Abstract
This review presents state-of-the-art knowledge about the roles of the basal ganglia (BG) in action-selection, cognition, and motivation, and how this knowledge has been used to improve deep brain stimulation (DBS) treatment of neurological and psychiatric disorders. Such pathological conditions include Parkinson’s disease, Huntington’s disease, Tourette syndrome, depression, and obsessive-compulsive disorder. The first section presents evidence supporting current hypotheses of how the cortico-BG circuitry works to select motor and emotional actions, and how defects in this circuitry can cause symptoms of the BG diseases. Emphasis is given to the role of striatal dopamine on motor performance, motivated behaviors and learning of procedural memories. Next, the use of cutting-edge electrochemical techniques in animal and human studies of BG functioning under normal and disease conditions is discussed. Finally, functional neuroimaging studies are reviewed; these works have shown the relationship between cortico-BG structures activated during DBS and improvement of disease symptoms.
Keywords: deep brain stimulation, striatum, subthalamic nucleus, globus pallidus, substantia nigra, electrochemistry, voltammetry, functional magnetic resonance imaging, pig, human
1. Introduction
Deep brain stimulation (DBS) of the basal ganglia (BG) is a well-established FDA-approved therapy for a variety of movement disorders such as Parkinson’s disease (PD), essential tremor, and dystonia (Benabid, 1989, 1994, 2003, 2006; Lang and Lozano, 1998a). Additionally, it is an emerging therapy for several psychiatric and neurological conditions, including epilepsy, Tourette’s syndrome (TS), major depression, and obsessive compulsive disorder (OCD) (Mayberg, 2005; Fisher et al., 2010). Despite its clinical success on these and other neurologic and psychiatric disorders, there is a limited understanding of the therapeutic mechanism behind DBS. In this review we discuss the current theories of DBS behind modulating BG dysfunction. Here, we explore current theories regarding BG dysfunction and the mechanisms underlying the associated motor deficit and neuropsychiatric symptoms in order to explore BG dysfunction improvement in response to DBS of cortico-BG targets.
A complete implanted DBS system consists of a pulse generator in the abdominal or infraclavicular region, which delivers the stimulation pulses via an intracerebrally implanted electrode. The stimulus is delivered via a clinical multi-contact electrode in which each contact is typically 1.5 mm in length and 1.27 mm in diameter (Godinho, 2006). The generator is connected to the electrode and battery powered. Stimulation parameters (pulse width, amplitude, frequency etc.) can be changed transdermally in order to optimize the therapeutic effects (Lyons, 2011).
There are several DBS targets within the basal ganglia (BG) that have been optimized to treat the aforementioned disorders. The subthalamic nucleus (STN) (Lyons, 2011 et al., 2006; Rodriguez-Oroz et al., 2005), internal part of the globus pallidum (GPi), or pedunculopontine tegmental nucleus (PPT) (Stefani et al., 2007) are shown to be effective in treating PD. To treat Huntington’s disease and primary dystonia, GPi has been effectively targeted (Moreno et al., 2014; Vidailhet et al., 2013). The nucleus accumbens (NAc), cingulate cortex, and anterior limb of the internal capsule are targets to treat major depression (Kuhn et al., 2014; Schlaepfer et al., 2008; Lozano et al., 2008; Baker et al., 2007; Bewernick et al., 2010; Pandya et al., 2012). The anterior limb of the internal capsule is targeted to treat OCD (Greenberg et al., 2006). Finally, the internal capsule/NAc, centromedian/parafascicularis (CMPf) and the GPi are used to treat TS (Hariz and Robertson, 2010; Neuner et al., 2009; Saleh et al., 2012; Welter et al., 2008; Williams and Okun, 2013). Here, we will discuss the rationale and potential mechanism behind the effective treatment of the disorders associated with these BG DBS targets.
In this review, we explain how BG dysfunction is thought to give rise to an array of motor-related and neuropsychiatric disease states, and present the current theories surrounding how DBS of cortico-BG targets may lead to symptom relief. The BG has been proposed as a system to make selections: action-selection, reasoning/cognition related selections, and motivational state selections (Da Cunha et al., 2009, 2012; Redgrave et al., 2011). Understanding how the cortico-BG circuitry is suited for such computations is critical to understand how electrical stimulation of parts of this system can improve and/or motor and psychicatric functions. Here, we present new emerging theories pertaining to the diverse roles of the BG in action-selection, cognition, and motivation that support the notion that BG function is highly complex, and may therefore be sub-optimally controlled by simple continuous large area electrical stimulation. We go on to discuss potential avenues for increasing the sophistication of future BG neuromodulation techniques. Finally, we review new research approaches that may be critical to the development of such advances, including electrochemical monitoring of neurochemicals, functional neuroimaging, and new large animal models of neuropsychiatric disorders. In summary, we emphasize that future research aimed at elucidating normal and pathological BG function, in combination with improved understanding of DBS mechanisms on a cellular and systems level, will open the door to more sophisticated and individualized DBS technologies.
2. BG cortico-thalamic loop
a. Circuits and connections
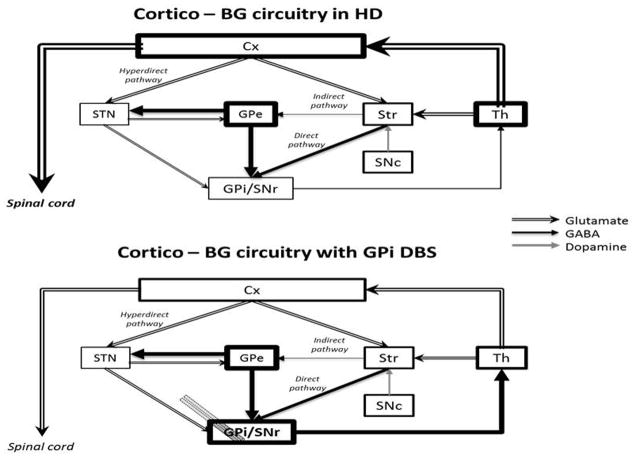
A growing body of evidence has shown that the BG forms a neural network dedicated to selection (Nicola, 2007; Redgrave et al., 2008, 2010; Da Cunha et al., 2009, 2012; Grillner et al., 2013). The strongest evidence is related to action-selection by the cortico-BG motor loop (Frank and Claus, 2006; Frank, 2011; Isoda and Hikosaka, 2011; Mink, 1996; Mogenson et al., 1980). In this loop, information from nearly all cortical and limbic subcortical areas flow into the BG input stations, including the dorsal striatum (Alexander et al., 1986) and STN (Nambu et al., 2002). The dorsal striatum also receives input from the thalamus (Parent and Hazrati, 1995a). More than 90% of the striatal neurons are GABAergic projection neurons named medium spiny neurons (MSNs) (Sesack and Grace, 2010). They compose two populations that make direct and indirect projections to the main output stations of the BG: the substantia nigra pars reticulata (SNr) and the GPi (Alexander et al., 1986). The external part of the globus pallidum (GPe) sends inhibitory projections to the SNr/GPi. Next, STN sends excitatory projections to the SNr/GPi (Alexander et al., 1986). The BG output stations (SNr/GPi) project mostly to motor areas of the thalamus (e.g., the ventrolateral thalamus), which in turn, project to motor areas of the neocortex (Alexander et al., 1986, Albin et al., 1989, Parent and Hazrati, 1995a,b). The inhibitory control of the BG over the thalamocortical neurons can be increased by the hyperdirect pathway formed by cortical projections that bypass the striatum by sending “hyperdirect” excitatory projections to the STN that stimulate the SNr/GPi (Nambu et al., 2002) (Figure 1). All BG nuclei are modulated by dopaminergic and GABAergic projections from the substantia nigra pars compacta (SNc), and by cholinergic, glutamatergic and, to a lesser extent, by GABAergic projections from the PPT (Parent and Hazrati, 1995b; Inglis and Winn, 1995).
Figure 1.
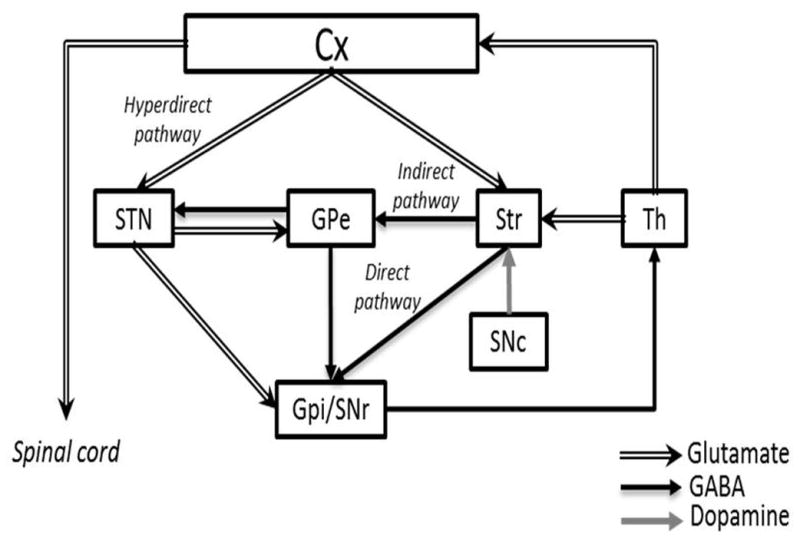
Normal functioning of cortico-BG motor loop as proposed by Alexander, Delong and Strick (1986), Albin, Young, and Penney (1989) and Nambu (2002). Cx, cerebral cortex; GPe, external globus pallidum; GPi, internal globus pallidum; SNc, subtantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Str, striatum; Th, thalamus. Illustration adapted with permission from Nambu (2011).
b. Dopamine as the main modulator of the BG
In the dorsal striatum, both direct and indirect pathways are modulated by dopaminergic neurons. Dopamine (DA) is released in the dorsal and ventral striatum (which includes the NAc) by SNc and VTA neurons, respectively (Bjorklund and Dunnett, 2007). Population release of DA produces a slow time course of changes in extra-synaptic DA concentrations (Grace, 1991). Under this tonic release of DA, the extra-synaptic DA concentration in the striatum may be as low as 40 to 50 nanomolar (Sharp et al., 1986; Church et al., 1987). Short duration bursts of high-frequency firing of DA neurons cause transient increases in the extracellular DA concentration to micromolar levels, which have been labeled as phasic DA release. While changes in tonic DA do not seem to impact behavior, phasic DA is proposed to cause robust changes in behavior and to be involved in prediction-error encoding and reinforcement learning (Kuczenski and Segal, 1989; Kalivas and Duffy, 1990; Shultz, 1997).
DA receptors have been classified into two subtypes designated D1- and D2-like DA receptors (Richfield et al, 1989). Tonic levels of extracellular DA concentrations can activate high affinity D2 receptors. In contrast, phasic activity of DA neurons is needed to increase the extracellular DA to a concentration necessary to activate D1 receptors (Richfield et al, 1989; Grace 1995; Floresco et al., 2003). D1-like receptors (D1 and D5) stimulate Gs proteins; D2-like receptors (D2, D3 and D4) stimulate Go or Gi proteins (Neve et al. 2004). The result is stimulation or inhibition of the cyclic adenosine monophospate (cAMP) dependent protein kinase A (PKA) (Stoof and Kebabian 1984). PKA, in turn, phosphorylates voltage-dependent K+ and Ca2+ channels, leading to changes in the resting potentials of pre- and post-synaptic membranes (Svenningsson et al. 2004). Although all five DA receptors are expressed in the striatum, D1 and D2 receptors are by far the most abundant (Surmeier et al. 1996). MSNs of the direct and indirect pathways (see Figure 1) express predominantly D1 and D2 DA receptors, respectively (Gerfen et al. 1990; Valjent et at al., 2009; Gertler et al., 2008; Hersch et al., 1995; Surmeier et al., 2007). D2 receptors are also expressed in the presynaptic terminals of nigral and VTA dopaminergic neurons (Benoit-Marand and Borrelli, 2001) and in the terminals of corticostriatal neurons (Wang and Pickel, 2002). As mentioned above, in the dorsal and ventral striatum, D1 and D2 receptors are mostly segregated into two populations of MSNs which send direct and indirect projections to the BG output stations (Robertson and Jian, 1995; Nicola, 2007). Although this has not been completely established, it has been proposed that MSNs in the NAc expressing predominantly D1 and D2 receptors are also segregated into two subpopulations of MSNs; both project to the ventral pallidum (the main output station of the ventral striatum) in a manner that might be equivalent to the direct and indirect pathways of the dorsal striatum (Nicola, 2007).
Striatal MSNs oscillate between the so-called down state (hyperpolarized) and up state (membrane potential closer to the depolarization threshold). MSNs fire in response to excitatory glutamatergic cortical and thalamic inputs only when they are in the up state and DA is released in a concentration enough to activate D1 receptors. Under these conditions activation of D1 receptors increase the likelihood of an MSN of the direct pathway to fire. In contrast, in the hyperpolarized down state activation of D1 receptors cause inhibition of MSNs (Flores-Barrera et al., 2011; for a review see Surmeier et al. 2011). This dual-condition mechanism probably works as a filter to increase the signal-to-noise ratio of corticostriatal neurotransmission. MSNs switch between down and up states depending on the activity of corticostriatal neurons. Stronger corticostriatal signals are supposed to encode relevant information for action-selection while weak signals are more likely to be irrelevant noise. This might increase the likelihood of the most proper action to be chosen by activation of specific MSNs. Activation of D2 receptors prevents the transition of MSNs from the down state to the up state (for a review see Surmeier et al., 2011).
3. Current theory of DBS on modulating BG dysfunction
a. DBS in Parkinson’s disease (PD)
PD is one of the most common neurological movement disorders. It is characterized by a progressive loss of dopaminergic neurons in the SNc, leading to a massive reduction of extracellular DA levels in the striatum (Lim and Lang, 2010; Wichmann and DeLong, 2011). This reduction prevents activation of both D1 and D2 dopaminergic post-synaptic receptors in the striatum that stimulate the direct and inhibit the indirect cortico-BG pathways. Thus, activity in the direct pathway is diminished and activity in the indirect pathway is increased (Alexander et al., 1986). High frequency neuronal discharges in GPi/SNr and STN and low frequency firing in the GPe are observed and this causes inhibition of motor nuclei in the thalamus (Bergman et al., 1994; Miller and DeLong, 1987; Soares et al., 2004; Wichmann et al., 1999) (Figure 2). The final result is the inhibition of movement initiation (akinesia) and increased inhibition of ongoing movements (muscular rigidity) (Okun, 2012).
Figure 2.
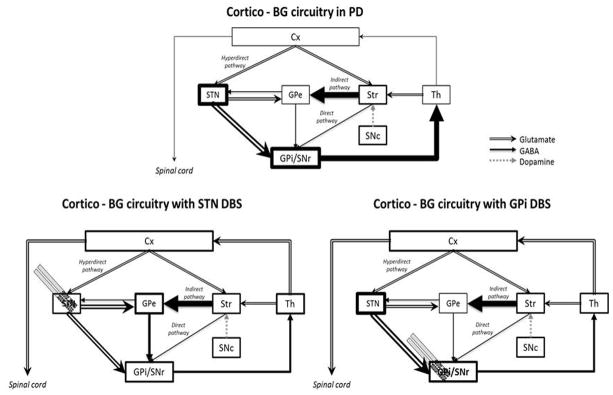
Hypothetical cortico-BG circuitry functioning in Parkinson’s disease (PD) before and after STN or GPi DBS. Cx, cerebral cortex; GPe, external globus pallidum; GPi, internal globus pallidum; SNc, subtantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Str, striatum; Th, thalamus. Illustration adapted from Nambu (2011).
