Abstract
Toll-like receptors (TLRs) are membrane-bound microbial sensors that mediate important host-to-microbe responses. Cell biology aspects of TLR function have been intensively studied in professional immune cells, in particular the macrophages and dendritic cells, but not well explored in other specialized epithelial cell types. The adult intestinal epithelial cells are in close contact with trillions of enteric microbes and engage in lifelong immune surveillance. Mature intestinal epithelial cells, in contrast to immune cells, are highly polarized. Recent studies suggest that distinct mechanisms may govern TLR traffic and compartmentalization in these specialized epithelial cells to establish and maintain precise signaling of individual TLRs. We, using immune cells as references, discuss here the shared and/or unique molecular machineries used by intestinal epithelial cells to control TLR transport, localization, processing, activation, and signaling. A better understanding of these mechanisms will certainly generate important insights into both the mechanism and potential intervention of leading digestive disorders, in particular inflammatory bowel diseases.
Keywords: Toll-like receptor, Intestinal epithelium, Microbe, Traffic
Introduction
The mammalian intestinal lumen is immediately colonized by trillions of microorganisms after the initiation of postnatal enteral feeding. This community of commensal microbes, also known as microbiota or microflora, lives in a symbiotic relationship with the host by engaging in food digestion and vitamin production. In return, host cells provide microbiota the essential surviving niches and nutrients [1–4]. Commensal microbes in healthy individuals critically promote the normal development of host immune system and prevent adverse colonization by enteric pathogens, thereby contributing to local immune homeostasis [5–7]. The mutualistic relationship between host and microbes may be disrupted by various factors that trigger microbial recognition by the host immune system. Such microbial recognition in turn activates local or systemic inflammatory responses [8–10]. After intestinal infection or injury being resolved, pro-inflammatory signaling responses must be down-regulated and ultimately withdrawn to avoid unwanted damage to host tissues. Otherwise, intestinal pathogenesis such as inflammatory bowel diseases develops. Thus, the immune homeostasis of mammalian intestines critically relies on cellular mechanisms that exquisitely balance the immune-activating and -suppressing cues. In this review, we conduct a comprehensive literature survey and elaborate on various molecular mechanisms, proposed in recent studies for the compartmentalization and activation of specific microbial receptors. In intestinal epithelia, emerging studies suggest that these mechanisms strategically regulate microbial receptor activation and are essential for a balanced microbe–host interaction.
Intestinal epithelial cells
The intestinal mucosa is lined by highly polarized epithelial cells whose apical plasma membrane domains face the lumen. The basolateral surfaces of these epithelial cells are associated with lamina propria where professional immune cells reside. A small population of intestinal epithelial stem cells is located at the bottom of crypts, from which functionally distinct epithelial cell types differentiate and mature [11, 12]. In contrast to enterocytes, goblet cells, enteroendocrine cells, and tufts cells that locate in the villus epithelia, mature Paneth cells reside at the crypt bottom. In addition, microfold cells with high phagocytotic and transcytotic activities account for 10 % of follicle-associated epithelia that overlie gut-associated lymphoid tissues [13]. Formation of a critical barrier by intestinal epithelial cells via tight junctions physically separates commensal microbiota from host tissues, thereby constituting one of the most important mechanisms to support mucosal innate immunity [14–16]. In addition to the barrier function, a growing body of evidence has suggested the critical contribution of pattern recognition receptors (PRRs) to the homeostatic interplay between intestinal epithelia and gut microbiota [14–16].
Toll-like receptors
Toll-like receptors (TLRs) are a group of type I transmembrane proteins that belong to the PRR family. To data, 13 TLRs have been identified in mammalian cells, including 10 human and 12 mouse functional receptors [17]. TLRs recognize conserved microbe or pathogen-associated molecular patterns (PAMPs) [18]. Different TLRs bind distinct ligands derived from either microbes or endogenous molecules. TLR2 forms heterodimer with TLR1 or TLR6, respectively, to sense bacterial lipoproteins [19, 20]. TLR4 specifically binds and recognizes lipopolysaccharide (LPS), TLR5 for flagellin, TLR3 for double-stranded RNA, TLR7 and TLR8 for single-stranded RNA, TLR9 for double-stranded DNA, TLR11 and TLR12 for profillin, and TLR13 for bacterial 23S rRNA [17, 21, 22]. In addition to microbial agonists, a large number of endogenous molecules derived from host tissues can also bind TLRs and trigger sterile inflammatory responses in pathological conditions [23, 24]. For example, heat shock proteins, extracellular matrix components, degraded intermediates, high-mobility group box 1(HMGB1), surfactant protein A, and uric acid crystal can be recognized and bound by TLR2 and TLR4 [24]. Human cardiac myosin can be recognized by TLR2 and TLR8 [25], and self-RNA and DNA, when loaded onto TLR3 or TLR7–9, stimulate autoimmune responses [23].
All TLRs contain multiple leucine-rich repeats in their ectodomains and use intracellular Toll/IL1 receptor (TIR) domains for signal transduction [18]. TLR signaling has been well characterized in professional immune cells, especially in macrophage and several immortalized cell lines. In general, TLRs recognition of PAMPs can occur in multiple cellular compartments including cell surface, endosomes, and lysosomes [26]. The proper cellular compartmentalization of TLRs appears to be critical for ligand binding, maintenance of immune tolerance, and initiation of downstream signaling [26]. In a generic TLR signaling cascade, TLR binding to its specific ligand initiates receptor dimerization and recruitment of unique adaptor proteins such as MyD88 adaptor-like (Mal, also called TIRAP)/MyD88 or TRIF-related adaptor molecule (TRAM)/TRIF, which in turn activates NF-κB or interferon regulatory factor (IRF) signaling pathways, respectively [17, 27]. Intracellular TLR signaling and molecular regulation of the signal transduction pathways have been adequately discussed elsewhere [26–30]. In contrast, a collective analysis of TLR transport and activation in polarized cells such as the intestinal epithelial cells has been incomplete.
