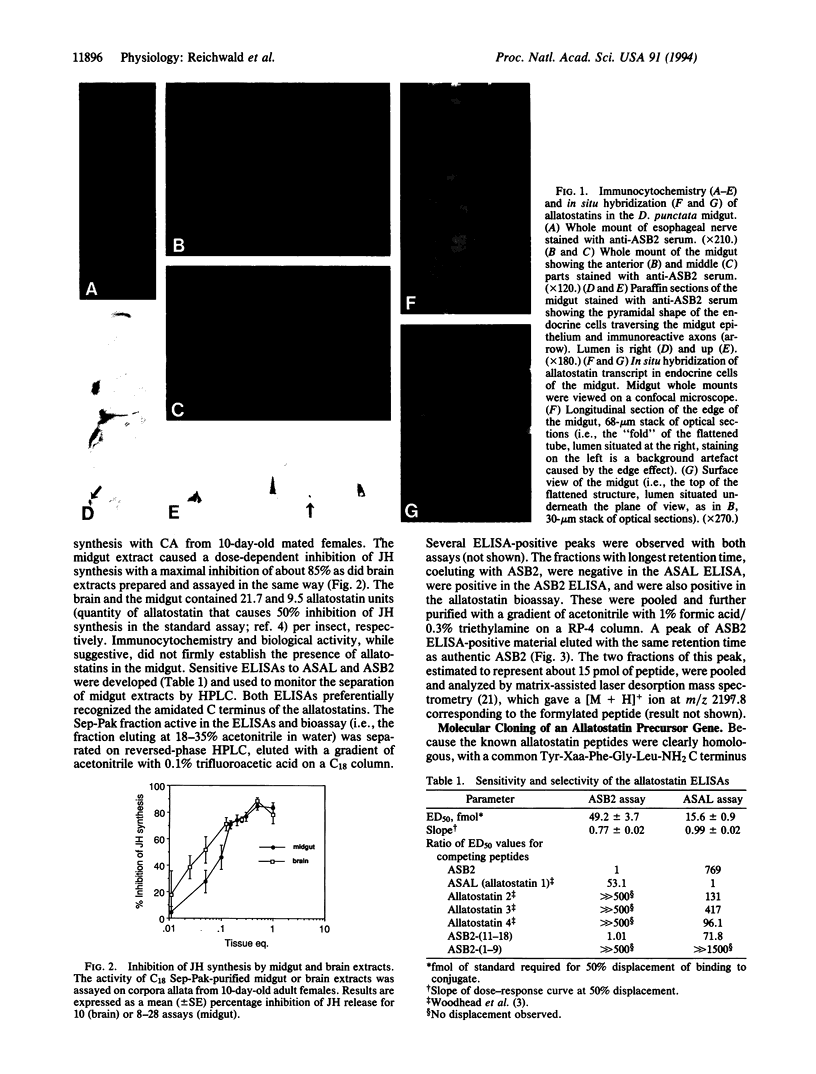
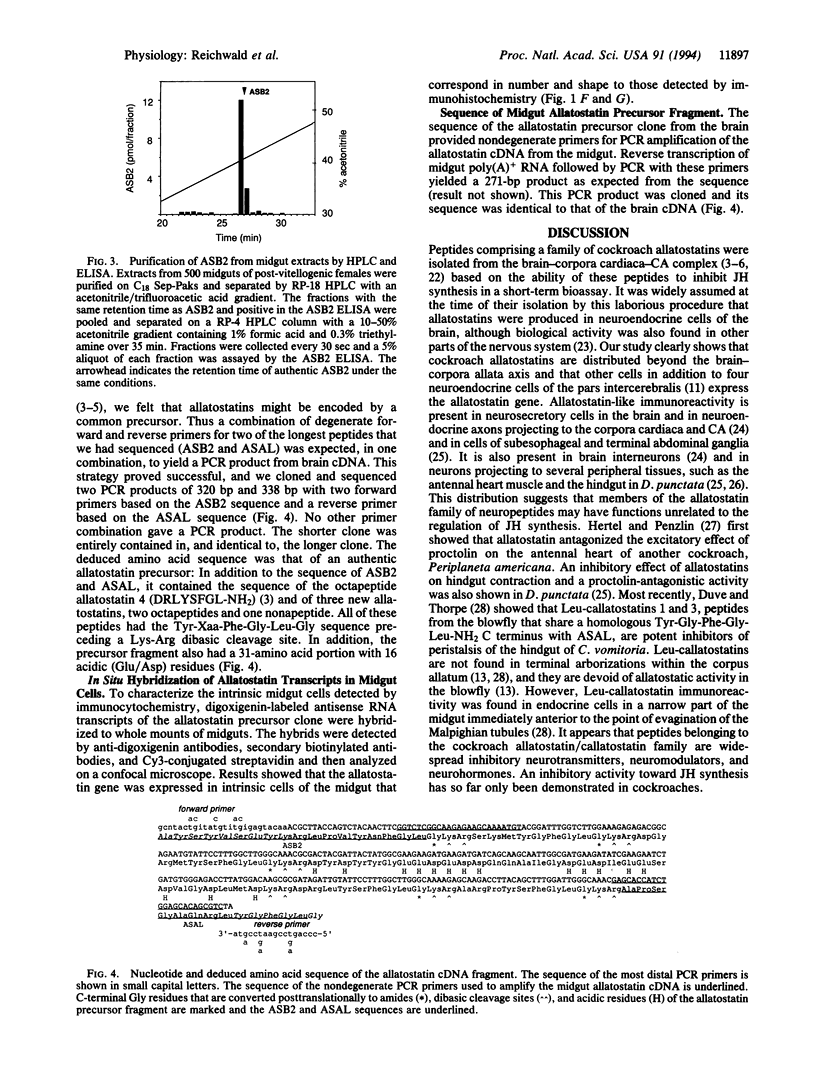
Cockroach allatostatins are neuropeptides that have been isolated from the brain of Diploptera punctata and shown to inhibit juvenile hormone production by the corpora allata. Enzyme-linked immunoassay and immunocytochemistry with antisera to two allatostatins, ASB2 (AYSYVSEYKRLPVYNFGL-NH2) and ASAL (APSGAQRLYGFGL-NH2), revealed that allatostatins were located not only in the insect brain but also in several peripheral tissues including the cockroach midgut and hindgut. Allatostatin-like immunoreactivity was found in nerve fibers of the stomatogastric nervous system as well as in intrinsic endocrine cells of the midgut. Midgut extracts were shown to be biologically active in an allatostatin bioassay and to contain several allatostatin-like peptides, including the octadecapeptide ASB2, which was identified by mass spectrometry following HPLC purification. Reverse transcription of brain mRNA followed by PCR with degenerate oligonucleotides for ASB2 and ASAL yielded a 338-bp fragment of the allatostatin gene that encoded six allatostatins. In situ hybridization with this probe confirmed that an allatostatin gene is expressed in intrinsic endocrine cells of the midgut. Reverse transcription of midgut mRNA followed by PCR and sequencing of the product revealed that the same gene is expressed in the midgut and in the brain. Allatostatins are thus an example of insect "brain-gut peptides" and we suggest that their function may not be restricted to the regulation of juvenile hormone production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donly B. C., Ding Q., Tobe S. S., Bendena W. G. Molecular cloning of the gene for the allatostatin family of neuropeptides from the cockroach Diploptera punctata. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8807–8811. doi: 10.1073/pnas.90.19.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve H., Johnsen A. H., Scott A. G., Yu C. G., Yagi K. J., Tobe S. S., Thorpe A. Callatostatins: neuropeptides from the blowfly Calliphora vomitoria with sequence homology to cockroach allatostatins. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2456–2460. doi: 10.1073/pnas.90.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve H., Thorpe A. Distribution and functional significance of Leu-callatostatins in the blowfly Calliphora vomitoria. Cell Tissue Res. 1994 May;276(2):367–379. doi: 10.1007/BF00306122. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nishiitsutsuji-Uwo J. Exocytotic release of secretory granules from endocrine cells in the midgut of insects. Cell Tissue Res. 1982;222(3):515–522. doi: 10.1007/BF00213851. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel W., Penzlin H. Function and modulation of the antennal heart of Periplaneta americana (L.). Acta Biol Hung. 1992;43(1-4):113–125. [PubMed] [Google Scholar]
- Kataoka H., Toschi A., Li J. P., Carney R. L., Schooley D. A., Kramer S. J. Identification of an allatotropin from adult manduca sexta. Science. 1989 Mar 17;243(4897):1481–1483. doi: 10.1126/science.243.4897.1481. [DOI] [PubMed] [Google Scholar]
- Kingan T. G. A competitive enzyme-linked immunosorbent assay: applications in the assay of peptides, steroids, and cyclic nucleotides. Anal Biochem. 1989 Dec;183(2):283–289. doi: 10.1016/0003-2697(89)90481-8. [DOI] [PubMed] [Google Scholar]
- Kramer S. J., Toschi A., Miller C. A., Kataoka H., Quistad G. B., Li J. P., Carney R. L., Schooley D. A. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9458–9462. doi: 10.1073/pnas.88.21.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A. B., Chan K. K., Stay B. Effect of allatostatin and proctolin on antennal pulsatile organ and hindgut muscle in the cockroach, Diploptera punctata. Arch Insect Biochem Physiol. 1993;24(2):79–92. doi: 10.1002/arch.940240203. [DOI] [PubMed] [Google Scholar]
- Paemen L., Tips A., Schoofs L., Proost P., Van Damme J., De Loof A. Lom-AG-myotropin: a novel myotropic peptide from the male accessory glands of Locusta migratoria. Peptides. 1991 Jan-Feb;12(1):7–10. doi: 10.1016/0196-9781(91)90158-l. [DOI] [PubMed] [Google Scholar]
- Pratt G. E., Farnsworth D. E., Feyereisen R. Changes in the sensitivity of adult cockroach corpora allata to a brain allatostatin. Mol Cell Endocrinol. 1990 Apr 17;70(2):185–195. doi: 10.1016/0303-7207(90)90158-5. [DOI] [PubMed] [Google Scholar]