DBS has grown considerably in the past decade as a therapeutic alternative for advanced PD and is considered an effective intervention to treat PD motor deficits (Wichmann and DeLong, 2011). The most common targets for this intervention include the motor portions of GPi or STN. In 2009, NIH COMPARE trial revealed no significant differences in STN or GPi DBS regarding motor deficit improvement (Williams and Okun, 2013). In addition, recent trials found comparable results for medication reduction, notably L-DOPA, in patients with STN or GPi DBS (Deuschl 2006; Okun 2012; Schuepbach et al., 2013; Odekerken et al., 2012; Follett, 2010). However, long-term cognitive problems have been reported in some STN DBS patients (Weaver, 2012). Hence, STN DBS is considered to be more suitable for patients with high dose medications and no significant cognitive deficits (Rodriguez-Oroz et al, 2004). GPi is a good target for patients with dyskinesia and/or preexisting cognitive issues (Williams and Okun, 2013). Thus, DBS in both STN and GPi significantly improves the quality of life for advanced PD patients, with consideration of psychiatric involvement, and is more effective than medication management (Weaver et al., 2009).
The mechanisms of action of DBS for motor improvement in PD patients are still unclear. In agreement with the hypothesis that DBS modulates neural activity, GPe and SNr neuronal firing records show that pathological low-frequency (~9 Hz) network oscillations are regularized by high-frequency STN DBS; and that neurons are entrained to fire at the stimulation frequency pattern (McConnel et al., 2012). In addition, STN DBS may inhibit STN neurons through activation of GABAergic neurons projecting from the GPe to directly activate axons of nearby neurons (McIntyre et al., 2004). Recent studies in rodents suggest that STN DBS influences cortical activity via antidromic activation of the hyperdirect pathway (Li et al., 2007; Gradinaru et al., 2009). In general terms, there are multiple putative mechanisms by which STN/GPi DBS may affect BG neural activity in a manner that improves PD motor deficits. DBS inhibits local neural firing while activating antidromic and orthodromic axonal conduction (Li et al., 2007; Dejean et al., 2009; Gradinaru et al., 2009). It alters concentrations of excitatory and inhibitory neurotransmitters, neural firing patterns, and may increase neurogenesis (Lee et al., 2009; Tye et al., 2009; Bourne et al., 2012). Thus, it appears that the multiple mechanisms of action of DBS in cortico-BG function culminate in the reinstatement of balance within BG connections (Wichmann and DeLong, 2011). One potential explanation for effective STN or GPi DBS is the idea that DBS normalizes inhibition from GPi to thalamus (Rubin et al., 2012).
A recent study investigated functional magnetic resonance imaging (fMRI) analysis of the effects of unilateral (single electrode) DBS of the STN and entopeduncular nucleus (EN), the non-primate analog of the primate GPi, in a normal large animal (swine). This study showed that STN and EN/GPi DBS significantly increased blood-oxygen-level dependent (BOLD) activation in the sensorimotor network (Min et al., 2012). Concomitant fMRI with DBS has also been described in rodents, revealing non-specific motor cortex BOLD increase (Lai et al., 2013; Younce et al., 2014). Finally, the network effects of DBS in nonhuman primates have been investigated, showing that STN DBS similarly increased BOLD activation in the sensorimotor cortex, supplementary motor area, caudate nucleus, PPT, cingulate, insular cortex and contralateral cerebellum. These results demonstrate that STN DBS evokes neural network grouping within the motor network and the BG (Min et al., 2014).
There is increasing evidence that DBS exerts both its therapeutic and adverse effects by modulating neural activity through anatomical and functional connections related to the target stimulation area and its surrounding structures (Chopra et al., 2011; Frankemolle et al., 2010; Kringelbach et al., 2007; Mallet et al., 2007; McIntyre and Hahn, 2010). The brain’s dense wiring makes it challenging to characterize the effect of electrical stimulation on neuronal communication beyond a few synapses. Functional brain imaging has the advantage of providing global assessment of simultaneous neural activity. Much as we have found in swine and non-human primates, studies in PD patients during STN DBS show modulation of motor and non-motor areas including the primary sensorimotor cortex, premotor cortex, sensory motor area (SMA), dorsolateral prefrontal cortex, thalamus, BG, insular cortex and contralateral cerebellum (Asanuma et al., 2006; Grafton et al., 2006; Haslinger et al., 2003; Kahan et al., 2012; Phillips et al., 2006; Stefurak et al., 2003). Positron emission tomography (PET) studies have implicated parietal and temporal cortices classically defined as associative and limbic structures (Hershey et al., 2003; Le Jeune et al., 2010).
There are several clinical observations pointing toward an additional mechanism of action of STN DBS in PD involving the indirect activation of surviving nigrostriatal dopaminergic neurons. For example, STN DBS typically decreases or eliminates the need for L-Dopa (Moro et al., 1999; Molinuevo et al., 2000). It is most effective in PD patients who respond well to L-Dopa (Breit et al., 2004) and is contraindicated for those who do not respond to L-Dopa (Kern and Kumar, 2007). This suggests that therapeutic DBS requires endogenous DA production in the BG. DBS may even elicit dyskinesias that resemble those observed with increased L-Dopa dosage (Limousin et al., 1998) and impulsivity, a DA-related behavior (Frank et al., 2007). These clinical observations point toward the hypothesis that STN DBS may evoke DA release from surviving nigrostriatal dopaminergic neurons projecting to the BG to contribute to the therapeutic effects of STN DBS. They also elicit unwanted side effects when combined with inappropriately high doses of L-Dopa. Using in vivo electrochemical recording techniques, it has been shown that high-frequency stimulation of the STN is capable of evoking striatal DA release in the intact and 6-OHDA DA lesioned rat (Lee et al., 2006; Covey et al., 2008; Blaha et al., 2008). An important question for future investigation is whether STN DBS improves PD symptoms via the release of DA in the BG.
Another STN DBS mechanism may be stimulation-induced adenosine (ADO) release. This important, but understudied, endogenous neuromodulator is present in all cells and plays a role in the regulation of physiological activity in various tissues (Latini and Pedata, 2001). ADO has been shown to be released near the DBS electrode in the thalamus, and appears to be critical for tremor relief (Bekar et al., 2008). In the central nervous system, ADO regulates cerebral blood flow by signalling at A2A receptors, and to a lesser extent at A2B receptors (Cechova and Venton, 2008). As it is a product of ATP degradation, its release from cells is a sign of a high metabolic rate (Masino and Dulla, 2005). Thus, increases in extracellular ADO appear to match elevations in cerebral blood flow that result from increases in neural activity, directly amenable to measurement with fMRI (Phillips, 2004; Brundege and Dunwiddie, 1997). ADO A2A and DA D2 receptors are found on striatal GABAergic MSNs that comprise the indirect striatal output pathway, whereas ADO A1 and DA D1 receptors are found on GABAergic MSNs that form the direct striatal output pathway (Fredholm et al., 2005). In the striatum, cholinergic interneurons are one of the main sources of ADO (James and Richardson, 1993). Dopaminergic input from the SNc into the striatum inhibits the release of acetylcholine through D2 receptors and also stimulates its release through DA D1 receptors (Damsma et al., 1990; Bertorelli and Consolo, 1990). In this regard, it is of interest to note that at the circuit level, using fast scan cyclic voltammetry (FSCV) to measure ADO release several groups have demonstrated that high-frequency stimulation of the SNc elicits ADO release in the striatum of the rat and pig (Cechova and Venton, 2008; Shon et al., 2010a, 2010b).
Current DBS practice is based on the idea that high-frequency stimulation acts as a functional lesion by inhibiting or exciting specific brain regions. However, as our understanding of the mechanism behind DBS expands, we understand that there is substantial variability in its therapeutic effect. A recent study comparing pre- and postoperative diffusion tensor imaging in a PD patient undergoing bilateral STN DBS showed changes in structural connectivity before and after DBS. Using a computational model of spontaneous brain activity in this patient, van Hartevelt et al. found significant localized structural changes after long-term DBS in sensory-motor, prefrontal/limbic, and olfactory brain regions. This suggests that long-term DBS affects global structural and functional connectivity and changes in neural plasticity (van Hartevelt et al., 2014). Although our general understanding is improving, there are still large gaps of knowledge regarding neural plasticity changes from DBS in neurologic and neuropsychiatric disease.
b. DBS in Huntington’s disease (HD)
HD is an autosomal dominant neurodegenerative disorder of striatal MSNs caused by the extensive repetition of the CAG sequence in the huntingtin gene (Sapp et al., 1997). The mutant form of the protein is responsible for dysfunctional cellular processes, such as gene transcription, protein trafficking, mitochondrial respiration, autophagy and calcium homeostasis (Eidelberg and Surmeier, 2011).
A predominant death of striatal MSNs in the indirect cortico-BG pathway is observed in HD (Reiner et al., 1988; Albin et al., 1989; Menalled et al., 2000; Raymond et al., 2011). Loss of this circuit leads to a condition where the ‘break’ provided by the indirect pathway is absent; therefore, improper movements are no longer inhibited. This is proposed to be the cause of chorea, the main observed motor disability, characterized by spasmodic irregular movements in arms, legs, or in face muscles (Albin et al., 1990). Motor incoordination and cognitive deficits are also observed. In addition, other hyperkinetic (dystonia, myoclonia, tics) and hypokinetic dysfunctions (akinesia and muscular rigidity) are present. These motoric deficits are claimed to result from increased DA function in the early phase and decreased DA function in the late phase of HD (Raymond et al., 2011).
The preferential loss of indirect pathway MSNs reduces inhibitory GABAergic input to the GPe leading to an increase in GABAergic inhibition of the STN from the GPe. This inhibition is thought to lead to a decrease in STN glutamatergic excitatory drive on the GPi/SNr. In turn, GPi/SNr GABAergic inhibition of the thalamus is reduced, inducing an overflow of glutamate in motor areas of the cortex, and resultant hyperkinetic movements (Chevalier and Deniau, 1990; Raymond et al., 2011). Although much more is understood into the mechanism of DBS and PD, the documented increased firing rates in GPe and decreased firing rates in GPi observed in a HD patient strongly point toward a mechanism behind effective GPi DBS for motor deficits in HD patients (Figure 3) (Starr et al., 2008; Edwards et al., 2012).
Figure 3.
Hypothetical cortico-BG functioning in Huntington’s disease (HD) before and after GPi DBS. Cx, cerebral cortex; GPe, external globus pallidum; GPi, internal globus pallidum; SNc, subtantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Str, striatum; Th, thalamus. Illustration adapted from Nambu (2011).
c. DBS in obsessive compulsive disorder (OCD)
OCD is a type of anxiety disorder that happens when habits become compulsions. Obsessions are failures to inhibit invasive thoughts or images and compulsions are failures in inhibiting certain behaviors (Bourne et al., 2012). Disturbances in the cortico-BG limbic loop, especially the orbitofrontal cortex, anterior cingulate cortex, NAc, and mediodorsal thalamus have been reported (Bourne et al. 2012; Kopell and Greenberg, 2008).
Functional imaging studies report a significant dysfunction in the orbitofrontal cortex (Chamberlain et al., 2008) in OCD patients, whereby the orbitofrontal cortex and the basal ganglia are hyper-connected (Beucke et al., 2013). Distinct dysconnectivity has also been shown in the default mode network between the prefrontal cortex and BG (Anticevic et al., 2014). Additional studies investigating OCD in adolescent-patient populations has helped in disease prediction revealing that the caudate nucleus volume correlates significantly with the OCD symptoms in early adulthood (Bloch et al., 2005).
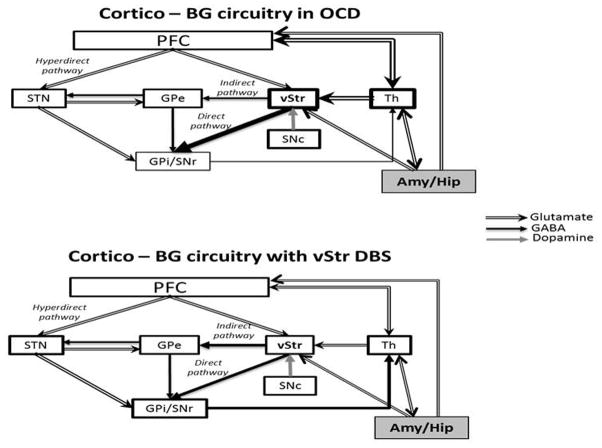
There is also a deficit of behavioral inhibition due to a misbalance between the direct and indirect BG pathways. Increase in the activity of the direct pathway, without the control of the indirect pathway, results in positive feedback where obsessive thoughts would permanently reverberate (Goodman et al., 2010) (Figure 4). Thus, abnormal activity in the amygdala and the hippocampus are linked to the anxiety generated by certain stimuli that often accompany the patient’s urge to perform compulsive behaviors (Bourne et al., 2012; Koppel and Greenberg, 2008).
Figure 4.
Hypothetical cortico-BG circuitry functioning in obsessive compulsive disorder (OCD) before and after ventral striatum DBS. According to this model, obsessions would permanently reverberate in the loop formed by the stimulating activity of the Amy/Hip to Th and then to ventral striatum. The original emotional information would be transmitted through the overactive direct pathway back to Th where it could become a compulsion through thalamocortical activation or return to ventral striatum or Amy/Hip, closing the circuit. Amy, amygdala; GPe, external globus pallidum; GPi, internal globus pallidum; Hip, hippocampus; PFC, prefrontal cortex; SNc, subtantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Str, striatum; vStr, ventral striatum (also as the nucleus accumbens); Th, thalamus. Illustration adapted from Nambu (2011).
A number of DBS targets including the anterior limb of the internal capsule (Greenberg et al., 2006), ventral capsule/NAc (Do-Monte et al., 2013; Rodriguez-Romaguera et al., 2012), STN (Welter et al., 2011), and NAc (Denys et al., 2010) have been investigated for the treatment of OCD. DBS of the anterior limb of the internal capsule in OCD patients is proposed to influence activity of the nearby NAc which, in turn, alters activity in other brain areas, predominantly in limbic areas of the cortex, BG and its projections to the thalamus (Nuttin et al., 2003; Rauch et al., 2006; Wichmann and DeLong, 2011; Okun et al., 2013). A double-blind cross-over study carried out by Denys et al. (2010) in which the NAc was targeted found a 25% improvement in OCD deficits and significant reductions in depression and anxiety. Interestingly, depression improved within seconds of stimulation, anxiety in minutes, obsessions in days and compulsions in months.