The consensus view has been that TLR signaling in the intestinal epithelia critically influences epithelial cell proliferation and differentiation, maintenance of tight junctions, synthesis and release of antimicrobial peptides, and induction of pro- or anti-inflammatory responses, respectively [15, 16]. As the intestinal epithelial cells are at the frontline of a microbe-rich environment, TLR signaling in intestinal epithelial cells appears to be critically regulated to not only maintain immune tolerance to host-friendly microbes, but effectively respond to invasive enteric pathogens. To serve such dual purposes, polarized distribution and activation of specific TLRs have been employed by intestinal epithelial cells as the most important mechanisms for immune balance. We have surveyed the recent progress in understanding the molecular basis of TLR compartmentalization and will focus the discussion on TLR transport, processing, and activation in intestinal epithelial cells.
Expression and polarized distribution of TLRs in intestinal epithelium
In response to the extremely diverse commensal microorganisms in host digestive tract, TLR expression and activation in intestinal epithelial cells are regulated in temporal and spatial manners to achieve immune defense and equilibrium [15]. Among the 13 TLRs discovered, TLR1–9 have been detected in human intestinal epithelial cells [31–33], while TLR1–11, with the exception of non-functional TLR10 due to a retrovirus insertion [28], are expressed in mouse intestinal epithelia [15]. Different from the high expression levels of TLRs in professional immune cells, TLRs are generally expressed at low level in intestinal epithelial cells [33, 34]. Furthermore, in contrast to non-polarized immune cells, in polarized intestinal epithelial cells TLRs demonstrate specific intracellular distributional features (Fig. 1).
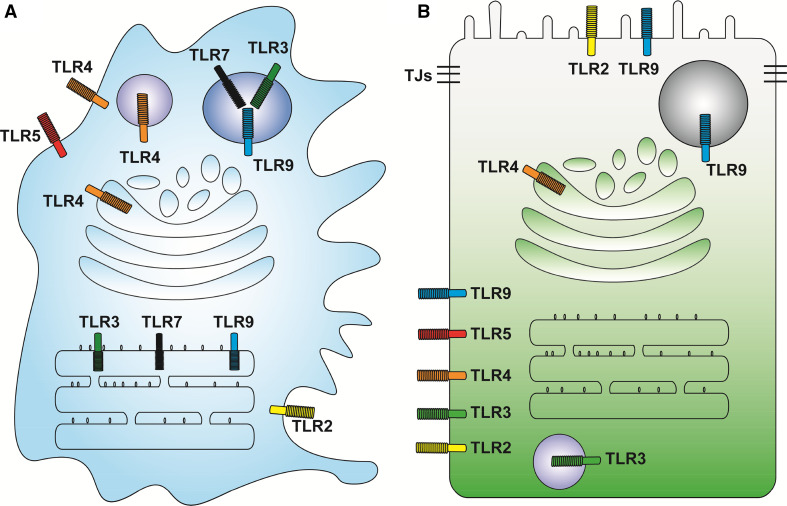
Fig. 1.
Compartmentalization of different TLRs in polarized intestinal epithelial cells in contrast to non-polarized professional immune cells. a Professional immune cells (such as macrophages and DCs) contain both cell surface (TLR1, 2, 4–6) and endosome (TLR3, TLR7–9) localized TLRs. TLR1 and 6 form heterodimer with TLR2 for bacterial lipoprotein recognition; to simplify the diagrams, both receptors are not shown. b Intestinal epithelial cells are typical polarized monolayers assembled through tight junctions (TJs). Apical surfaces are constantly confronted by commensal microbes and microbial products, which are ligands for specific TLRs. Classic surface TLR2, 4, and 5 are localized to the basolateral plasma membrane. Classic endosomal TLRs such as TLR3 and TLR9 are sometimes detected on the basolateral surfaces. Apically localized TLR2 and 9 have been reported. In Paneth cells; TLR9 has been found in secretory vesicles
Immunohistochemistry for TLR2 in human fetal ileum has demonstrated that this receptor is mainly localized to the basolateral membranes of crypt epithelial cells and only weakly detected at the brush border [35]. In human colon cancer T84 cells, TLR2 is distributed from the cytoplasm to apical surface, once the cells become polarized in culture [32]. In mouse intestine, TLR2 was shown as apically distributed in villus and crypt epithelial cells; however, the localization appeared at both apical and basolateral domains in follicle-associated epithelia [36], suggesting that the localization of TLR2 depends on cell types as well as the surrounding tissue environment. TLR3 has been shown to be distributed in the cytoplasm of human small intestinal and colonic epithelial cells; however, its localization changes to the basolateral surface in colonic epithelial cells in ulcerative colitis patients [37].
Immunofluorescent analysis of TLR4 in human intestine samples has demonstrated that this receptor is localized to the basolateral side of fetal ileal crypts and adult colon [35]. In addition, the expression levels of TLR4 in ulcerative colitis and Crohn’s disease tissues, when compared with those in normal non-inflammatory bowel disease mucosa, are elevated. However, in contrast to the basolateral localization of TLR4 in ulcerative colitis mucosa, active Crohn’s disease patient samples show apical enrichment of TLR4 in both ileum and colon [37]. In line with these studies, TLR4 is also found to be localized to the apical surface of polarized human colon cancer T84 cells [32]. In mouse intestines, TLR4 is apically localized in terminal ileal epithelium, but basolaterally in colonic epithelium where a lower expression level was detected [36, 38]. Interestingly, functional TLR4 has also been reported in Golgi compartments of the mouse small intestinal epithelial cell line m-IC [39], collectively suggesting that TLR4 is compartmentalized into a variety of cellular components.