- Pratt G. E., Farnsworth D. E., Fok K. F., Siegel N. R., McCormack A. L., Shabanowitz J., Hunt D. F., Feyereisen R. Identity of a second type of allatostatin from cockroach brains: an octadecapeptide amide with a tyrosine-rich address sequence. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2412–2416. doi: 10.1073/pnas.88.6.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt G. E., Farnsworth D. E., Siegel N. R., Fok K. F., Feyereisen R. Identification of an allatostatin from adult Diploptera punctata. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1243–1247. doi: 10.1016/0006-291x(89)91111-x. [DOI] [PubMed] [Google Scholar]
- Stay B., Chan K. K., Woodhead A. P. Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res. 1992 Oct;270(1):15–23. doi: 10.1007/BF00381875. [DOI] [PubMed] [Google Scholar]
- This week in science. Science. 1992 Aug 28;257(5074):1185–1185. doi: 10.1126/science.257.5074.1185. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. Immunocytochemical demonstration of vertebrate peptides in invertebrates: the homology concept. Neuropeptides. 1988 Aug-Sep;12(2):49–54. doi: 10.1016/0143-4179(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., Lehman H. K., Davis N. T. Allatotropin is a cardioacceleratory peptide in Manduca sexta. J Exp Biol. 1994 Mar;188:347–354. doi: 10.1242/jeb.188.1.347. [DOI] [PubMed] [Google Scholar]
- Woodhead A. P., Khan M. A., Stay B., Tobe S. S. Two new allatostatins from the brains of Diploptera punctata. Insect Biochem Mol Biol. 1994 Mar;24(3):257–263. doi: 10.1016/0965-1748(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Woodhead A. P., Stay B., Seidel S. L., Khan M. A., Tobe S. S. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5997–6001. doi: 10.1073/pnas.86.15.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead A. P., Stoltzman C. A., Stay B. Allatostatins in the nerves of the antennal pulsatile organ muscle of the cockroach Diploptera punctata. Arch Insect Biochem Physiol. 1992;20(4):253–263. doi: 10.1002/arch.940200403. [DOI] [PubMed] [Google Scholar]