Some reports suggest that excessive connectivity between striatum and prefrontal cortex is normalized with NAc DBS (Figee et al., 2013, 2014). A relatively small number of clinical studies have investigated the efficacy of DBS for OCD. These do not permit a sufficiently detailed hypothesis of how DBS may work to improve OCD deficits. However, it has been proposed that the mechanisms of action behind the therapeutic effects of DBS in OCD result from a complex combination of effects on the cortico-BG circuit as proposed for the mechanisms of DBS in PD discussed above.
d. DBS in major depression
Major depression disorder is one of the most severe and prevalent neuropsychiatric disorders and the most common cause of disability. It is as debilitating as coronary heart disease and more debilitating as diabetes mellitus or arthritis (Prince et al., 2007). DBS might be of some help to treat the nearly 30% of major depression disorder patients who are unresponsive to traditional antidepressants, behavioral therapy, vagus nerve stimulation and electroconvulsive therapy (Rush et al., 2006). To date, there is no consensus on which brain regions are responsible for the major depression disorder deficits. However, ablation and imaging studies have indicated some structures that might be involved in the pathophysiology of the disease: the cingulate cortical area 25 (Dougherty et al., 2003; Mottaghy et al., 2002); the anterior limb of the internal capsule and the NAc (Malone et al., 2009; Hauptman et al., 2008); the inferior thalamic peduncle (Jimenez et al., 2005); and the lateral habenula (Sartorius et al., 2010). Improvement of depression deficits has been reported in patients receiving DBS in some of these brain areas. Decreased blood flow in the medial and frontal orbital areas and in the hypothalamus has been observed in major depression patients with DBS in cortical area 25 (Lozano et al., 2008). Stimulation of the anterior limb of the internal capsule has been shown to result in activational changes in the ipsilateral striatum, medial thalamus, anterior cingulate and contralateral cerebellum (Baker et al., 2007), while stimulation of the NAc decreased metabolism in orbitofrontal and dorsolateral prefrontal cortex and amygdala (Bewernick et al., 2010). Although the number of available up-to-date clinical studies is relatively small, it has been speculated that DBS of the targeted nuclei may lead to activation or deactivation of adjacent white matter projections in other cortical and subcortical areas involved in mood regulation, which includes the limbic loop of the BG (Pandya et al., 2012). Data from a recent fMRI study of NAc DBS in a large animal (swine) has shown alterations in the ipsilateral prefrontal cortex, insula, cingulate and bilateral parahippocampal gyrus along with a decreased BOLD signal in the ipsilateral dorsal region of the thalamus (Knight et al., 2013). This large animal model may offer a new and effective approach for identifying the cerebral nuclei and brain stuctures involved in DBS treatment of intractable drug-resistant depression.
e. DBS in Tourette syndrome (TS)
Tourette syndrome is a neuropsychiatric disorder with childhood onset that manifests itself in repetitive, stereotyped, involuntary movements and vocalizations called tics (Kuhn et al., 2011; Tye et al., 2009). These tics reach a peak in adolescence but tend to alleviate in adulthood (Wichmann and DeLong, 2011; Williams and Okun, 2013). Comorbidities can also be observed such as OCD, attention-deficit hyperactivity disorder, depression and psychosocial difficulties (Ludolph et al., 2012).
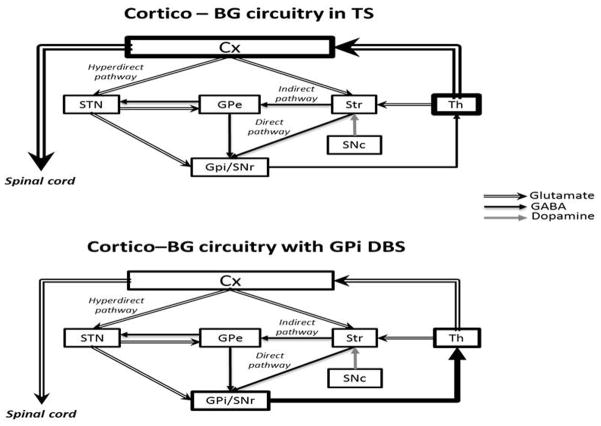
It has been postulated that an overactive thalamocortical system is responsible for TS deficits. However, the exact location within these pathways remains unclear (Singer and Minzer, 2003; Singer et al., 1993). Research has indicated that stimulation of various neural targets promotes amelioration of some of the deficits of Tourette syndrome, such as diminishing frequency of tics and improving comorbid psychiatric disorders. The main target areas proposed for DBS in TS are the thalamic centromedial/parafascicular nucleus (Savica et al., 2013), the motor and limbic portions of the GPi (Diederich et al., 2005; Ackermans et al., 2008), and the NAc (Kuhn et al., 2007).
A clinical study involving 18 refractory TS patients who underwent bilateral DBS in the thalamic centromedial/parafascicular nucleus reported decreased tics, self-injurious behaviors and anxiety (Servello et al. 2008). In this regard, a recent study investigating centromedial/parafascicular DBS in a large animal model supports that thalamic DBS has an inhibitory effect in regions that contribute to impaired sensory-motor and emotional processing (Kim et al., 2013). Only more recently the GPi has also been considered as a DBS target to treat Tourette syndrome. Some studies have shown great efficacy of GPi DBS in reducing motor tics and comorbid psychiatric deficits (Welter et al., 2008; Williams and Okun, 2013). Saleh et al. (2012) observed amelioration of hyperkinetic states in TS patients under GPi DBS. They proposed that this was a result of inhibition of thalamic neurons that project to motor areas of the cortex, as described in Figure 5.
Figure 5.
Hypothetical cortico-BG circuitry functioning in Tourette syndrome before and after GPi DBS. Cx, cerebral cortex; GPe, external globus pallidum; GPi, internal globus pallidum; SNc, subtantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Str, striatum; Th, thalamus. Illustration adapted from Nambu (2011).
4. Negative effects in DBS of the BG
DBS is an emerging treatment option to improve both motor disabilities and psychiatric symptoms observed in neuropsychological diseases, such as PD, OCD and depression. However, negative effects have been described following electrode implantation and/or after long-term stimulation which may directly worsen DBS outcome. Relative to pharmacological treatment, DBS is substantially more intrusive as is any surgical procedure where technical devices are implanted into the brain. Moreover, these devices are constantly active and may differentially affect brains regions as the disease progresses and the overall structure of the brain changes (Whoopen et al., 2013). Hence, DBS negative side effects may result from procedure-related complications, hardware implant and/or stimulation-related issues (Martinez-Ramires et al., 2014).
a. STN DBS and GPi DBS side effects in PD patients
Stimulation of the motor portions of the STN and GPi has near equivalent benefits in improving parkinsonian symptoms of PD patients. The site for DBS is therefore chosen based on clinical aspects, such as the intention to reduce medication intake (STN DBS) or preexistence of dyskinesia or cognitive symptoms (GPi DBS), and the incidence of negative side effects associated to the stimulation of each of these targets (Williams and Okun, 2013). The most commom procedure-related complications found for STN and GPi DBS are psychiatric side effects, such as confusion and delirium. Moreover, these symptoms appear more prevalent in patients with STN DBS than GPi DBS (Videnovic and Metman, 2008). Also, surgical-site pain, low-risk intracerebral hemorrhaging (1–2%), and 10% risk of infection associated with the surgery or with the device have been reported for DBS regardless of the targeted nucleus (Weaver et al., 2010).
In a randomized controlled trial conducted by Weaver and co-workers (2010), patients undergoing DBS surgical intervention had a 3.8 times higher risk of experiencing serious side effects than patients on medical therapy. However, these serious side effects were resolved in 99% of cases by 6 months follow-up. They also reported other serious side effects that were device-related, such as lead migration and defective lead wire, and stimulation-related, such as delusions and hallucinations (Weaver et al., 2010).
Stimulation of the STN may produce weight-gain (Deuschl et al., 2006; Videnovic and Metman, 2008), cognitive side effects (e.g., reduced verbal fluency and deficits in executive functions) (Stefurak et al., 2003; Funkiewiez et al., 2004; Parsons et al., 2006), and psychiatric symptoms, such as depression (with suicide attempts or completed suicide), mania, anxiety and apathy (Bejjani et al., 1999; Stefurak et al., 2003; Funkiewiez et al., 2004; Anderson et al., 2005; Borgohaim et al., 2012). Adverse motor symptoms are also observed during STN stimulation adjustment or after long-term STN stimulation and include induction of paresthesias, severe dysarthria (Rodrigues-Oroz et al., 2004, Okun et al., 2013), and other parkinsonian-like effects, such as bradykinesia, rigidity, swallowing difficulties and worsening of gait or speech (Anderson et al., 2005).
GPi DBS presents relatively insignificant neuropsychiatric impairments compared to STN DBS (Borgohain et al., 2012). This is likely due to the more extensive separation of motor and non-motor functions in the GPi relative to the STN (Wichmann and DeLong, 2011). However, weigth gain, gait ignition failure, dysarthria (Videnovic and Metman, 2008), and mild visual field defects have been reported (Anderson et al., 2005). The apparent better “risk-benefit” of GPi DBS may lead this structure to be the preferred target to treat PD in the future.
b. GPi DBS side effects in HD patients
Stimulation of the GPi has been used to treat motoric symptoms in HD patients based on improvement of choreic dyskinesias of PD patients under GPi DBS (Anderson et al., 2005; Weaver et al., 2010). Although this improvement has been substantially observed, bradykinesia can be aggravated by GPi DBS depending on the stimulation frequency (Spielberger et al., 2012; Cislaghi et al., 2013; Gruber et al., 2014; Moro et al., 2004). Other motoric symptoms occasionally exacerbated in HD patients under GPi DBS are gait disturbance and dystonia of the feet (Gruber et al., 2014). Impairments in cognition (decline in executive functions and working memory) have been described, but they are likely related to disease progression, rather than from GPi stimulation solely (Fasano et al., 2008; Kang et al., 2010; Gruber et al., 2014). There are relatively few clinical data about the effects of GPi DBS in HD and critical parameters such as patient selection criteria and stimulation settings need to be carefully considered (Chen et al., 2013).
c. Limbic targets - DBS side effects in OCD, depression and TS patients
Stimulation of limbic targets, such as the anterior limb of the internal capsule/ventral striatum, the NAc, the ventral NST and limbic portions of the GPi, has improved psychiatric symptoms of compulsion, obsession, depression, anxiety, and motor and vocal tics (Wichmann and DeLong, 2011). Neverthless, DBS of the anterior limb of the internal capsule and the NAc have been shown to produce forgetfulness and word-finding difficulties (Mallet et al., 2008; Denys et al., 2010; Greenberg et al., 2013). In addition, several other psychiatric symptoms such as mania, fear, irritability, and anger may result from this DBS therapy (Shapira et al., 2006; Denys et al., 2010; Haq et al., 2010). Furthermore, the occurrence of suicidal feelings has been reported in some some patients with anxiety and depression under DBS treatment (Greenberg et al., 2013; Williams and Okun, 2013).
5. Emerging theories in BG mechanism
a. Role of the BG motor loop in action-selection
MSNs of the direct pathway are proposed to be in position to select actions encoded in the motor cortex, while MSNs of the indirect pathway can inhibit improper (e.g., concurrent) actions. A clear means of understanding this process is by the cortico-BG circuitry backwards:
Actions are encoded in motor areas of the cortex that include the area M1, the premotor cortex, and the supplementary motor cortex.
Cortical neurons encoding an action are selectively activated by neurons of the motor thalamus.
Thalamic neurons are under tonic inhibition of GABAergic neurons of the BG output stations, providing a distinct selective threshold prior to activation (e.g., the SNr/GPi).
In order to trigger the onset of this action, selective striatal MSNs of the direct pathway disinhibit the thalamic neurons that project to the cortical neurons. At the same time, firing rates of a large number of MSNs of the indirect pathway and neurons of the hyperdirect pathway increase inhibition of the SNr/GPi on the other thalamocortical neurons, thus preventing initiation of the not selected actions.
Fundamentally, BG connectivity literature supports the model where activation of the direct pathway facilitates movements and activation of the hyperdirect and indirect pathways prevents movement (Nambu, 2011, Obeso et al., 2013). However, although there is extensive evidence that action-selection depends on the cortico-BG loop, such evidence is still insufficient to clearly support that action selection occurs as proposed above.
It is largely unknown how motor actions are encoded in motor areas of the cortex. Recent evidence suggests that it is not as simple as the selective control of all muscles or joint movements as represented in Penfield’s functional maps. Instead, it has been proposed that broader areas of the motor cortex encode stereotyped behaviors such as defensive movements of the arms or purposeful actions such as pointing to and reaching for a specific place (Schieber, 2001, Capaday et al., 2013).
Tracing (Asanuma et al., 1983; Holsapple et al., 1991; Flaherty and Graybiel, 1993) and probabilistic tractography (Hyam et al., 2012) evidence have consistently shown that neurons of the motor thalamus (e.g., central anterior/ventrolateral thalamus) receive hyperdepolarizing inputs from GPi neurons (Hoover and Strick, 1993) and send projections that can activate motor areas of the cortex (primary motor cortex, premotor cortex, and supplementary motor area). Unitary cell recording studies in awake behaving monkeys show thalamocortical neurons presenting direction-related sustained change in activity during cue-guided motor action (Kurata, 2005), and before the onset of self-generated movements (van Donkelaar et al., 1999). This suggests that these neurons play a role in initiation and execution preparation of instructed and spontaneous motor actions. Studies have also revealed that the nucleus ventralis lateralis, pars oralis (VLo) which is a part of the motor thalamus that receives projections from the BG, is the thalamic region presenting the highest percentage of neurons responding to active movements and that these neurons are organized in a somatotopic manner (Vitek et al., 1994,1996). In addition, it has been shown that electrical microstimulation of the motor thalamus evokes movements in the contralateral limbs, trunk or face. Nearly 20% of the VLo neurons evoked movements when stimulated (Vitek et al., 1996). These findings are coherent with the hypothesis that activation of a subset of striato-thalamic neurons can selectively trigger motor actions carried out by specific body parts.
The general model of the direct and indirect pathways controlling movement vs. non-movement predicts that when an animal is at rest the GPi/SNr neurons fire tonically at a high rate and that they decrease their activity just before a movement starts. It has been shown that in most monkeys GPi neurons present firing rates which vary from 20–140 spikes/sec (Filion and Temblay, 1991; Miller and DeLong, 1987); most of the time they present firing rates of approximately 60–80 spikes/sec (Delong, 1971; De long et al., 1985). A study by Oviedo et al. (2008) showed that electrical stimulation or infusion of glutamate into the dorsal striatum of rats produced a significant spike rate reduction in pallidal neurons. However, studies did not confirm the prediction that GPi neurons present a pause before the onset of a motor action. Instead, these studies have shown that most pallidal neurons alter their firing rate after the action was selected, i.e., after the firing rates of neurons in the primary motor cortex and supplementary motor area are increased (Crutcher and Alexander, 1990). In addition, most GPi neurons increased firing rate at movement onset (for a review see Goldberg and Bergman, 2011). This might be due to a subset of GPi neurons that pause to select a specific action while the majority increases firing to prevent concurrent actions. Indeed, most of the studies have shown movement related to decreased activity in a subpopulation of GPi neurons (Georgopoulos et al., 1983; Mitchell et al., 1987; Anderson and Horak, 1985; Turner and Anderson, 1997). Alhough less frequent, these studies report that a few GPi neurons fired before the movement onset. However, more problematic to the action-selection hypothesis is the finding that after inactivation of the GPi, as in pallidotomy or GPi DBS treatment of patients with Parkinson’s disease, Huntington disease, or Tourette syndrome, patients maintain the ability to avoid improper actions (Turner and Desmurget, 2010).
The Alexander et al. (1986) and Albin et al. (1989) general model of the BG circuitry predicts that activation of the direct (D1+) or indirect (D2+) pathway facilitates or inhibits movement, respectively. Such predictions have been confirmed by several approaches. In a study by Kravitz et al. (2010) optogenetic control of MSNs of the direct and indirect pathways of mice expressing Cre recombinase under control of regulatory elements for the DA D1 and D2 receptor was achieved through Cre-dependent viral expression of channelrhodopsin-2 (ChR2) in the striatum. Bilateral activation of the indirect pathway resulted in increased freezing, bradykinesia and decreased initiation of locomotor episodes. This picture is also seen in PD patients and in animal models of PD (DeLong, 1990). These motor deficits result from decreased striatal DA and, consequently, reduced activation of the direct pathway and reduced inhibition of the indirect pathway by D1 and D2 DA receptors, respectively. Conversely, optogenetic activation of direct pathway MSNs increased locomotion and rescued deficits in freezing, bradykinesia and akinesia in D2-Cre mice pre-treated with 6-OHDA (Kravitz et al., 2010). Coherently, unilateral activation of D2 receptors in hemiparkinsonian rats caused misbalanced locomotor activation in the contralateral side of the body causing turning behavior (Da Cunha et al., 2008; Dombrowki et al., 2010; Kravitz et al., 2010).