In contrast to TLR4, TLR5 has been consistently and predominantly detected at the basolateral cell surface and cytoplasm in colonic cells (Fig. 1b) [37, 40–42]. This unique distribution pattern of TLR5 prevents luminal bacterial flagellin access and activates this receptor, thus avoiding unnecessary immune response in steady-state conditions. However, mucosal injuries that impair epithelial barrier function will expose TLR5 to bacterial flagellin, eliciting TLR5-mediated inflammatory response [42]. Apical TLR5 has been reported in mouse ileal and follicle-associated epithelial cells, but its function has not been well characterized [36, 43].
To avoid recognition of self-DNA, TLR9 is almost exclusively distributed in the endosomal compartments in professional immune cells including macrophages and dendritic cells (Fig. 1a) [44], with certain exceptions such as the splenic dendritic cells, demonstrating some cell surface TLR9 localizations [45]. In addition, in mouse RAW264.7 macrophages, TLR9 requires proteolytic processing at its ectodomain in endolysosomal compartments before its activation [46–48]. Reinforcing TLR9 onto cell surface and circumventing its proteolytic processing by mutating the transmembrane domain still leads to MyD88 recruitment and CpG-stimulated NFκB signaling activation [49]. Transferring hematopoietic stem cells carrying this transmembrane domain mutant TLR9 to lethally irradiated recipient mice causes expansion of CD11c+ cells, reduction of CD19+ B cells, and lethal auto-inflammation [49]. These studies suggested that internalization of TLR9 mediated by its transmembrane domain is one of the important strategies for peripheral immune tolerance. In polarized intestinal epithelial cells, TLR9 is detected at both the apical and basolateral plasma membrane domains in cultured human colonic cells with immune-suppressive and immune-stimulative functions, respectively (Fig. 1b) [50]. Intriguingly, activation of the basolateral TLR9 induces IκBα degradation and NFκB activation, whereas activation of apical TLR9 causes IκBα ubiquitination and accumulation but not degradation, thereby preventing NFκB signaling [50]. Consistent with these reports, localization of TLR9 to plasma membranes has also been observed in mouse small intestinal epithelia [51]. In mouse intestinal Paneth cells, TLR9 is found in granules (Fig. 1b), and its activation by CpG triggers degranulation of this cell type [52, 53].
TLR transport in polarized intestinal epithelial cells
Being highly polarized after maturation, adult intestinal epithelial cells have multifaceted functions including absorption of nutrients, electrolytes, and vitamins, as well as secretion of digestive enzymes, mucin, neuropeptides, and antimicrobial peptides. Intestinal epithelial cells also communicate with the submucosal immune system through transcytosis of secreted IgA and sampling luminal antigens for presentation to professional immune cells [54]. Efficient coordination of these biological processes requires highly selective and directional cargo transports that are guided by specific small GTPases, motor proteins, adaptors, and SNAREs [55–58]. The homeostatic distributions of membrane-bound receptors, such as TLRs, are established and maintained by dynamic trafficking processes consisting biosynthetic, endo-, and exocytotic, and protein turnover pathways. In the biosynthetic pathway, newly synthesized TLRs, after proper folding assisted by endoplasmic reticulum (ER) chaperone gp96 and protein associated with TLR4 A (PRAT4A) [59–61], are exported from ER lumen to Golgi apparatus via coat protein complex II (COPII) vesicles (Fig. 2). Additional post-translational modifications occur in Golgi, from where the cargos are transported to trans-Golgi network (TGN) for segregation into various vesicular compartments prior to delivery to final destinations [55, 62]. In parallel to the biosynthetic pathway, transmembrane proteins can be reutilized through endocytotic pathways [63, 64]. Membrane-bound subcellular vesicles typically travel along actin or microtubule filaments for short- or long-range cargo transport, respectively. The specific directionality of individual vesicles is controlled by their associated motor proteins that power the polarized movement along cytoskeletal tracks [65–68]. On actin cables, the myosin family of motor proteins comprises the primary motors driving vesicular deliveries, which are typically regulated by Rab small GTPases [67, 69]. On microtubule tracks, plus-end-directed kinesin superfamily and minus-end-directed motor proteins such as dynein are the major effectors whose activities are also controlled by Rab small GTPases [56, 68, 70].
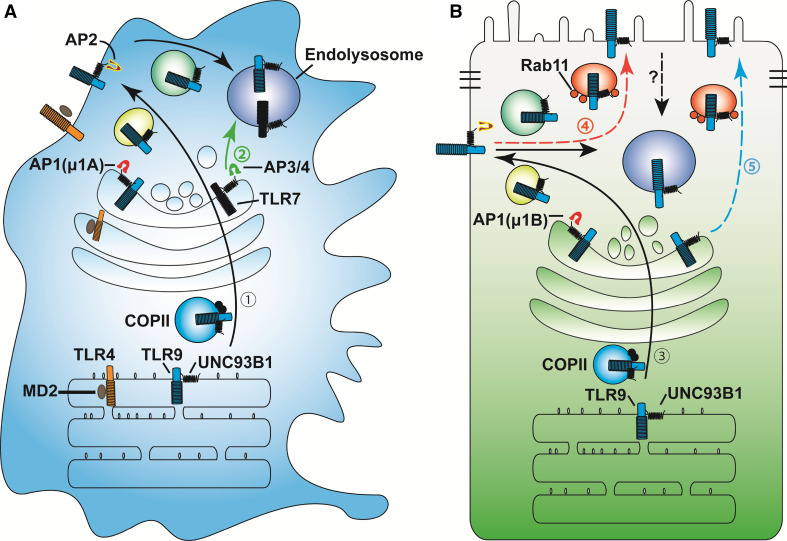
Fig. 2.