It is currently recognized that other important connections exist making the BG connectivity more complex than initially proposed. First, it is now understood that cortical neurons synapse onto not only the MSNs but also onto GABAergic interneurons in the striatum. Second, MSNs of the direct pathway are known to have branching collateral fibers that terminate in the GPe. Third, in addition to the striatum, cortical and subcortical inputs target the STN, which is now recognized as another important input station of the BG (see above hyperdirect pathway). Fourth, it is now recognized that the GPe projects not only to the STN but also sends branched collaterals to the GPi, SNr and SNc. Finally, instead of parallel cortico-BG loops, the striatum is now acknowledged to integrate functionally diverse information derived from cortex and subcortical areas (Bolam et al., 2000; Nambu et al., 2000; Miwa et al., 2001; Jaeger and Kita, 2011; Obeso et al., 2013; Surmeier, 2013; Ullsperger et al., 2014; Woolley et al., 2014).
There are, however, emerging pieces of evidence that challenge the general view that activation of the direct pathway promotes movement and activation of the indirect pathway inhibits movement. Contrary to this model, bilateral ablations of the main output station, the GPi, are not detrimental but rather therapeutic to action-selection in BG diseases such as PD and dystonia. Critics of the action-selection hypothesis for the BG function have been taking this as evidence that the BG plays a role for the motor cortex areas responsible for action-selection (Turner and Desmurget, 2010).
The general model based on movement/non-movement mediated by the direct/indirect pathways has also been challenged by recent optogenetic studies and by studies based on optical fiber recordings. These studies have shown that while specific motor actions are carried out, both the direct and the indirect pathways are activated concomitantly (e.g., Cui et al., 2013).
Based on evidence that some MSNs of the primate putamen and rodent dorsolateral striatum are activated by sensory and motor stimulation of the same body part and that they increase their firing when an object projects to that body part (see below), it has been proposed in the mosaic of broken mirrors model (MBMM) that some MSNs can trigger movement of specific body parts towards specific objects and other MSNs can initiate locomotion to specific places (Figure 6) (Da Cunha et al., 2009). This means that all neurons of the direct or indirect pathway do not act as a switch between movement and non-movement. Rather, the MBMM considers that these neurons form an action-selection mechanism with a great number of channels, each being able to start or prevent specific actions. While interpreting the above-mentioned data under the logic of the MBMM, it is important to note that it does not predict activation of most MSNs of the direct pathway at the onset of a motor action as the general model of the BG does (Alexander et al., 1986). Instead, the MBMM predicts that selection of a motor action depends on activation of a few specific MSNs of the direct pathway, and inhibition of the few MSNs of the indirect pathway that inhibits that specific action. It also requires activation of a large number of MSNs of the indirect pathway that inhibit initiation of the concurrent actions. Such predictions are difficult to be tested because they require recording the activation of only a few neurons of the direct pathway among a huge population of the remaining neurons that remain silent.
Figure 6.
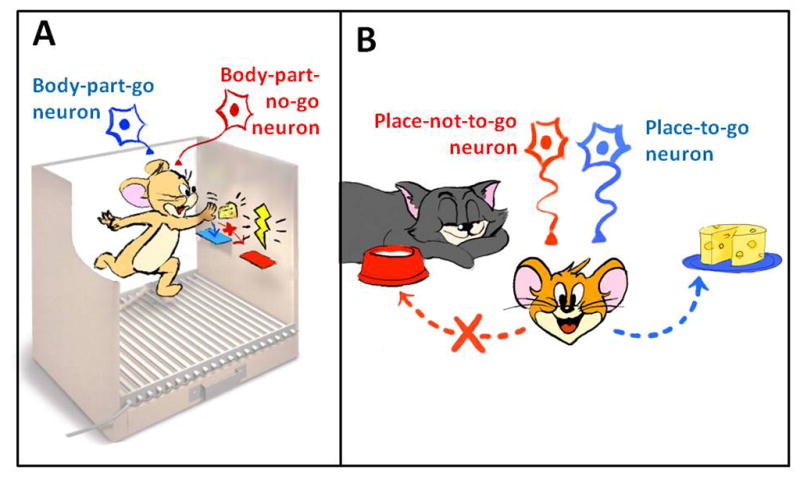
Cartoon illustrating two postulates of the mosaic of broken mirrors model (MBMM). (A) Activation of a striatal “body-part-go cell” selects and initiates a motor action of a body part towards an object. (B) Activation of a striatal “place-to-go cell” selects and initiates the approach to a specific place; activation of a “place-not-to-go cell” prevents approaching to a place.
Though collecting this type of evidence is difficult, there is some evidence that supports the MBMM. Optogenetic studies investigating D1-Cre and D2-Cre mice expressing ChR2 in the dorsolateral striatum showed that unilateral activation of D1+ MSNs at a decision-making point in a nose-poke reinforcement-learning task shifted the response toward the contralateral side while activation of D2+ MSNs shifted the response toward the ipsilateral side (Tai et al., 2012). Without activation the same mice presented right/left responses according to their previous reward history. These findings are in agreement with the MBMM in that selective MSNs of the direct pathway can trigger motor actions directed to specific places. An additional study revealed that stimulation of D1+ MSNs in the dorsomedial striatum promoted place preference, while stimulation of D2+ MSNs did not promote place preference or aversion (Kravitz et al., 2012).
A more recent study by Cui et al. (2013) challenged the general BG model prediction that the MSNs of the direct pathway are active just before movement initiation and the MSNs of the indirect pathway are active when movement stops. They used Cre-dependent viral expression of a calcium indicator in the dorsal striatum of D1-Cre and A2A-Cre to monitor neural activity in MSNs of the direct and indirect pathways, respectively. Time-correlated single-photon counting registered transient increases in activity of both direct and indirect pathways just before initiation and with motor action. These results can be explained by the MBMM as reflecting the activation of a few MSNs of the direct pathway to choose and initiate motor action and activation of the MSNs of the indirect pathway to prevent the initiation of concurrent actions.
The MBMM is also supported by evidence correlating activation of MSNs in response to the approach and/or object touch in nonhuman primates (Graziano and Gross, 1993) and rats (West et al., 1990; Carelli and West, 1991). The same studies showed correlation between the firing of specific MSNs of the nonhuman primate putamen or the rat dorsolateral striatum and passive or self-generated movements of specific body parts.
Causal correlations between activation of MSNs in the dorsal striatum and motor action onset have been provided by old studies showing that electrical microstimulation in different regions of the nonhuman primate putamen evokes movement of specific body parts (Alexander and De Long, 1985a,b). These studies also suggest redundancy in the representation of body parts in the striatum as proposed by the MBMM, because they showed that stimulation at different depths of the putamen evoked movement of the same body part, as if MSNs encoding the same body part were organized in columns. The main problem with this evidence is that it does not discard the alternative explanation that such movements resulted from antidromic stimulation of the motor cortex neurons that project to the striatum. The same cannot be said about the evidence provided by Pisa (1988) showing that neurotoxic lesions of the rat lateral striatum caused severe and chronic impairment of tongue and forelimb reach movements.
b. Roles of the BG associative and limbic loops in cognition and affect
In addition to motor control regulation through the motor loop, the BG is also involved in some non-motor aspects of behavior (Leisman, 2014). There are other loops connecting cortical areas to function-related regions in the BG. More specifically, these loops involve the prefrontal and limbic cortices through which the BG is thought to play a role in cognitive and emotional function, respectively (Yin and Knowlton, 2006). The cortico-BG associative loop originates in the dorsolateral prefrontal cortex and projects to the head of the caudate nucleus and to the rostral part of the putamen anterior to the anterior commissure. This prefrontal territory in the striatum projects to the rostral GPe, to the dorsal parts of the caudal GPe and GPi, and to the rostromedial SNr. Projections from these structures have their terminals in the ventral anterior and medial dorsal thalamic nuclei, which in turn project back to the dorsolateral prefrontal cortex (Alexander et al., 1986; Voorn et al., 2004, Nambu, 2011, Leisman et al., 2014). This associative loop is implicated in “executive functions” that include attention, spatial orientation and in cognitive “working memory” tasks (Cagliori et al., 2013).
The selection of an action, among many possibilities stored along the frontal cortex, would be driven by the goal and/or by an intentional signal represented by prefrontal cortex projections to the striatum (Caligiori et al., 2013; Thill et al., 2013). Within the BG circuitry, information originating from various sources is somatotopically filtered and supported by prefrontal cortex signals in a way to properly select the correct action. Damage to areas forming this associative loop is associated with a variety of behavioral abnormalities related to these cognitive functions, such as attention-deficit and hyperactivity disorder, OCD, schizophrenia and autism (Leisman et al., 2014).
The limbic loop has its origin in the pre-limbic, infra-limbic and lateral orbitofrontal cortices, along with the hippocampus and subcortical amygdala. Projections from these structures reach the ventromedial caudate nucleus, including the NAc, and the ventromedial part of the pre-commissural putamen. The ventral pallidum, the rostral part of GPe and the medial GPi/SNr receive those striatal projections and, in turn, project to the paramedian portion of the medial dorsal nucleus of the thalamus and then back to the limbic regions in the cortex (Haber et al., 1990; Ono et al., 2000; Nakano et al., 2000; Nambu, 2011).
Connectivity between the hippocampus and the NAc also provide some support for the MBMM hypothesis that “place-to-go” cells might exist in the NAc. The NAc receives afferents from the hippocampal formation, a region implicated in mapping the animal’s location and in spatial memory (Groenewegen et al., 1987; van Groen and Wyss, 1990; Witter et al., 1990). In addition, the NAc receives a dopaminergic projection from the ventral tegmental area (VTA) which is known to carry information about motivational value and salience (Bromberg-Martin et al., 2010). Furthermore, the NAc projects to mesencephalic areas related to locomotor functions (Voorn et al., 2004). These connections put the NAc in a strategic position to select places to go based on expected outcomes (Redish and Touretzky, 1997).
The NAc is modulated by DA and is known as the main brain system that integrates emotional and cognitive information into behavioral actions known as motivated behaviors (Berridge and Robinson, 1998; Ono et al., 2000). In general, the limbic loop is involved in the selection of final reward-guided goals based on motivational aspects of the stimuli. Apathy, irritability, social and emotional inability, and lack of empathy are some of the symptoms present in neuropsychiatric disorders associated with damage to areas in the limbic loop, such as OCD and depression (Leisman et al., 2014).
The functionality of each of the three cortico-BG loops is given by a hierarchically-based flow of information according to their respective roles in the action-selection process (Yin and Knowlton, 2006). The sensorimotor loop controls motor actions oriented by decisions made in the current context of the animal. Associative loop-driven decisions are guided by motivational aspects of reward processed by the limbic loop. At the highest level, the NAc, enriched with motivational value information provided by other subcortical structures (e.g., amygdala, hippocampus), helps the limbic and the associative cortex to select the more biologically relevant goals. At the lowest level, the dorsolateral striatum selects the more adequate motor action encoded in the pre-motor and primary motor cortices (Haber et al., 2000; Cagliori et al., 2013). Such selection depends critically on DA alterations in the striatum as reviewed below.
c. Role of NAc DA in motivation
DA release in the NAc is related to motivation and drive states. It has been shown that high-arousal states are induced by DA release in the NAc shell/olfactory tubercle (Ikemoto and Panksepp, 1999; Hebb, 1955; Ikemoto, 2002, 2007). This state is particularly important to an organism’s survival because it promotes approach and avoidance behaviors to unconditioned stimuli (US) and conditioned stimuli (CS) (see section 4.d), depending on the reward or aversive nature of the stimulus (Parkinson et al., 1999). Such affective and drive states of mind/body interaction modulated by the NAc DA are referred to as “action-arousal.” The limbic system supplies midbrain DA neurons with information about environmental stimuli that are important for self-preservation and procreation; this effects DA release in the NAc in a manner that motivates or “energizes” actions related to self-preservation (e.g., eating, drinking, mating, hiding from predators) (MacLean, 1990; Ikemoto, 2007). This DA system is sensitized by regulatory imbalances, such as hunger, and activated when animals detect incentive stimuli (Ikemoto, 2007). Another important function of DA release in the NAc shell/olfactory tubercle is acquisition and consolidation of stimulus-outcome associations (CS-US). When DA is released in the NAc core and lateral parts of the NAc shell/olfactory tubercle, the organism selects previously learned behaviors through CS-US associations (Ikemoto, 2007).
d. Role of striatal DA in associative learning
Subjects’ motor behaviors are driven by changes in unconditioned responses (URs) to biologically relevant stimuli (i.e., USs such as food, sex, and painful or dangerous situations). Motor behavior is also driven by expectations about appetitive and aversive outcomes (USs) based on prediction cues (i.e., CSs). The predictive value of different CSs is learned through classic (Pavlovian) conditioning (Pavlov, 1927; Rescorla, 1988; Li et al., 2014). In addition, behavior is also driven by habitual (automatic) responses to neutral stimuli and by goal-directed actions, both learned through instrumental (operant) conditioning (Yin and Knowlton, 2006; Domjan, 2010). During instrumental conditioning learned under appetitive motivation, early responding appears to be goal-directed and slowly progresses to habitual responding (Mishkin et al., 1982; Knowlton et al. 1996; Packard and Knowlton, 2002). Conversely, during extinction (when a response is no longer rewarded), goal-directed responding of appetitive motivated actions rapidly fade while habitual responses persist for a relatively longer time (Devan and White, 1999; Yin et al., 2006; Balleine and O’Doherty, 2010).
An action is considered to be goal-directed if it is sensitive to outcome devaluation; for example, by pre-feeding the animal (Dickinson and Balleine, 1994). In contrast, stimulus-response (S-R) habits are considered to be insensitive to outcome devaluation, being performed not with an intended goal but as an automatic response to a stimulus that precedes the response’s outcome (Yin et al., 2008). Therefore, the memory traces of habits are the S-R associations, meaning that, after a habit is acquired, the motor response is automatically triggered by the neutral stimulus, independent of the outcome. In contrast, the memory traces of goal-directed actions are the action-outcome (A-O) associations, meaning that goal-directed actions are selected based on expectation of a rewarding (e.g., appetitive) outcome (Dickinson and Balleine, 1994). Another relevant element in selection of motor responses is related to interaction between classical and instrumental conditioning. This association activates an emotional state that motivates the instrumental behavior where the emotional state is assumed to be either positive or negative in valence, depending on the hedonic property of the US. Conditioned cues that predict relevant stimuli can greatly enhance instrumental responding. This process is known as Pavlovian-to-instrumental transfer (PIT) (Lovibond, 1983). PIT is highly influenced by DA activity (Dickinson et al., 2000; Wyvell and Berridge, 2000; Niv et al., 2006; Belin et al., 2009).
There is also compelling evidence that the striatum and other regions of the BG play a role in reward-motivated action selection learning (Schultz et al., 1997; Alderson et al., 2004; Yin et al., 2004, 2006; Da Cunha et al., 2009; Wilson at al., 2009; Haber and Knutson, 2010; Redgrave et al., 2010; Flagel et al., 2011; Da Cunha et al., 2012; Dezfouli and Balleine, 2012; Kravitz et al., 2012; Liljeholm and O’Doherty, 2012). In addition, the striatum and the other regions play a role in learning how to select responses instrumental to avoid aversive stimuli (Wadenberg et al., 2010; La Lumiere et al., 2005; Manago et al., 2009; Darvas et al., 2011; Dombrowski et al., 2013; Wendler et al., 2014). Striatal DA also plays a key role in associative learning (Schultz, 1997; Da Cunha et al., 2009; Niv, 2006; Bromberg-Martin et al., 2010; Da Cunha et al., 2012; Fiorillo et al., 2013; Schultz, 2013; Lak et al., 2014).
Strong evidence exists that acquisition of S-R and action-outcome (A-O) memory traces depend on strengthening synapses between cortical or limbic neurons with MSNs. These cortical neurons encode the stimulus or the outcome and the striatal MSNs trigger the proper motor response/motor action. Furthermore, many studies suggest that selection of USs occurs in the medial NAc shell, CRs in the NAc core, S-R habits in the dorsolateral striatum (putamen in primates), and goal-directed actions in the dorsomedial striatum (head of caudate nucleus in primates) (Wendler et al., 2014; for a review see Ikemoto 2007; Da Cunha et al., 2012). Subregions of the dorsal striatum and NAc are also known to play different roles in learning appetitive-motivated actions (Yin et al., 2004; Yin and Knowlton, 2006; Yin et al., 2006; Redgrave et al., 2010; Dezfouli and Balleine, 2012).