TLR trafficking in polarized IECs in reference to professional immune cells. a In professional immune cells, newly synthesized TLRs are trafficked with their accessary proteins along the secretory pathway (route 1). TLR4 requires MD2, while endosomal TLRs need UNC93B1 (route 1) for transport from ER to COPII vesicles to cis-Golgi, and to trans-Golgi network (TGN). Clathrin- associated adaptor protein AP1A, including β, γ, σ1 and μ1A subunits, recognizes sorting motifs in the cytosolic tail of TLRs or in sorting transporter UNC93B1, for segregating TLRs into exocytic vesicles. Exocytic transport of these vesicles is regulated by Rab small GTPases and their corresponding motor proteins that power vesicular movement along the microtubule and/or actin filaments to the plasma membrane. Endosomal TLRs are internalized via the endocytotic pathway to the endolysosomal compartments. TLR7 has also been shown to use direct route from TGN to endolysosomes (route 2) with the aid of AP3 and/or AP4. b In polarized intestinal epithelial cells, TLR9 is observed at both the apical and basolateral plasma membrane domains. Basolateral targeting of TLR9 probably follows a similar pathway (route 3) as in professional immune cells, except that the epithelial cell-specific AP1B with β, γ, σ1, and μ1B subunits are utilized. Apical targeting of TLR9 is suggested to occur through the transcytotic pathway (route 4) or via TGN (route 5)
In polarized epithelial cells, the transporting directionalities of intracellular cargos are established via multiple mechanisms. First, cells with apical–basolateral polarity form polarized actin and microtubule cytoskeleton [71], directing the unidirectional motor movements. Second, the lipid components of distinct plasma membrane domains also demonstrate disparities with the apical membrane enriched with phosphatidylinositol-4,5-bisphosphate (PtdIns (4,5)P2), and the basolateral membrane with PtdIns (3,4,5)P3 [72]. Such distinct lipid constituents serve as critical targeting sites for polarized vesicular transport and cargo delivery [73]. Third, distinct intracellular trafficking machineries have also been developed to differentiate basolateral and apical cargos. It has been proposed that specific sorting motifs for basolateral transport are contained frequently at the cytosolic tails of those basolaterally destined cargos and cargo receptors (please see below).
Two canonical sorting motifs for basolateral trafficking, one tyrosine-based NPXY (X is any amino acid) or YXXØ (Ø presents a bulky hydrophobic amino acid) and another dileucine-based [D/E]XXXL[L/I] or DXXLL motifs, have been reported [55, 62]. Notably, these motifs also mediate the endocytosis as well as delivery of related cargos to lysosomes [62]. A tyrosine-based motif (YNEL) in TLR9 cytosolic tail has been proposed as essential for TLR9 endolysosomal localization; deletion of this motif causes cell surface retention of TLR9 in HeLa cells [74]. Likewise, a tyrosine-based sorting motif (YDAF) of TLR7 is critical for its trafficking to endosome; mutating tyrosine to alanine (Y892A) impairs TLR7’s processing and ligand response [75].
Some basolateral cargos contain non-canonical sorting motifs. For example, the basolateral transport of transferrin receptor depends on GDNS [62], while the basolateral traffic of polymeric immunoglobulin receptor is determined by a 17-amino acid motif containing an HRRNV core sequence, the mutation of which abolishes basolateral targeting, resulting in non-polarized or apical delivery of the mutant protein [62]. Of note, these basolateral motifs described above appear to be recognized by adaptor protein complexes (APs). A total of six different AP complexes participate in cargo selection and formation of coated vesicles [76]. Among them, AP2 is uniquely critical for endocytotic cargo trafficking, while other AP complexes primarily regulate cargo traffic from TGN or recycling endosome to cell surface or endosomes. AP1 μ1B subunit that is specifically expressed by some polarized, including the intestinal, epithelial cells directly and preferentially recognizes basolateral sorting motifs, thereby mediating basolateral sorting (Fig. 2b). AP1 μ1B knockout mouse intestinal epithelial cells display disrupted cellular polarity evident by misdistributed basolateral cargos, including the low-density lipoprotein receptor (LDLR), ephrin receptor Eph2B, E-cadherin, and interleukin 6 signal transducer (IL6st) [77, 78]. Nevertheless, trafficking of some apical cargos such as the ion exchanger NHX-2 is also affected in intestinal epithelia of AP1 μ1B knockout mice and AP1-deficient C. elegans [77, 79]. In line with these studies, reduced expression of AP1 μ1B has been reported in patients with Crohn’s disease [78], indicating plausible occurrence of disrupted polarized cargo trafficking during the course of inflammation.
Since most TLRs are basolaterally distributed in intestinal epithelial cells (Fig. 1b), it is highly possible that AP complexes participate in TLR trafficking; however, the direct in vivo evidence has been absent at this moment. Studies using other cell types do support the critical involvement of AP complexes in TLR trafficking [80–83]. In keratinocytes, AP1σ1C subunit regulates TLR3 trafficking; pustular psoriasis mutations of AP1σ1C reduces TLR3 trafficking and the induction of anti-inflammatory interferon β (IFNβ) [80]. In human kidney HEK293T cells, TLR9 trafficking from plasma membrane to endolysosomes requires AP-2 complex (Fig. 2); knockdown of AP2μ1 accumulates TLR9 on the cell surface [75]. Two recent studies, using plasmacytoid dendritic cells and bone marrow-derived macrophages, have demonstrated that AP-3 regulates the delivery of TLR7 and TLR9 to lysosomal compartments (Fig. 2a) for type I IFN induction [82, 83]. Consistent with these results, AP-3 genetic ablation in plasmacytoid dendritic cells impairs TLR9 trafficking to lysosomal compartments, thereby decreasing type I IFN production [82]. AP-3 has also been implicated in phagosome recruitment of TLR4 and promoting MHC class II antigen presentation in bone marrow-derived dendritic cells [81]. Moreover, a recent study has shown that TLR7 trafficking from TGN to endosome needs AP-4 in 293T cells and bone marrow-derived macrophage [75].