The dorsomedial striatum (DMS) and the dorsolateral striatum (DLS) of rodents are thought to be necessary for selection of goal-directed and S-R habits learned under appetitive reinforcement (Yin et al., 2006; Ikemoto, 2007). Although this is well established for appetitive motivated learning, it is not clear whether the same striatal regions play equivalent roles in aversively-motivated learning. There is also evidence that the NA core plays a role in Pavlovian conditioning (Riedel et al., 1997; Ikemoto and Panksepp, 1999; Berridge, 2012; Bossert et al., 2012; Klucken et al., 2012), but there is some uncertainty about the specific roles the NAc core and other limbic structures have in Pavlovian conditioning (for a review see Da Cunha et al., 2012).
Potentiation of corticostriatal synapses depends on three events happenning concomitantly: depolarization of the pre- and post-synaptic membranes of the involved neurons and phasic release of DA (for review see Da Cunha et al., 2009). Phasic release of DA is evoked by appetitive USs that are better than expected (positive prediction error), CSs that are predictive of appetitive USs and salient stimuli independent of their rewarding or aversive nature (Ramnani et al., 2004; Schultz, 2007; Bromberg-Martin et al., 2010; Berridge, 2012). Phasic DA drops in extracellular DA concentrations happen when something rewarding does not occur as expected and when something less rewarding than expected happens (negative prediction error) (Schultz et al., 1998; Tobler et al., 2003). Different subpopulations of midbrain DA neurons respond to aversive stimuli with phasic increases or phasic decreases in DA release, respectively (Ljungberg et al., 1992; Mirenowicz and Schultz, 1996; Matsumoto and Hikosaka, 2009; Brischoux, et al., 2009; Budygin et al., 2012; Ilango et al., 2014). This supports the view that actions or responses to neutral stimuli that result in better than expected outcomes promote phasic release of DA that, in turn, strengthens the S-R and response outcome (R-O) memory traces. This increases the likelihood that a response will be selected as a result of coming in contact with the same stimulus or that an action will be selected. In contrast, phasic decrease of DA release is known to weaken the synapses between the cortical and striatal neurons encoding S-R and A-O associations. This drives avoidance behaviors in situations in which the same stimulus is presented or the subject wants to avoid an aversive outcome (for review see Da Cunha et al., 2009, 2012).
Strong evidence supports the view that activation of DA neurons in the VTA is critical for associative appetitive learning (Cheng et al., 2003; Nicola et al., 2005; Day et al., 2007; Ikemoto, 2007; Berridge and Kringelbach, 2013; Ouachikh et al., 2013; Steinberg et al., 2013;) Moreover, it has been shown that not only VTA, but also SNc DA neurons are implicated in unconditioned and conditioned responses to appetitive stimuli. Mice bar-press to stimulate either SNc DA neurons (Rossi et al., 2013) or dorsal striatum neurons expressing D1 receptors (Kravitz et al., 2012). This suggests that DA release in the dorsal striatum has reinforcing properties. A recent study presented strong evidence that DA neurons of the SNc are as critical to appetitive and aversive associative learning as the DA neurons of the VTA (Ilango et al., 2014). The latter study showed that optogenetic activation of DA neurons of the SNc sustains conditioned-place preference.
Aversive-driven learning has also been shown to depend on striatal DA. Optogenetic inactivation of DA neurons in the SNc or VTA induces conditioned-place aversion (Ilango and et al, 2014). Studies have demonstrated impaired conditioned-avoidance learning in rats with SNc lesions induced by MPTP (Da Cunha et al., 2001; Gevaerd et al., 2001a, b; Perry et al., 2004; Bortolanza et al., 2010) or 6-OHDA (Cooper et al., 1973) and in rats with dorsal striatum lesions (Wendler et al., 2014), dorsal striatum DA depletion (Rane and King, 2011), and intra-dorsolateral striatum infusion of D1 (Wietzikoski et al., 2012) and D2 DA receptor antagonists (Boschen et al., 2011). A recent microdialysis study by Dombrowski et al. (2013) found that during conditioned-avoidance learning, DA release in the striatum increased only in the first trials in which rats avoided footshocks but not after they had learned the task. However, no alteration in DA release was observed when the footshocks were presented in an unpredictable, unavoidable and inescapable manner. In contrast, MPTP-lesioned rats did not learn the task. Another recent study showed that inactivation of the tyrosine hydroxylase gene in the dorsal striatum, and consequent lack of DA synthesis, impaired the ability of mice to learn conditioned-avoidance responses (Darvas et al., 2011). Impairment in rats with these SNc lesions has also been observed in the cued- and working-memory version of the Morris Water maze (Bellissimo et al., 2004; Da Cunha et al., 2001; Ferro et al., 2005; Miyoshi et al., 2002).
The understanding that striatal DA is involved not only in motor control, but also in action-selection, learning and memory, and affective states is critical to explain and treat the non-motor symptoms of BG diseases such as PD (Conte et al., 2010), drug addiction (Rovinson et al., 2009; Wanat et al., 2009), bipolar disorder (Cousins et al., 2009), schizophrenia (Carlsson et al., 2004), and attention deficit/hyperactive disorder (Del Campo et al., 2011), obsessive-compulsive disorder and Tourrette syndrome. Deficits in aversive-driven learning related to the inability of striatal DA to encode negative-prediction errors may also explain why depression and gambling is much more prevalent in PD (Rosa et al., 2013).
6. New approaches for BG-DBS research
a. Electrophysiological signal as a feedback for DBS
The DBS field has advanced at a rapid pace and supporting technology to improve current use has evolved with it. Advanced DBS systems such as those that rely on real-time feedback from electrophysiological signals, provide the first generation of implantable devices that identify symptomatic and healthy brain states (Gunduz et al., 2014). These devices include the Medtronic Activa PC+S (Ryapolova-Webb et al., 2014) and Neuroscan RNS (Sun et al., 2008). With the understanding that changes in neuronal firing frequency occur in a pathogenic state, this approach shows potential in many disorders that are currently treated with DBS (Gunduz et al., 2014). Exaggerated beta band (8–35 Hz) synchrony in STN local field potentials occurs in PD patients, which is attenuated during therapeutic DBS, and returns when stimulation stops (Bronte-Stewart et al, 2009 Kuhn, 2008). Other studies have shown beta activity in the motor cortex (Whitmer et al., 2012), and phase-amplitude coupling (PAC) between beta and high gamma (>70 Hz), observed in PD, are also potential targets for closed-loop DBS (de Hemptinne et al., 2013). Electrophysiological measurements may also hold promise in DBS for the treatment of psychiatric disorders. For example, symptom provocation in the setting of OCD has been correlated with increased low frequency (2–5 Hz) activity over the frontal cortex (Pogarell et al., 2006), and DBS of the ventral striatum has been shown to attenuate these low frequency oscillations (Figee et al., 2013).
Although electrophysiological feedback is a promising modality for a closed-loop DBS device, it is important to acknowledge potential pitfalls as well. DBS has been shown to decrease beta synchrony in PD patients, but this has also been demonstrated during voluntary movements and during tremor. Despite its association with PD, PAC occurs during rest and also with voluntary movement (Miller et al., 2012; de Hemptinne et al., 2013). Additionally, frontal low frequency oscillations, while implicated in OCD, are correlated with normal goal-directed behavior (Knyazev, 2007). Preliminary trials will demonstrate the efficacy of these first generation neural stimulation feedback devices, which may provide direction in development of future generations of closed-loop DBS systems (Gunduz et al., 2014).
b. Electrochemical methods to determine neural circuitry underlying DBS
The use of in vivo electrochemical methods to investigate the neural circuitry underlying DBS makes it possible to circumvent the assumptions that are necessary with the use of alternative methods. The electrochemical procedures FSCV and fixed potential amperometry (FPA) offer the best temporal resolution of all in vivo electrochemical methods to date (5–10 samples/sec for FSCV; 10K samples/sec for FPA) (Kimble et al., 2009; Venton et al., 2002).
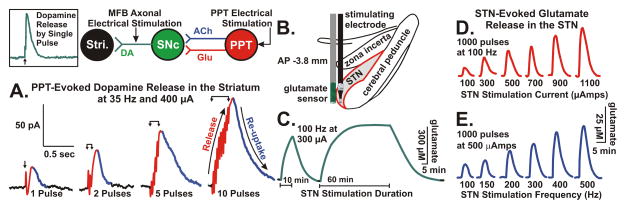
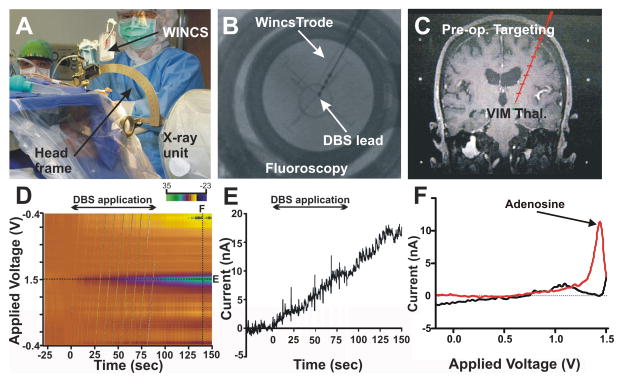
In Figure 7, FPA in combination with CFMs permitted quantitative detection of striatal DA overflow (efflux) evoked by electrical stimulation of excitatory inputs to DA cells in the SNc, such as those originating in the hindbrain PPT (Forster and Blaha, 2003). Overflow of a synaptic transmitter is referred to as “release” throughout this section. Dommett et al. (2005) have shown that FPA can be used to monitor striatal DA release in response to natural stimuli, such as light pulses. Visual stimuli evoked increases in striatal DA release via a direct input to the superior colliculus from the retina that, in turn, activated midbrain dopaminergic cells at a short latency. Collectively, these studies have confirmed the utility of fast electrochemical recording procedures to measure DA transmission driven by polysynaptic pathways, such as those we have proposed to mediate DBS-evoked DA neurotransmission (Lee et al., 2006, 2009; Shah et al., 2010).
Figure 7.
(A.) DA release in the striatum (Stri.) of urethane anesthetized rats evoked by 1 to 10 pulses of electrical stimulation of pedunculopontine tegmental nucleus (PPT) glutamatergic and cholinergic projections to substantia nigra pars compacta (SNc) DA cells. Note that the increase in DA release is time locked to the stimulation (pulse artifacts are superimposed on the rising portion of the signal) and the recovery to baseline is a reflection of clearance of DA mainly via presynaptic re-uptake. INSET: rapid response of DA to stimulation of DA axons in the medial forebrain bundle (MFB) illustrating that the relatively slower PPT evoked response is trans-synaptically mediated. Glutamate release in the STN evoked by electrical stimulation of the STN at various durations (C.), intensities (D.), and frequencies (E.). (B.) Positioning of a glutamate sensor adjacent to a bipolar stimulating electrode in the STN (see Lee et al., 2007).
With respect to quantifying glutamate release using FPA, recent development of enzyme-coated platinum microelectrodes based on work by Hu et al. (1994) have shown a high degree of reliability as a selective, sensitive and rapidly responding glutamate sensor in vivo (Wilson and Gifford, 2005). This glutamate sensor system provides an additional quantitative measure of potential glutamatergic transmission in the dorsal striatal complex circuitry interfacing with SNc DA cells. Our preliminary studies have shown that electrical stimulation can evoke frequency- and intensity-dependent increases in glutamate release recorded locally at the site of stimulation (STN) using these procedures (Figure 7B–E).
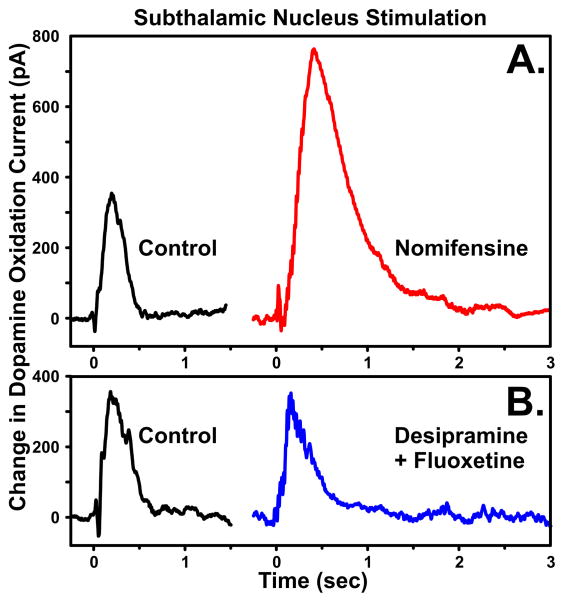
In agreement with the hypothesis that STN DBS improves motor symptoms of PD by striatal DA release, several animal studies have shown that STN DBS increases striatal DA levels. For example, in vivo microdialysis studies have shown that STN DBS increases the striatal DA metabolites (DOPAC and HVA) and tyrosine hydroxylase activity in normal and 6-OHDA lesioned rats (Meissner et al., 2001, 2002, 2003; Paul et al., 2000). With one exception (Bruet et al., 2001), STN DBS-evoked increases in striatal DA dialysate could not be detected without first inhibiting DA reuptake with nomifensine and stimulating for prolonged durations (20 mins) (Meissner et al., 2003). In vivo monitoring of slow (min-hrs) changes in DA release is easily accomplished using these conventional microdialysis techniques. However, analysis of more rapid changes in DA release in the absence of DA reuptake inhibition that may result from STN DBS requires an equally rapid ‘real-time’ detection and monitoring system, such as FSCV and FPA. For detection, sensitive carbon-fiber microelectrodes (CFM) and enzymatic sensors used with these methods permit submicromolar monitoring of central DA and glutamate release. As such, to establish the functional characteristics of the dorsal striatal complex circuitry, we have utilized electrochemical recording procedures established for reliable monitoring of DA and glutamate release and reuptake in dopaminergic and glutamatergic terminal sites in the brain in vivo (Blaha and Phillips, 1996; Michael and Wightman, 1999; Suaud-Chagny, 2004; Wilson and Gifford, 2005).
Our neurochemical studies have focused on determining the functional consequences of STN DBS in terms of rapid changes in striatal DA release elicited by relatively brief and prolonged electrical stimulation of the STN in the urethane anesthetized rat. Brief STN stimulation (15 pulses at 300 μA and 50 Hz) resulted in a stimulus time-locked increase in striatal DA release as measured by FPA in combination with CFMs (Figure 8). The selectivity of the recording microelectrode to STN stimulation-evoked DA release was confirmed by systemic injection of the DA reuptake inhibitor nomifensine, which resulted in a significant increase in DA oxidation current, compared to serotonin (fluoxetine) and noradrenaline (desipramine) reuptake inhibitors, which did not alter the STN stimulation-evoked striatal response (Lee et al., 2006). These results support the hypothesis that STN DBS results in quantifiable striatal DA release. However, as these studies were performed in rats with intact SNc dopaminergic neurons, it is crucial to perform systematic measurements in an animal model of PD, such as 6-OHDA lesions, where the SNc neurons are selectively and partially destroyed.
Figure 8.
Striatal DA is increased with brief STN stimulation. The selectivity of the response was confirmed by (A.) systemic injection of the DA reuptake inhibitor nomifensine (red line), which resulted in a significant increase in DA oxidation current, compared to (B.) serotonin (fluoxetine) and norepinephrine (desipramine) reuptake inhibitors (blue line) which did not significantly increase the STN stimulation-evoked response.
In relation to an animal model of PD, our preliminary results have shown that, in combination with L-DOPA, repetitive stimulations in 6-OHDA lesioned rat are capable of facilitating DA release to levels comparable to that seen with stimulation in intact rats and are consistent with recent findings that high-frequency stimulations modulate the action of L-DOPA (Oueslati et al., 2007). Microinfusion of the neurotoxin 6-OHDA onto DA cell bodies in the SNc resulted in the selective degeneration of dopaminergic cells in that region (Ferro et al., 2005). As shown in Figure 9, compared to intact rats, 6-OHDA lesions resulted in rats exhibiting a marked, but clearly detectable, attenuation in medial forebrain bundle stimulation-evoked DA release in the striatum (16.4±8.3% of 100% intact responses) as monitored using FPA in combination with CFMs. Most significantly, compared to intact animals receiving a systemic injection of saline, systemic administration of a relatively low dose of L-DOPA to lesioned rats resulted in a near complete recovery in the magnitude of the evoked DA responses (84.87±16.22% of 100% intact responses at 30 min post-injection). Since L-DOPA increased the DA response in the lesioned animals to the statistical equivalent of the non-lesioned (saline-treated) animals, it can be inferred that on-line FPA or FSCV would be capable of (1) detecting depleted extracellular levels of DA in the striatum and (2) enhancing these levels in response to L-DOPA treatment across various dose ranges in PD patients (Blaha et al., 2008). These data highlight the relevance of monitoring dopaminergic transmission during STN DBS; these data also provide a framework for the development of a closed-loop neuroprosthesis with chemical sensing feedback and neuromodulation to maintain neurotransmitter levels consistent with optimal therapeutic efficacy.
Figure 9.
(A.) Represetative example of medial forebrain bundle stimulation-evoked (20 pulses at 100 Hz applied every 10 min) striatal DA release in a urethane anesthetized intact rat and a rat sustaining neurotoxic 6-OHDA lesioning of the nigrostriatal dopaminergic pathway before and following systemic injection of L-DOPA (100 mg/kg i.p.). Note the recovery of stimulated DA release to 100% baseline levels of evoked striatal DA release following administration of L-DOPA in lesioned animals. (B.) Mean ± SEM time courses of effects of L-DOPA injections, compared to saline administration, on striatal DA release in intact and lesioned rats before and following systemic injection of L-DOPA. Solid and dashed blue lines: intact mice that received L-DOPA and saline, respectively. Percentage changes refer to intact pre-saline treated mice.
Stimulation frequencies in the range of 37 to 75 Hz and current intensities in the range of 200–600 μA evoked maximal DA responses in the striatum (Figure 10A and B) (see Lee et al., 2006). More prolonged STN stimulation evoked a DA response that peaked within 15–20 applied pulses and fell off to ~30% of pre-stimulus baseline levels despite continuous stimulation. Stimulation dorsomedial to STN, corresponding to a portion of the medial forebrain bundle containing ascending nigrostriatal dopaminergic axons, resulted in an increase in striatal DA release that plateaued 5 sec into stimulation (Figure 10C). These results suggest that DBS in PD patients typically applied to the STN and adjacent regions increases brain extracellular DA levels, but also indicate that the pattern and magnitude of DA release varies significantly depending on the site and nature of stimulation. This latter finding has therapeutic implications for the site and pattern of stimulation in PD patients, which so far have not been fully explored.
Figure 10.
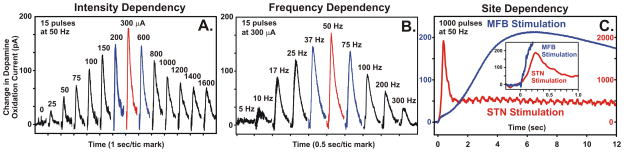
Intensity, frequency, and site dependency of STN stimulation evoked striatal DA release of the urethane anesthetized rat. (A.) The optimal current intensity for STN stimulation was 300 μA, with the stimulation evoked DA response falling off at 600 μA and greater. (B.) The optimal frequency for STN stimulation was 50 Hz, with the stimulation evoked DA response falling off at 75 Hz and greater. (C.) Prolonged STN stimulation evoked a transient response that peaked within 20 applied pulses (red line), as compared to a 10-fold greater and more sustained DA response to stimulation of the medial forebrain bundle (MFB) (blue line). INSET: DA release evoked by stimulation of the MFB (blue line) was significantly faster at onset compared to STN stimulation when viewed on the same scale as the STN response (red line).
We have demonstrated our ability to measure in vivo real-time oxygen, DA, ADO, histamine, and serotonin release with CFMs and glutamate release with an enzyme-linked biosensor during DBS (Lee et al., 2007; Agnesi et al., 2009; Bledsoe et al., 2009; Chang et al., 2012a; Shon et al., 2010a; 2010b). We have also shown that we can use the Mayo Clinic Engineering Department developed Wireless Instantaneous Neurotransmitter Concentration Sensing system (WINCS) to electrochemically codetect in vivo changes in ADO and DA concentrations in small and large animal models of DBS (Shon et al., 2010a). More importantly, our results (Shon et al., 2010b) have shown that STN DBS elicits DA and ADO release in the caudate nucleus of isoflurane anesthetized pigs. Additionally, we examined striatal DA release evoked by STN DBS in awake monkeys. We identified a site-dependency of DA release by stimulating at multiple points along a trajectory passing through the thalamus and STN. Greater DA release was observed when the stimulating electrode was within the dorsal region of the STN. Our results showed that the amount of DA release depends critically on the location of the stimulating electrode (Gale et al., 2013).
Our pig experiments have demonstrated that STN DBS elicits DA and ADO release distally in the caudate nucleus, shown in Figure 11 (see Shon et al., 2010b). For these experiments we implanted a single CFM into the caudate nucleus of isoflurane (1%) anesthetized pigs; FSCV recordings were taken during brief (2 sec) electrical stimulations of the human Medtronic 3389 DBS electrode implanted in the ipsilateral STN. With high-frequency stimulation (140 Hz), ADO and DA were clearly released (Figure 11A). The temporal patterns and magnitude of DA (black line) and ADO release (1st peak blue line and oxidation product 2nd peak red line) evoked by STN DBS are shown in Figure 11B. The voltammograms obtained with WINCS in the pig revealed one peak at +0.6 V for DA oxidation and two oxidation peaks for ADO (1st peak near +1.5 V and 2nd peak near +1.0 V), as shown in Figure 11C and D, respectively. Our now established pig model has permitted testing of our human neurochemical recording electrodes, as well as study of distal neurotransmitter release by STN DBS.
Figure 11.
In vivo dopamine (DA) and adenosine (ADO) release measured with WINCS-based FSCV at CFMs in the CN of the isoflurane anesthetized pig. (A.) Electrical stimulation (140 Hz, 0.5 ms- pulse width, for 2 sec) of the STN evoked both DA and adenosine release in the CN. The color plot shows the appearance of DA release immediately during and after stimulation, while the peak corresponding to adenosine release was delayed. (B.) Current versus time plot at +1.5V (blue), +1.0V (red), and +0.6V (black line) shows adenosine first and second oxidation (ADO - 1st, blue and ADO – 2nd, red) and DA (DA, black line) release following electrical stimulation (yellow box). (C.) and (D.) Background subtracted voltammograms for DA and adenosine, respectively, demonstrate simultaneous measurements of DA and adenosine releases (black and blue vertical dashed lines in A).
From knowledge and experience gained from tests conducted in our large animal (pig) experiments, we have successfully used WINCS to monitor DA and ADO release in the hippocampus of human patients undergoing resective surgery for medically intractable epilepsy (Van Gompel et al., 2014). A CFM was implanted 5 μm into the temporal lobe cortical surface of each patient for 15 min during intraoperative electrocorticography (ECoG) and 10 min of FSCV recordings prior to resection. One, out of ten patients tested, expressed a lateral neocortical seizure in which ADO release was observed and this was time-locked with the onset and termination of the seizure. These findings support a potential role of ADO in seizure termination during temporal lobe seizures.
In addition, WINCS-based FSCV measurements were taken in the thalamus of essential tremor patients during DBS neurosurgery (Figure 12); all the patients were awake during surgery. Arm and hand tremor were measured using a triaxial accelerometer which patients held in the hands opposite to the implanted hemisphere. Upon implantation of the DBS electrode, but prior to activation of the pulse generator, tremor amplitude was significantly reduced (n = 7; Figure 12A). Referred to as the microthalamotomy effect, symptom relief prior to neurostimulation has been observed in as many as 53% of patients undergoing DBS surgery for essential tremor (Tasker, 1998). Our results showed that, as expected (Lakie et al., 1992), there was no change in tremor frequency, but average tremor amplitude was reduced by 61.2±13.7% upon DBS electrode insertion (n = 7 patients).
Figure 12.
Intraoperative FSCV recordings during monitoring of tremor in essential tremor patients. (A) FSCV color plot shows increases in oxidation current at +1.4 V (black arrow) and +1.2 V (white arrow) time-locked with the insertion of the DBS electrode into the ventral intermediate nucleus (VIM) of the thalamus (n = 7 patients). (B) Oxidation currents at +1.4 V (black) and +1.2 V (gray) plotted against time. (C) Representative tremor monitoring with a hand- held accelerometer shows tremor was decreased upon DBS electrode insertion. Time scale is the same as in (B). (D) Representative handwriting sample before and after DBS electrode implantation (L, left hand; R, right hand).
Coincident with tremor reduction, DBS electrode implantation evoked a large increase in the FSCV oxidation current peak at +1.45±0.03 V (n=7; Figure 12B). In four patients, this peak was followed by a second significantly smaller oxidation current peak at +1.19±0.06 V (white arrow in Figure 12B). Post-calibration analyses showed that the two oxidation current peaks recorded by FSCV matched those for authentic ADO and its oxidation byproduct (second peak). In accordance with post-calibration of the human CFM (see Chang et al., 2012b), the maximal increase in ADO at the first oxidation peak potential corresponded to an increase of 1.76±0.12 μM. These results demonstrate the first application of WINCS during DBS neurosurgery in patients (see Chang et al., 2012b; Kasasbeh et al., 2013).
To monitor real-time neurochemical changes associated with DBS, we performed similar FSCV measurements (−0.4 to +1.5 V every 100 ms) in the VIM of the thalamus of essential tremor patients during DBS neurosurgery using WINCS (Figure 13A–C). A FSCV color plot (Figure 13D) revealed that, during DBS, an oxidation peak current was detected at +1.4 V, corresponding to ADO oxidation (Swamy and Venton, 2007; Cechova and Venton, 2008; Pajski and Venton, 2010). Although a hand held accelerometer to measure tremor was not used in this patient shown in Figure 13, the rise in the ADO signal during and after DBS (Figure 13E) was visually observed to correlate with a marked reduction in tremor.
Figure 13.
Neurochemical changes evoked by DBS in the VIM of the thalamus in patients with essential tremor. (A) Surgical set-up. (B) X-ray of the position of the human CFM and DBS lead in patient’s thalamus. (C) MR image of implantation trajectory. (D) Color plot shows the appearance of oxidation current at +1.4V immediately upon application of DBS (135 Hz, 60 μsec pulse width, 0.5–2.0 V, slowly increased). (E) Current versus time plot at +1.4V following DBS. (F) Background subtracted cyclic voltammogram shows the oxidation peak of adenosine at +1.4 V.
c. Translational importance on new large animal DBS models
Large animal models provide a necessary bridge between the fundamental discoveries made in small animal models and the translation into clinical practice. The preferred large animal model in neuroscience has been the non-human primate due to the higher cognitive function and behavioral similarities to the human. The rhesus macaque, for example, is a model with highly conserved when anatomy is compared to human. The Atlas of the Rhesus Monkey Brain (Saleem, 2007), combined with high-precision stereotactic head frame and high-resolution MRI coil allows translational DBS studies in this large animal model (Min et al., 2014).
There are also known similarities between porcine and human biology that have led to the proposal to use the porcine model as an excellent way to study human disease (Lind et al., 2007). Easy to get and maintain, the white domestic pig (Sus scrofa) is proving to be an economically viable alternative to the expensive non-human primate, and thus is becoming an increasingly popular animal for neuroscience research. Several characteristics make the pig an excellent model for mock human DBS surgery: (1) the pig has a gyrencephalic cortex, which is more similar to the human and non-human primate structure than that of commonly used lissencephalic rodent brains (Hofman, 1985, 1988); (2) the adult pig brain (~160 g) is comparable in size to that of the rhesus monkey (~100 g) and baboon (~140 g) and closer in size to the human brain (1300–1400g) than the rodent brain; (3) a high-resolution pig brain atlas is available allowing for precision DBS studies in pigs to advance our understanding of the underlying mechanism using functional imaging (Saikali et al., 2010); (4) pig brain development is complete by ~5 months (Dobbing, 1964), which enables the use of younger pigs (20 – 50 kg) with adult-sized brains for easier mobilization and dissection during cranial surgery; (5) MPTP produces an effective pig PD model (Danielsen et al., 2000; Dall et al., 2002; Cumming et al., 2003); (6) several high-precision stereotactic head frames for pig neurosurgical studies have been developed establishing this system for DBS mechanistic studies (Bjarkam et al., 2009; Min et al., 2012); and (7) the pig genome has been decoded, which has revealed many key similarities between human and porcine genomes, further reinforcing the translational potential of this large animal model (Groenen et al., 2012). The advent of high-precision imaging tools such as functional magnetic resonance imaging (fMRI), combined with a recent increase in our understanding of cross-species neuroanatomical similarities among pig, human, and non-human primates highlight the potential of these large animal models to further our understanding of the mechanism behind DBS.
d. Use of functional neuroimaging in BG-DBS
Damage to the BG produces well-characterized changes that are related to movement disorders and motor deficits, including tremor, rigidity, and akinesia (Bhatia and Marsden, 1994; DeLong, 1983). Functional imaging is often used to indirectly monitor biochemical and anatomical changes within BG structures in movement and affective disorders due to its wide clinical availability and its global assessment of neural activity. Techniques including single photon emission computed tomography (SPECT), fMRI, and PET are used for this application. SPECT imaging has shown promise as a diagnostic tool for movement disorders revealing that changes in the caudate and putamen may help to differentiate early-stage PD from dystonia or essential tremor (Song et al., 2014). Other imaging studies, including PET, have been used to show decreased dopaminergic innervation within the BG in PD patients (Song et al., 2013). Longitudinal fMRI studies have also revealed heterogeneity in PD patients. These latter studies have shown differential changes in BG structures when patient populations are presented with motor tasks whether they are responding or not responding to pharmacological therapy (Holiga et al., 2013). In addition to movement disorders, BG dysfunction is now accepted as playing a role in a variety of neuropsychiatric diseases, including TS (DeLong and Wichmann, 2010).
When mapping this dysfunction, fMRI studies in TS patients have shown a relationship between tic onset and paralimbic and sensory-association areas (Bohlhalter et al., 2006). fMRI has also revealed a role for cortico-BG network dysfunction in TS (Peterson et al., 1998; Worbe et al., 2010; Worbe et al., 2012). Using diffusion tensor imaging (DTI), studies have also shown dysfunction in motor, associative, and limbic pathways (Neuner et al., 2009, 2010). Taken together, these studies show a role for neuroimaging in disease diagnosis and evaluating functionality of DBS for treating BG diseases.