In contrast to basolateral cargos, apical cargos contain even more diverse sorting motifs in transmembrane domains or luminal regions [84]. Typically, apical sorting depends on glycosylation modification at the ectodomain, glycosyl phosphatidylinositol (GPI) anchorage, lipid raft-associated transmembrane domain, or certain specialized determinant motifs in the cytosolic domain [55, 84]. Both N-linked and O-linked glycosylations are considered to be apical sorting signal [55, 84]. However, this type of apical sorting signal by glycosylation is recessive to cytosolic basolateral sorting motifs [55]. In the case of TLRs, TLR2–4 have been identified as highly glycosylated proteins [85, 86], whereas other TLRs may contain potential glycosylation sites in their ectodomain [87], hinting at their potential apical trafficking activities. However, most TLRs locate at the basolateral side of polarized IECs at steady-state conditions, suggesting that basolateral sorting of these TLRs or their transporting receptors may play a dominant role. Of note, polarized TLR distribution also appears to be cell-type dependent. Immunofluorescent analysis for TLR5 detected its exclusive distribution at the basolateral side of polarized enterocytes [42]; however in microfold cells, TLR5 is found at the apical poles and supranuclear structures [36]. This cell type-dependent polarization of TLR5 may attribute to specific trafficking machinery that requires further investigations.
Rab small GTPase family proteins have been well characterized in apical trafficking in the recent years [73, 84]. In polarized epithelia, Rab11a is located in the apical recycling endosome to modulate apical trafficking [88, 89]. Genetic ablation of Rab11a in mouse intestinal epithelia led to abnormal TLR9 trafficking and processing [51]. In wild-type intestinal epithelial cells, TLR9 is detected by immunofluorescent analysis at both basolateral and apical domains as small vesicles, whereas TLR9 is accumulated into larger puncta of vacuolar-like intracellular compartments in Rab11a-deficient cells. In Rab11a-deficient intestines, abnormal activation of NFκB signaling and overproduction of inflammatory cytokines (IL6, IL1β, etc.) have been observed. Histopathologically, Rab11a mutant mice developed blunting villi, hyperproliferative crypts, and infiltration of immune cells. These phenotypes resemble inflammatory bowel diseases and collectively suggest that Rab11a vesicles contribute to a homeostatic TLR9 intracellular compartmentalization to sustain intestinal epithelial and immune homeostasis [51]. In human monocytes, Rab11a is also found to regulate TLR4 transport to E. coli phagosomes [90], hinting at a broader involvement of Rab11a in TLR trafficking and innate immunity. In addition, Rab10 has also been shown to control TLR4 transport from TGN to plasma membrane to regulate the macrophage response to LPS stimulation [91]. In polarized Madin-Darby canine kidney cells and C. elegans intestines, Rab10 is proposed as an important regulator of basolateral transport [92, 93]. Whether or not Rab10 also regulates TLR4 transport in intestinal epithelial cells is currently not clear.
In addition to TGN to apical membrane trafficking, transcytosis is another important route for apical delivery of some macromolecules in the intestinal epithelial cells [94]. During transcytosis, cargos are transported from the basolateral to apical cellular domains (Fig. 2b), or vice versa, via intracellular compartments without affecting tight junction integrity. Studies of transcytosis have shown that basolateral proteins travel through basal sorting endosome, common recycling endosome, and apical recycling endosome before reaching apical plasma membrane [84]. On this long journey, apical recycling endosome resident proteins, Rab11a and its effectors Rab11a-FIP5 and myosin V, appear to play critical roles [88, 95]. However, it remains unclear whether apical TLR traffic intersects the route of transcytosis. In the intestinal epithelial cell-specific Rab11a knockout mice, accumulation of TLR9 at the subapical cytoplasmic compartments affirms the role of Rab11a vesicles in TLR9 apical transport [51]; however, whether the observed impairment of TLR9 traffic represented a defective biosynthetic pathway, transcytotic pathway, or both has not been determined. Careful dissection of the molecular mechanism of TLR9 trafficking by Rab11a will facilitate a better understanding of this microbial receptor and its contribution to intestinal immune homeostasis.
Ubiquitylation of cargos, such as TLRs, can also serve as an endosome targeting signal to influence the trafficking destination [96]. Genome-wide RNAi screening studies have identified hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) as being able to recognize ubiquitinated TLR9, thereby regulating TLR9 endolysosome localization [97]. Knockdown of Hrs in Raw 264.7 mouse macrophages inhibits the proteolytic cleavage of TLR9. Compared to control cells, Hrs-deficient cells show significant reduction of NFκB promoter activation in response to TLR7- or TLR9-specific ligand challenge. Furthermore, mutating the lysine residues of mouse TLR9 cytosolic tail disrupts an interaction between TLR9 and Hrs, causing poor response to CpG stimulation, highlighting the positive role of Hrs in TLR7 and TLR9 trafficking and signaling. Negative regulation of TLRs signaling by Hrs has also been reported in HEK293 cells and monocytes, where Hrs interacts with the ubiquitinated TLR4 and delivers it to lysosome for degradation upon LPS stimulation [98]. It will be interesting to test Hrs-mediated TLR transport in intestinal epithelial cells and determine the specific mechanisms attributed to the distinct, potentially TLR-/ligand-dependent transport by Hrs.