As DBS is an effective treatment option for PD (Benabid, 1994, 2003, 2006), its application has now been applied to other neurological and psychiatric disorders (Mayberg et al., 2005). The incomplete understanding of the therapeutic mechanisms of DBS and lack of objective methods to measure its central global effects inhibit the advancement of a more individualized approach to DBS therapy. The brain’s dense wiring makes characterizing the effect of electrical stimulation on neuronal communication beyond a few synapses extremely challenging. Functional neuroimaging appears well suited to this task due to its wide clinical availability and its global assessment of neural activity. Many imaging studies in PD patients during STN DBS show modulation of motor and non-motor areas including the primary sensorimotor cortex, premotor cortex, SMA, dorsolateral prefrontal cortex, thalamus, BG, insular cortex, and contralateral cerebellum (Asanuma et al., 2006; Grafton et al., 2006; Haslinger et al., 2003; Kahan et al., 2012; Phillips et al., 2006; Stefurak et al., 2003). Other PET studies have implicated parietal and temporal cortices, classically defined as associative and limbic structures (Hersh et al., 2003; Le Jeune et al., 2010). These studies help to elucidate what makes DBS effective for treating motor and non-motor symptoms of PD.
Functional imaging studies of DBS have recently been aimed at identifying the circuitry elements that underlie effective, as opposed to ineffective, stimulation. For example, a recent tractography study of PD patients undergoing STN DBS identified a direct relationship between the positive clinical outcome and the targeting within white matter tracts that involve the cerebellum (Sweet et al., 2014). In a longitudinal SPECT study, a significant increase was shown in cerebral blood flow in the anterior cingulate/supplementary motor cortex in PD patients who responded to STN DBS treatment (Antonini et al., 2003). PET has shown that long-term effective STN stimulation of PD patients increases frontal motor/associative area blood flow compared to baseline (Sestini et al., 2005). A regional cerebral blood flow (rCBF) SPECT study reported a correlation between improved motor scores and an increase in rCBF in the pre-SMA and primary motor cortex in PD patients receiving STN DBS treatment (Paschali et al., 2013). These studies suggest that neuroimaging biomarkers of effective DBS may exist for PD treatment.
Imaging has already identified structural and functional correlates of cognitive and affective deficits associated with PD (Kalbe et al., 2009; Kostic et al., 2010; Reijnders et al., 2010). In a case report in which STN DBS elicited several reproducible episodes of acute depressive dysphoria in PD patient, fMRI revealed increases in the superior prefrontal cortex, anterior cingulate, anterior thalamus, caudate and brainstem with widespread decreases in medial prefrontal cortex activation (Stefurak et al., 2003). Another case study identified STN DBS stimulation parameters which produced a hypomanic state in PD patient. A PET investigation showed involvement of limbic and association cortex, including areas of the anterior cingulate gyrus and the ventral anterior nucleus of the thalamus (Mallet et al., 2007). The fact that STN DBS can elicit cognitive and emotional side effects is unsurprising given our current understanding of the STN as a structure with anatomically-defined subregions which connect to motor, associative and limbic structures (Benarroch, 2009). A recent clinical report showed that using model-based optimization of DBS stimulation parameters to avoid the current spreading to non-motor areas reduced cognitive and cognitive-motor impairments while maintaining therapeutic motor benefits in STN DBS PD patients (Frankemolle et al., 2010). These data suggest that anatomical target-placement decisions during human DBS surgery could be augmented by structural and functional neuroimaging techniques which intraoperatively assess the motor and non-motor networks affected by STN DBS.
7. Concluding remarks
The last decade has held impressive developments in understanding the mechanism of BG function and how targeting different brain sites can improve motor, cognitive and motivional aspects of BG diseases. However, we are still far from having the ability to fundamentally describe the mechanism behind therapeutically effective DBS. Deepened understanding of BG connectivity and the resultant computational modeling of functional motor, motivational and cognitive processes have emerged in the last decade. However, these models are still insufficient to support the idea that different striatal-GPi/SNr-thalamus-motor cortex neurons can initiate or prevent a particular movement or a sequence of movements that compose specific actions. Additionally, these studies have revealed paradoxical challenges to the current BG action-selection hypothesis. DBS has indeed emerged as a therapy with wide applications expanding from STN DBS to treat PD, to cover many neurological and psychiatric diseases; however, our fundamental understanding of BG DBS is limited in its current state. A great deal of effort from the neuroscience community will be required to advance BG DBS to the proposed level of understanding and clinical application.
Highlights.
We review the circuits and connections of the basal ganglia cortico-thalamic loop.
Deep brain stimulation theories on modulating basal ganglia dysfunction are discussed.
We discuss new research approaches on basal ganglia-deep brain stimulation mechanisms.
Acknowledgments
The authors are grateful for the English revision by Alice Burns, cartoon drawing by Ariel Morais Da Cunha, and financial support from CNPq, Fundação Araucária, and CAPES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette’s syndrome. Neurotherapeutics. 2008;5:339–344. doi: 10.1016/j.nurt.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, Bennet KE, Garris PA, Blaha CD, Lee KH. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009;111:701–711. doi: 10.3171/2009.3.JNS0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington’s disease - implications for the functional-anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–365. doi: 10.1002/ana.410270403. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional-anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience. 2004;125:349–358. doi: 10.1016/j.neuroscience.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR. Microstimulation of the primate neostriatum .1. Physiological-properties of striatal microexcitable zones. J Neurophysiol. 1985a;53:1401–1416. doi: 10.1152/jn.1985.53.6.1401. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR. Microstimulation of the primate neostriatum .2. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985b;53:1417–1430. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Horak FB. Influence of the globus pallidus on arm movements in monkeys .3. Timing of movement-related information. J Neurophysiol. 1985;54:433–448. doi: 10.1152/jn.1985.54.2.433. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CR, Pittenger C. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Landi A, Benti R, Mariani C, De Notaris R, Marotta G, Pezzoli G, Gaini SM, Gerundini P. Functional neuroimaging (PET and SPECT) in the selection and assessment of patients with Parkinson’s disease undergoing deep brain stimulation. J Neurosurg Sci. 2003;47:40–46. [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Anatomical evidence for segregated focal groupings of efferent cells and their terminal ramifications in the cerebello-thalamic pathway of the monkey. Brain Res Rev. 1983;5:267–269. doi: 10.1016/0165-0173(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kopell BH, Malone D, Horenstein C, Lowe M, Phillips MD, Rezai AR. Deep brain stimulation for obsessive-compulsive disorder: Using functional magnetic resonance imaging and electrophysiological techniques: Technical case report. Neurosurgery. 2007;61:367–368. doi: 10.1227/01.neu.0000303995.66902.36. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2009;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215:20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Bejjani B-P, Damier P, Arnulf I, Thivard l, Bonnet A-M, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bellissimo MI, Kouzmine I, Ferro MM, Oliveira BH, Canteras NS. Is the unilateral lesion of the left substantia nigra pars compacta sufficient to induce working memory impairment in rats? Neurobiol Learn Mem. 2004;82:150–158. doi: 10.1016/j.nlm.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Deuschl G, Lang AE, Lyons KE, Rezai AR. Deep brain stimulation for Parkinson’s disease - Introduction. Mov Disorders. 2006;21:S168–S170. doi: 10.1002/mds.20954. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Hommel M, Gaio JM, Derougemont J, Perret J. Treatment of parkinsonian tremor by chronic VIM-thalamic stimulation. Revue Neurologique. 1989;145:320–323. [PubMed] [Google Scholar]
- Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2009;72:1110–1110. doi: 10.1212/01.wnl.0000346321.31603.4e. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, Delong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Behav Brain Res. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorelli R, Consolo S. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J Neurochem. 1990;54:2145–2148. doi: 10.1111/j.1471-4159.1990.tb04922.x. [DOI] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kathmann N. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bhatia K, Marsden C. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Bjarkam CR, Cancian G, Glud AN, Ettrup KS, Jorgensen RL, Sorensen JC. MRI-guided stereotaxic targeting in pigs based on a stereotaxic localizer box fitted with an isocentric frame and use of SurgiPlan computer-planning software. J Neurosci Methods. 2009;183:119–126. doi: 10.1016/j.jneumeth.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blaha CC, Phillips AA. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav Pharmacol. 1996;7:675–708. [PubMed] [Google Scholar]
- Blaha CD, Lester DB, Ramsson ES, Lee KH, Garris PA. Striatal dopamine release evoked by subthalamic stimulation in intact and 6-OHDA-lesioned rats: Relevance to deep brain stimulation in Parkinson’s disease. In: Phillips PEM, Sandberg SG, Ahn S, Phillips AG, editors. Monitoring Molecules in Neuroscience; Proceedings of the 12th International Conference on In Vivo Methods; Vancouver, Canada: University of British Columbia; 2008. pp. 395–397. [Google Scholar]
- Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, Whitlock S, Johnson DM, Horne A, Bennet KE, Lee KH, Garris PA. Development of the Wireless Instantaneous Neurotransmitter Concentration System for intraoperative neurochemical monitoring using fast-scan cyclic voltammetry Technical note. J Neurosurg. 2009;111:712–723. doi: 10.3171/2009.3.JNS081348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PAC, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgohain R, Kandadai RM, Jabeen A, Kannikannan MA. Nonmotor outcomes in Parkinson’s disease: is deep brain stimulation better than dopamine replacement therapy? Ther Adv Neurol Disord. 2012;5:23–41. doi: 10.1177/1756285611423412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolanza M, Wietzikoski EC, Boschen SL, Dombrowski P, Latimer M, MacLaren DAA, Winn P, Da Cunha C. Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task. Neurobiol Learn Mem. 2010;94:229–239. doi: 10.1016/j.nlm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen SL, Wietzikoski EC, Winn P, Da Cunha C. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol Learn Mem. 2011;96:254–262. doi: 10.1016/j.nlm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne S, Eckhardt C, Sheth S, Eskandar E. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. FNINT. 2012;6:1–14. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Schulz JB, Benabid AL. Deep brain stimulation. Cell Tissue Re. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of DA neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci. 2009;106:4893–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruet N, Windels F, Bertrand A, Feuerstein C, Poupard A, Savasta M. High-frequency stimulation of the subthalamic nucleus increases the extracellular contents of striatal DA in normal and partially dopaminergic denervated rats. J Neuropathol Exp Neurol. 2001;60:15–24. doi: 10.1093/jnen/60.1.15. [DOI] [PubMed] [Google Scholar]
- Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv Pharmacol. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond DA release in reward regions. Neuroscience. 2012;201:331–337. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliori D, Pezzulo G, Miall RC, Baldasarre G. The contribution of brain sub-cortical loops in the expression and acquisition of action understanding abilities. Neurosci Biobehav Rev. 2013;37:2504–2515. doi: 10.1016/j.neubiorev.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Ethier C, Van Vreeswijk C, Darling WG. On the functional organization and operational principles of the motor cortex. Front Neural Circuits. 2013;7 doi: 10.3389/fncir.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309:231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- Carlsson ML, Carlsson A, Nilsson M. Schizophrenia: From dopamine to glutamate and back. Curr Med Chem. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105:1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Chang S-Y, Jay T, Muñoz J, Kim I, Lee KH. Wireless fast-scan cyclic voltammetry measurement of histamine using WINCS--a proof-of-principle study. Analyst. 2012a;137:2158–2165. doi: 10.1039/c2an16038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-Y, Kim I, Marsh MP, Jang DP, Hwang S-C, Van Gompel JJ, Goerss SJ, Kimble CJ, Bennet KE, Garris PA, Blaha CD, Lee KH. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin Proc. 2012b;87:760–765. doi: 10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Xiong YY, Xu GL, Liu XF. Deep Brain Stimulation. Interv Neurol. 2013;1:200–212. doi: 10.1159/000353121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JJ, de Bruin JPC, Feenstra MGP. Dopamine efflux in nucleus accumbens shell and core in response to appetitive classical conditioning. Eur J Neurosci. 2003;18:1306–1314. doi: 10.1046/j.1460-9568.2003.02849.x. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Chopra A, Tye SJ, Lee KH, Matsumoto J, Klassen B, Adams AC, Stead M, Sampson S, Kall BA, Frye MA. Voltage-dependent mania after subthalamic nucleus deep brain stimulation in Parkinson’s disease: A case report. Biol Psychiatry. 2011;70:E5–E7. doi: 10.1016/j.biopsych.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Neill DB. Detecting behaviorally relevant changes in extracellular DA with microdialysis. Brain Res. 1987;412:397–399. doi: 10.1016/0006-8993(87)91150-4. [DOI] [PubMed] [Google Scholar]
- Cislaghi G, Capiluppi E, Saleh C, Romano L, Servello D, Mariani C, Porta M. Bilateral globus pallidus stimulation in Westphal variant of Huntington disease. Neuromodulation. 2013;17:502–505. doi: 10.1111/ner.12098. [DOI] [PubMed] [Google Scholar]
- Conte A, Fabbrini G, Belvisi D, Rothwell J, Berardelli A. Electrical activation of the orbicularis oculi muscle does not increase the effectiveness of botulinum toxin type A in patients with blepharospasm. Eur J Neurol. 2010;17:449–55. doi: 10.1111/j.1468-1331.2009.02840.x. [DOI] [PubMed] [Google Scholar]
- Cooper IS. Effect of chronic stimulation of anterior cerebellum on neurological disease. Lancet. 1973;1:206. doi: 10.1016/s0140-6736(73)90042-1. [DOI] [PubMed] [Google Scholar]
- Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- Covey DP, Ramsson ES, Heidenreich BA, Blaha CD, Lee KH, Garris PA. Monitoring subthalamic nucleus-evoked dopamine release in the rat striatum using fast-scan cyclic voltammetry in vivo. In: Phillips PEM, Sandberg SG, Ahn S, et al., editors. Monitoring Molecules in Neuroscience; Proceedings of the 12th International Conference on In Vivo Methods; Vancouver: University of British Columbia Institute of Mental Health; 2008. pp. 398–400. [Google Scholar]
- Crutcher MD, Alexander GE. Movement-related neuronal activity selectively coding either direction or muscle pattern in three motor areas of the monkey. J Neurophysiol. 1990;64:151–163. doi: 10.1152/jn.1990.64.1.151. [DOI] [PubMed] [Google Scholar]
- Cui GH, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Gillings NM, Jensen SB, Bjarkam C, Gjedde A. Kinetics of the uptake and distribution of the DA D-2,D-3 agonist (R)-N-[1-C-11]n-propylnorapomorphine in brain of healthy and MPTP-treated Gottingen miniature pigs. Nucl Med Biol. 2003;30:547–553. doi: 10.1016/s0969-8051(02)00448-1. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gevaerd MS, Vital Mabf, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS. Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav Brain Res. 2001;124:9–18. doi: 10.1016/s0166-4328(01)00211-x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gomez-A A, Blaha CD. The role of the basal ganglia in motivated behavior. Rev Neurosci. 2012;23:747–767. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Packard MG. Preface Special Issue on the role of the basal ganglia in learning and memory. Behav Brain Res. 2009;190:1–2. doi: 10.1016/j.bbr.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski EC, Ferro MM, Martinez GR, Vital M, Hipolide D, Tufik S, Canteras NS. Hemiparkinsonian rats rotate toward the side with the weaker dopaminergic neurotransmission. Behav Brain Res. 2008;189:364–372. doi: 10.1016/j.bbr.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Dall AM, Danielsen EH, Sorensen JC, Andersen F, Moller A, Zimmer J, Gjedde AH, Cumming P, DaNe XG. Quantitative [F-18]fluorodopa/PET and histology of fetal mesencephalic dopaminergic grafts to the striatum of MPTP-poisoned minipigs. Cell Transplant. 2002;11:733–746. [PubMed] [Google Scholar]