In inflammatory bowel diseases, e.g., Crohn’s disease and ulcerative colitis, persistent inflammation may compromise epithelial polarity and barrier integrity. For example, proinflammatory tumor necrosis factor α (TNFα) signaling suppresses atypical protein kinase C (PKCζ) level via NFκB-dependent abrogation of Hsp70/Hsc70 chaperoning activity and interrupts PKCζ–Par3–Par6 polarity complex activity that is critical for the maintenance of epithelial polarity [99, 100]. PKCζ activity can be rescued by NFκB inhibition [99]. However, NFκB activity is also critical for maintenance of intestinal epithelial barrier [101]. When epithelial polarity is compromised, polarized trafficking of TLRs can be disrupted, consequently altering their access to microbial ligands and activation of pro-inflammatory signaling. In addition, persistent inflammation can also reshape local membrane phospholipid composition, especially phosphorylated phosphatidylinositol through activating or deactivating related kinases, phosphatase, and/or phospholipase activity, and subsequently affect the recruitment of TLR adaptors and signaling pathways [102–104]. Further investigation of polarized trafficking and regulation in the milieu of inflammation will optimize therapeutic target selection and facilitate the re-establishment of normal TLR localization and signaling.
Role of UNC93B1 in TLR3, 7, 8, and 9 trafficking
Unc93 homolog B1 (UNC93B1) is a multi-transmembrane chaperone protein regulating TLR trafficking from ER to targeting destinations [105]. Previous studies have established the critical contribution of UNC93B1 to the delivery of endosomal TLRs, including TLR3, 7, 8, and 9 to endosomes via the ER–Golgi–endosome pathway [106]. Of note, cell surface-localized TLRs, such as TLR2 and TLR4, are generally UNC93B1 independent with only a few exceptions (please see below). Endosomal TLRs interact with UNC93B1 using distinct domains [107–109], deletion or mutation of which cause trafficking defects exhibited as ER accumulation or cell surface retention [49, 109, 110]. Conversely, point mutation (H412R) of UNC93B1 also completely abolishes ER exit of endolysosomal TLRs [105]. Serial truncation of UNC93B1 has identified its N terminus as critical for TLR9’s ER exit [75]. In macrophages, expressing N-terminal UNC93B1 truncates or D34A mutants prevent TLR9 exit from the ER compartment, leading to significant reduction of EndoH-resistant TLR9 precursor and proteolytically cleaved TLR9 forms. C-terminal truncation of UNC93B1 causes an accumulation of EndoH-resistant TLR9 precursor and a decrease of cleaved TLR9. Further analyses suggest that TLR9 trafficking to endocytic compartment requires adaptor protein 2 (AP2), indicating that TLR9 delivery is through endocytic pathway in macrophage cell line (Fig. 2a). Immunoprecipitation studies confirmed that UNC93B1 recruits AP2 to facilitate TLR9 internalization [75]. In parallel, AP3 and AP4 are involved in UNC93B1-mediated escort of TLR7 to endolysosomes, suggesting a direct transportation of TLR7 from Golgi to endolysosomes [75, 82].
UNC93B1 appears also involved in transport of cell surface-localized TLR3 and TLR5 [111, 112]. Overexpression of UNC93B1 in human 293T cells promotes surface-associated TLR3 that shows different glycosylated pattern and is sensitive to peptide N-glycosidase F treatment [111, 113]. Knockdown of UNC93B1 in macrophage decreases TLR5 cell surface distribution without affecting the total level of TLR5 or TLR2 plasma membrane localization, suggesting that UNC93B1 is an essential regulator for surface TLR5 transport [112]. Although UNC93B1 contains a C-terminus localized YXXØ motif that mediates AP1- and AP2-dependent UNC93B1 traffic, it does not contain any domain that may power the trafficking of TLR/UNC93B1 on actin or microtubule filaments. Disruption of this YXXØ motif leads to different effects of endosomal TLR signaling that is dependent on the cell types and the ligands [114]. Identification of additional trafficking components, such as the motors and the specific small GTPases that engage in UNC93B1-mediated TLR transport, will elucidate additional mechanisms of endosomal TLR compartmentalization and signaling.
Roles of MD2, CD14, and TMED7 in TLR4 trafficking
In resting cells, TLRs are distributed in a cell type-dependent manner. For example, TLR4 is mainly localized in Golgi and plasma membrane in human monocytes [115]. A secreted glycosylated protein, myeloid differentiation factor 2 (MD2), has been shown to associate with TLR4 (Fig. 2a) and regulates the dynamic trafficking of TLR4 between Golgi and plasma membrane [116]. In the absence of MD2, TLR4 is trapped in the Golgi apparatus and fails to reach the cell surface.
CD14, a glycosylphosphatidylinositol-anchored membrane protein, can interact with multiple TLR ligands and facilitate TLR recognition [117]. In addition to ligand delivery, CD14 also controls TLR4 endocytosis upon LPS stimulation and is required for endosomal TLR4/TRIF-mediated induction of type I interferon [118, 119]. Interestingly, the expression levels of MD2 and CD14 are relatively low in IECs at steady-state conditions, but are increased under inflammatory conditions [120, 121].
The transmembrane Emp24 domain-containing protein 7 (TMED7) functions as another adaptor protein that contributes to packaging of TLR4 into COPII vesicles [122]. Knocking down TMED7 in HEK293T cell or human THP-1 monocytes reduces surface TLR4 level and inhibits the activation of TLR4/MyD88/NFκB without affecting the TLR4/TRIF/IRF pathway.
Closing remarks
TLRs are membrane-bound microbial sensors that mediate important host-to-microbe responses. The TLR biology has been intensively studied in macrophage, dendritic cell, and human embryonic kidney cells, but not fully explored in mammalian intestinal epithelial cells. In close contact with enteric microbes and engaging in constant immune surveillance, these special epithelial cells may use similar molecular machineries, as found in immune cells, to control TLR transport, localization, processing, activation, and signaling (Fig. 2b). However, in contrast to immune cells, these cells, being highly polarized, may have developed distinct trafficking and regulatory mechanisms to establish and maintain the precise location and signaling of individual TLR. In pathological conditions, these trafficking routes can be disrupted. Continued exploration of these mechanisms will require combined inputs from both cell biology and genetic studies and is expected to generate important insights into the mechanism and therapeutic intervention of inflammatory bowel diseases.
Acknowledgments
This work was supported by the National Institute of Health (NIH) Grants DK085194, DK093809, DK102934, and CA178599; Charles and Johanna Busch Memorial Award (659160); NSF/BIO/IDBR (1353890) and Rutgers University Faculty Research Grant (281708). S.Y. was supported by New Jersey Commission on Cancer Research Postdoctoral Fellowship (DFHS13PPC016).
References
- 1.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashyap PC, et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA. 2013;110(42):17059–17064. doi: 10.1073/pnas.1306070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkosar B, et al. Host-intestinal microbiota mutualism: “learning on the fly”. Cell Host Microbe. 2013;13(1):8–14. doi: 10.1016/j.chom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature. 2014;514(7524):638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14(7):676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier L, et al. Microbiota-derived hydrogen fuels Salmonella typhimurium invasion of the gut ecosystem. Cell Host Microbe. 2013;14(6):641–651. doi: 10.1016/j.chom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Clemente JC, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekirov I, et al. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 11.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7(5):349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 12.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 13.Mabbott NA, et al. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6(4):666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JM, et al. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 16.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19.Kang JY, et al. Recognition of lipopeptide patterns by toll-like receptor 2-toll-like receptor 6 heterodimer. Immunity. 2009;31(6):873–884. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Jin MS, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130(6):1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Koblansky AA, et al. Recognition of profilin by toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38(1):119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldenburg M, et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337(6098):1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 23.Rifkin IR, et al. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 24.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, et al. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183(1):27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton GM, Kagan JC. A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9(8):535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay NJ, et al. Assembly and localization of toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14(8):546–558. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 30.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126(4):1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Cario E, et al. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol. 2002;160(1):165–173. doi: 10.1016/S0002-9440(10)64360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melmed G, et al. Human intestinal epithelial cells are broadly unresponsive to toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170(3):1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 34.Lavelle EC, et al. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3(1):17–28. doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusunyan RD, et al. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49(4):589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Chabot S, et al. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176(7):4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 37.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68(12):7010–7017. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega-Cava CF, et al. Strategic compartmentalization of toll-like receptor 4 in the mouse gut. J Immunol. 2003;170(8):3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 39.Hornef MW, et al. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195(5):559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortega-Cava CF, et al. Epithelial toll-like receptor 5 is constitutively localized in the mouse cecum and exhibits distinctive down-regulation during experimental colitis. Clin Vaccine Immunol. 2006;13(1):132–138. doi: 10.1128/CVI.13.1.132-138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gewirtz AT, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167(4):1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 42.Rhee SH, et al. Pathophysiological role of toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102(38):13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bambou JC, et al. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem. 2004;279(41):42984–42992. doi: 10.1074/jbc.M405410200. [DOI] [PubMed] [Google Scholar]
- 44.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7(1):49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 45.Onji M, et al. An essential role for the N-terminal fragment of toll-like receptor 9 in DNA sensing. Nat Commun. 2013;4:1949. doi: 10.1038/ncomms2949. [DOI] [PubMed] [Google Scholar]
- 46.Ewald SE, et al. The ectodomain of toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of toll-like receptor 9. Nat Immunol. 2008;9(12):1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewald SE, et al. Nucleic acid recognition by toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208(4):643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouchess ML, et al. Transmembrane mutations in toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity. 2011;35(5):721–732. doi: 10.1016/j.immuni.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8(12):1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 51.Yu S, et al. TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. EMBO J. 2014;33(17):1882–1895. doi: 10.15252/embj.201487888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rumio C, et al. Degranulation of Paneth cells via toll-like receptor 9. Am J Pathol. 2004;165(2):373–381. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rumio C, et al. Induction of Paneth cell degranulation by orally administered toll-like receptor ligands. J Cell Physiol. 2012;227(3):1107–1113. doi: 10.1002/jcp.22830. [DOI] [PubMed] [Google Scholar]
- 54.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34(4):155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 55.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9(4):273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 56.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 57.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi K, et al. A protein associated with toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204(12):2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B, et al. Folding of toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26(2):215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonifacino JS. Adaptor proteins involved in polarized sorting. J Cell Biol. 2014;204(1):7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu VW, Bai M, Li J. Getting active: protein sorting in endocytic recycling. Nat Rev Mol Cell Biol. 2012;13(5):323–328. doi: 10.1038/nrm3332. [DOI] [PubMed] [Google Scholar]
- 65.Hirokawa N, et al. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 66.Roberts AJ, et al. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14(11):713–726. doi: 10.1038/nrm3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammer JA, 3rd, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2012;13(1):13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- 68.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol. 2009;10(11):765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 69.Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5(6):393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 70.Hancock WO. Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol. 2014;15(9):615–628. doi: 10.1038/nrm3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128(2):383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15(4):225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leifer CA, et al. Cytoplasmic targeting motifs control localization of toll-like receptor 9. J Biol Chem. 2006;281(46):35585–35592. doi: 10.1074/jbc.M607511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee BL, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohno H. Clathrin-associated adaptor protein complexes. J Cell Sci. 2006;119(Pt 18):3719–3721. doi: 10.1242/jcs.03085. [DOI] [PubMed] [Google Scholar]
- 77.Hase K, et al. AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology. 2013;145(3):625–635. doi: 10.1053/j.gastro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi D, et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 2011;141(2):621–632. doi: 10.1053/j.gastro.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 79.Shafaq-Zadah M, et al. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development. 2012;139(11):2061–2070. doi: 10.1242/dev.076711. [DOI] [PubMed] [Google Scholar]
- 80.Setta-Kaffetzi N, et al. AP1S3 Mutations are associated with pustular psoriasis and impaired toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94(5):790–797. doi: 10.1016/j.ajhg.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantegazza AR, et al. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4+ T cells. Immunity. 2012;36(5):782–794. doi: 10.1016/j.immuni.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasai M, Linehan MM, Iwasaki A. Bifurcation of toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329(5998):1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blasius AL, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107(46):19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122(Pt 23):4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weber AN, Morse MA, Gay NJ. Four N linked glycosylation sites in human toll-like receptor 2 cooperate to direct efficient biosynthesis and secretion. J Biol Chem. 2004;279(33):34589–34594. doi: 10.1074/jbc.M403830200. [DOI] [PubMed] [Google Scholar]
- 86.Sun J, et al. Structural and functional analyses of the human toll-like receptor 3. Role of glycosylation. J Biol Chem. 2006;281(16):11144–11151. doi: 10.1074/jbc.M510442200. [DOI] [PubMed] [Google Scholar]
- 87.Istomin AY, Godzik A. Understanding diversity of human innate immunity receptors: analysis of surface features of leucine-rich repeat domains in NLRs and TLRs. BMC Immunol. 2009;10:48. doi: 10.1186/1471-2172-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu S, et al. A Rab11a-enriched subapical membrane compartment regulates a cytoskeleton-dependent transcytotic pathway in secretory epithelial cells of the lacrimal gland. J Cell Sci. 2011;124(Pt 20):3503–3514. doi: 10.1242/jcs.088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roland JT, et al. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci USA. 2011;108(7):2789–2794. doi: 10.1073/pnas.1010754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Husebye H, et al. The Rab11a GTPase controls toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33(4):583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D, et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107(31):13806–13811. doi: 10.1073/pnas.1009428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuck S, et al. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8(1):47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 93.Chen S, et al. SEC-10 and RAB-10 coordinate basolateral recycling of clathrin-independent cargo through endosomal tubules in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2014;111(43):15432–15437. doi: 10.1073/pnas.1408327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 95.Su T, et al. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2010;12(12):1143–1153. doi: 10.1038/ncb2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007;19(4):459–465. doi: 10.1016/j.ceb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiang CY, et al. Cofactors required for TLR7- and TLR9-dependent innate immune responses. Cell Host Microbe. 2012;11(3):306–318. doi: 10.1016/j.chom.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husebye H, et al. Endocytic pathways regulate toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25(4):683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mashukova A, Wald FA, Salas PJ. Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Mol Cell Biol. 2011;31(4):756–765. doi: 10.1128/MCB.00811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141(5):757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 101.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9(11):778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 102.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31(1):5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 103.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell. 2006;125(5):943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 104.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7(2):156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 106.Lee BL, Barton GM. Trafficking of endosomal toll-like receptors. Trends Cell Biol. 2014;24(6):360–369. doi: 10.1016/j.tcb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brinkmann MM, et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177(2):265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim J, et al. Acidic amino acid residues in the juxtamembrane region of the nucleotide-sensing TLRs are important for UNC93B1 binding and signaling. J Immunol. 2013;190(10):5287–5295. doi: 10.4049/jimmunol.1202767. [DOI] [PubMed] [Google Scholar]
- 109.Itoh H, et al. UNC93B1 physically associates with human TLR8 and regulates TLR8-mediated signaling. PLoS One. 2011;6(12):e28500. doi: 10.1371/journal.pone.0028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Funami K, et al. The cytoplasmic ‘linker region’ in toll-like receptor 3 controls receptor localization and signaling. Int Immunol. 2004;16(8):1143–1154. doi: 10.1093/intimm/dxh115. [DOI] [PubMed] [Google Scholar]
- 111.Pohar J, et al. The role of UNC93B1 protein in surface localization of TLR3 receptor and in cell priming to nucleic acid agonists. J Biol Chem. 2012;288(1):442–454. doi: 10.1074/jbc.M112.413922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huh JW, et al. UNC93B1 is essential for the plasma membrane localization and signaling of toll-like receptor 5. Proc Natl Acad Sci USA. 2014;111(19):7072–7077. doi: 10.1073/pnas.1322838111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qi R, Singh D, Kao CC. Proteolytic processing regulates toll-like receptor 3 stability and endosomal localization. J Biol Chem. 2012;287(39):32617–32629. doi: 10.1074/jbc.M112.387803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pelka K, et al. Cutting edge: the UNC93B1 tyrosine-based motif regulates trafficking and TLR responses via separate mechanisms. J Immunol. 2014;193(7):3257–3261. doi: 10.4049/jimmunol.1301886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Latz E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277(49):47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 116.Ishihara S, et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol. 2004;173(2):1406–1416. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- 117.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zanoni I, et al. CD14 controls the LPS-induced endocytosis of toll-like receptor 4. Cell. 2011;147(4):868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang Z, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6(6):565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 120.Abreu MT, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277(23):20431–20437. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- 121.Frolova L, et al. Expression of toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56(3):267–274. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liaunardy-Jopeace A, Bryant CE, Gay NJ. The COP II adaptor protein TMED7 is required to initiate and mediate the delivery of TLR4 to the plasma membrane. Sci Signal. 2014;7(336):ra70. doi: 10.1126/scisignal.2005275. [DOI] [PMC free article] [PubMed] [Google Scholar]