- Damsma G, Tham CS, Robertson GS, Fibiger HC. Dopamine D1 receptor stimulation increases striatal acetylcholine release in the rat. Eur J Pharmacol. 1990;186:335–338. doi: 10.1016/0014-2999(90)90456-g. [DOI] [PubMed] [Google Scholar]
- Danielsen EH, Cumming P, Andersen F, Bender D, Brevig T, Falborg L, Gee A, Gillings NM, Hansen SB, Hermansen F, Johansen J, Johansen TE, Dahl-Jorgensen A, Jorgensen HA, Meyer M, Munk O, Pedersen EB, Poulsen PH, Rodell AB, Sakoh M, Simonsen CZ, Smith DF, Sorensen JC, Ostergard L, Zimmer J, Gjedde A, Moller A. The DaNeX study of embryonic mesencephalic, dopaminergic tissue grafted to a minipig model of Parkinson’s disease: Preliminary findings of effect of MPTP poisoning on striatal dopaminergic markers. Cell Transplant. 2000;9:247–259. doi: 10.1177/096368970000900210. [DOI] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Requirement of DA signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Memory. 2011;18:136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and pavlovian reward learning. Neuroscientist. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced Akinesia. Cereb Cortex. 2009;19:1055–1063. doi: 10.1093/cercor/bhn149. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2011;69:e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- DeLong M. The neurophysiologic basis of abnormal movements in basal ganglia disorders. Neurotoxicol Teratol. 1983;5:611–616. [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement-disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider G, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J German Parkinson Study G. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Devan B, White N. Parallel information processing in the dorsal striatum: relation to hippocampal function. The J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli A, Balleine BW. Habits, action sequences and reinforcement learning. Eur J Neurosci. 2012;35:1036–1051. doi: 10.1111/j.1460-9568.2012.08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of pavlovian and instrumental incentive learning under DA antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: A case report. Mov Disorders. 2005;20:1496–1499. doi: 10.1002/mds.20551. [DOI] [PubMed] [Google Scholar]
- Dobbing J. The influence of early nutrition on the development and myelination of the brain. Proc Roy Soc. 1964;159:503. doi: 10.1098/rspb.1964.0016. [DOI] [PubMed] [Google Scholar]
- Dombrowski P, Maia TV, Boschen SL, Bortolanza M, Wendler E, Schwarting RKW, Brandao ML, Winn P, Blaha CD, Da Cunha C. Evidence that conditioned avoidance responses are reinforced by positive prediction errors signaled by tonic striatal DA. Behav Brain Res. 2013;241:112–119. doi: 10.1016/j.bbr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Dombrowski PA, Carvalho MC, Miyoshi E, Correia D, Bortolanza M, dos Santos LM, Wietzikoski EC, Eckart MT, Schwarting RKW, Brandao ML, Da Cunha C. Microdialysis study of striatal DA in MPTP-hemilesioned rats challenged with apomorphine and amphetamine. Behav Brain Res. 2010;215:63–70. doi: 10.1016/j.bbr.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Domjan M. The principles of learning and behavior. 6. Belmont, CA: Wadsworth/Cengage Learning; 2010. [Google Scholar]
- Dommett E, Coizet W, Blaha CD, Martindale J, Lefebvre W, Walton N, Mayhew JEW, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. FNBEH. 2013;7 doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, Price BH, Mayberg HS, Fischman AJ, Rauch SL. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–1017. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- Edwards TC, Zrinzo L, Limousin P, Foltynie T. Deep brain stimulation in the treatment of chorea. Mov Disord. 2012;27:357–363. doi: 10.1002/mds.23967. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Surmeier DJ. Brain networks in Huntington disease. J Clin Invest. 2011;2:484–492. doi: 10.1172/JCI45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Mazzone P, Piano C, Quaranta D, Soleti F, Bentivoglio AR. GPi-DBS in Huntington’s disease: results on motor function and cognition in a 72-year-old case. Mov Disord. 2008;23:1289–1292. doi: 10.1002/mds.22116. [DOI] [PubMed] [Google Scholar]
- Félix B, Léger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Felix B, Leger M-E, Albe-Fessard D. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–138. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci MEM, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148:78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Figee M, Koninga P, Klaassena S, Vulinka M, Mantionea M, van den Munckhofc P, Schuurmanc R, van Wingena G, van Amelsvoorta T, Booijb J, Denysa D. Deep brain stimulation induces striatal dopamine release in Obsessive-compulsive disorder. Biol psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, Van Wingen G, De Kwaasteniet B, Mantione M, Ooms P, Koning P, Vulink N, Levar N, Droge L, van den Munckhof P, Schuurman P, Nederveen A, van den Brink W, Mazaheri A, Vink M, Denys D. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, De Koning P, Vulink N, Levar N, Droge L, Van den Munckhof P, Schuurman PR, Nederveen A, Van den Brink W, Mazaheri A, Vink M. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nature Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:143–151. [PubMed] [Google Scholar]
- Fiorillo CD, Yun SR, Song MR. Diversity and homogeneity in responses of midbrain dopamine neurons. J Neurosci. 2013;33:4693–4709. doi: 10.1523/JNEUROSCI.3886-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Krishnamurthy KB, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Grp SS. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for DA in stimulus-reward learning. Nature. 2011;469:53–U63. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13:1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra-Chacón BJ, Bargas J, Tapia D, Galarraga E. Dopaminergic modulation of corticostriatal responses in medium spiny projection neurons from direct and indirect pathways. Front Syst Neurosci. 2011;5:15. doi: 10.3389/fnsys.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of DA neuron firing differentially regulates tonic and phasic DA transmission. Nature Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Follett KA. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;364:886–886. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal DA efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011;21:381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankemolle AMM, Wu J, Noecker AM, Voelcker-Rehage C, Ho JC, Vitek JL, McIntyre CC, Alberts JL. Reversing cognitive-motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain. 2010;133:746–761. doi: 10.1093/brain/awp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. International Review of Neurobiology. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, Chabardes S, Foote K, Benabid AL, Pollak P. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JT, Lee KH, Amirnovin R, Roberts DW, Williams ZM, Blaha CD, Eskandar EN. Electrical stimulation-evoked dopamine release in the primate striatum. Stereotact Funct Neurosurg. 2013;91:355–363. doi: 10.1159/000351523. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Delong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 DA receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaerd MS, Miyoshi E, Silveira R, Canteras NS, Takahashi RN, Da Cunha C. L-dopa restores striatal dopamine level but fails m to reverse MPTP-induced memory deficits in rats. Int J Neuropsychopharmacol. 2001a;4:361–370. doi: 10.1017/S1461145701002619. [DOI] [PubMed] [Google Scholar]
- Gevaerd MS, Takahashi RN, Silveira R, Da Cunha C. Caffeine reverses the memory disruption induced by intra-nigral MPTP-injection in rats. Brain Res Bull. 2001b;55:101–106. doi: 10.1016/s0361-9230(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Godinho F, Thobois S, Magnin M, Guenot M, Polo G, Benatru I, Xie J, Salvetti A, Garcia-Larrea L, Broussolle E, Mertens P. Subthalamic nucleus stimulation in Parkinson’s disease - Anatomical and electrophysiological localization of active contacts. J Neurol. 2006;253:347–1355. doi: 10.1007/s00415-006-0222-z. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Bergman H. Computational physiology of the neural networks of the primate globus pallidus: function and dysfunction. Neuroscience. 2011;198:171–192. doi: 10.1016/j.neuroscience.2011.08.068. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, Shapira NA, Wu SS, Hill CL, Rasmussen SA, Okun MS. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535–542. doi: 10.1016/j.biopsych.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic DA release and the modulation of DA system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of DA system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M. Normalizing motor-related brain activity - Subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. A bimodal map of space: somatosensory receptive fields in the macaque putamen with corresponding visual receptive fields. Exp Brain Res. 1993;97:96–109. doi: 10.1007/BF00228820. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2013;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Grillner S, Robertson B, Stephenson-Jones M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J Physiol (London) 2013;591:5425–5431. doi: 10.1113/jphysiol.2012.246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen MAM, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, Li S, Larkin DM, Kim H, Frantz LAF, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Zhan B, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi SH, Chow W, Clark RC, Clee C, Crooijmans RPMA, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du ZQ, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JGR, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu ZL, Humphray SJ, Hunt T, Hornshoj H, Jeon JT, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim JH, Kim KW, Kim TH, Larson G, Lee K, Lee KT, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, McLaren S, Morozumi T, Murtaugh MP, Narayan J, Dinh Truong N, Ni P, Oh SJ, Onteru S, Panitz F, Park EW, Park HS, Pascal G, Paudel Y, Perez-Enciso M, Ramirez-Gonzalez R, Reecy JM, Rodriguez-Zas S, Rohrer GA, Rund L, Sang Y, Schachtschneider K, Schraiber JG, Schwartz J, Scobie L, Scott C, Searle S, Servin B, Southey BR, Sperber G, Stadler P, Sweedler JV, Tafer H, Thomsen B, Wali R, Wang J, Wang J, White S, Xu X, Yerle M, Zhang G, Zhang J, Zhang J, Zhao S, Rogers J, Churcher C, Schook LB. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393–398. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulenvanderzee E, Kortschot AT, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Gruber D, Kuhn AA, Schoenecker T, Kopp UA, Kivi A, Huebl J, Lobsien E, Mueller B, Schneider GH, Kupsch A. Quadruple deep brain stimulation in Huntington’s disease, targeting pallidum and subthalamic nucleus: case report and review of the literature. J Neural Transm. 2014;121:1303–1312. doi: 10.1007/s00702-014-1201-7. [DOI] [PubMed] [Google Scholar]
- Gunduz A, Morita H, Rossi PJ, Allen WL, Alterman RL, Bronte-Stewart H, Butson CR, Charles D, Deckers S, de Hemptinne C, DeLong M, Dougherty D, Ellrich J, Foote KD, Giordano J, Goodman W, Greenberg BD, Greene D, Gross R, Judy JW, Karst E, Kent A, Kopell B, Lang A, Lozano A, Lungu C, Lyons KE, Machado A, Martens H, McIntyre C, Min H, Neimat J, Ostrem J, Pannu S, Ponce F, Pouratian N, Reymers D, Schrock L, Sheth S, Shih L, Stanslaski S, Steinke GK, Stypulkowski P, Tröster AI, Verhagen L, Walker H, Okun MS. Proceedings of the Second Annual Deep Brain Stimulation Think Tank: What’s in the Pipeline. Int J Neurosci Dec. 2014;19:1–31. doi: 10.3109/00207454.2014.999268. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WK, Ricciuti N, Ward H, Sudhyadhom A, Jacobson CE, Siddiqui MS, Okun MS. A case of mania following deep brain stimulation for obsessive compulsive disorder. Stereotact Funct Neurosurg. 2010;88:322–328. doi: 10.1159/000319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariz MI, Robertson MM. Gilles de la Tourette syndrome and deep brain stimulation. Eur J Neurosc. 2010;32(7):1128–1134. doi: 10.1111/j.1460-9568.2010.07415.x. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Boecker H, Buchel C, Vesper J, Tronnier VM, Pfister R, Alesch F, Moringlane JR, Krauss JK, Conrad B, Schwaiger M, Ceballos-Baumann AO. Differential modulation of subcortical target and cortex during deep brain stimulation. Neuroimage. 2003;18:517–524. doi: 10.1016/s1053-8119(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Hauptman JS, DeSalles AAF, Espinoza R, Sedrak M, Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg Focus. 2008;25 doi: 10.3171/FOC/2008/25/7/E3. [DOI] [PubMed] [Google Scholar]
- Hebb D. Drives and the C.N.S. (conceptual nervous system) Psychol Rev. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Hersch S, Ciliax B, Gutekunst C. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Size and shape of the Cerebellar cortex in mammals. I. The cortical surface. Brain Behav Evol. 1985;27:28–40. doi: 10.1159/000118718. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Size and shape of the cerebral cortex in mammals. II. The cortical volume. Brain Behav Evol. 1988;32:17–26. doi: 10.1159/000116529. [DOI] [PubMed] [Google Scholar]
- Holiga S, Mueller K, Moeller HE, Sieger T, Schroeter ML, Vymazal J, Ruzicka E, Jech R. Motor matters: tackling heterogeneity of Parkinson’s Disease in Functional MRI studies. Plos One. 2013;8 doi: 10.1371/journal.pone.0056133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 1991;11:2644–2654. doi: 10.1523/JNEUROSCI.11-09-02644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hu YB, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–125. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- Hyam JA, Owen SLF, Kringelbach ML, Jenkinson N, Stein JF, Green AL, Aziz TZ. Contrasting connectivity of the ventralis intermedius and ventralis oralis posterior nuclei of the motor thalamus demonstrated by probabilistic tractography. Neurosurgery. 2012;70:162–169. doi: 10.1227/NEU.0b013e3182262c9a. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, DA and D-1/D-2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens DA in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Cortico-basal ganglia mechanisms for overcoming innate, habitual and motivational behaviors. Eur J Neurosci. 2011;33:2058–2069. doi: 10.1111/j.1460-9568.2011.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Kita H. Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience. 2011;198:44–53. doi: 10.1016/j.neuroscience.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Richardson PJ. Production of adenosine from extracellular ATP at the striatal cholinergic synapse. J Neurochem. 1993;60:219–227. doi: 10.1111/j.1471-4159.1993.tb05841.x. [DOI] [PubMed] [Google Scholar]
- Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–592. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- Kahan J, Mancini L, Urner M, Friston KJ, Hariz M, Holl E, White M, Ruge D, Jahanshahi M, Boertien T, Yousry T, Thornton JS, Limousin P, Zrinzo L, Foltynie T. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson’s disease. Plos One. 2012;7:e50270. doi: 10.1371/journal.pone.0050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E, Voges J, Weber T, Haarer M, Baudrexel S, Klein JC, Kessler J, Sturm V, Heiss WD, Hilker R. Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease. Neurology. 2009;72:42–49. doi: 10.1212/01.wnl.0000338536.31388.f0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kang GA, Heath S, Rothlind J, Starr PA. Long-term follow-up of pallidal deep brain stimulation in two cases of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2011;82:272–277. doi: 10.1136/jnnp.2009.202903. [DOI] [PubMed] [Google Scholar]
- Kasasbeh A, Lee K, Bieber A, Bennet K, Chang S-Y. Wireless neurochemical monitoring in humans. Stereotact Funct Neurosurg. 2013;91:141–147. doi: 10.1159/000345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DS, Kumar R. Deep brain stimulation. Neurologist. 2007;13:237–252. doi: 10.1097/NRL.0b013e3181492c48. [DOI] [PubMed] [Google Scholar]
- Kim JP, Min HK, Knight EJ, Duffy PS, Abulseoud OA, Marsh MP, Kelsey K, Blaha CD, Bennet KE, Frye MA, Lee KH. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry. 2013;74:917–926. doi: 10.1016/j.biopsych.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble CJ, Johnson DM, Winter BA, Whitlock SV, Kressin KR, Horne AE, Robinson JC, Bledsoe JM, Tye SJ, Chang S-Y, Agnesi F, Griessenauer CJ, Covey D, Shon Y-M, Bennet KE, Garris PA, Lee KH Ieee. Wireless instantaneous neurotransmitter concentration sensing system (WINCS) for intraoperative neurochemical monitoring. 2009 Annual International Conference of the Ieee Engineering in Medicine and Biology Society; 2009. pp. 4856–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, Merz CJ, Kagerer S, Walter B, Sammer G, Vaitl D, Stark R. Neural correlates of disgust- and fear-conditioned responses. Neuroscience. 2012;201:209–218. doi: 10.1016/j.neuroscience.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Knight EJ, Min H-K, Hwang S-C, Marsh MP, Paek S, Kim I, Felmlee JP, Abulseoud OA, Bennet KE, Frye MA, Lee KH. Nucleus Accumbens Deep Brain Stimulation Results in Insula and Prefrontal Activation: A Large Animal fMRI Study. Plos One. 2013;8 doi: 10.1371/journal.pone.0056640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton B, Mangels J, Squire L. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscie Biobehav Rev. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: Implications for DBS in psychiatry. Neuroscience and Biobehavioral Reviews. 2008;32:408–422. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Agosta F, Petrovic I, Galantucci S, Spica V, Jecmenica-Lukic M, Filippi M. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75:857–863. doi: 10.1212/WNL.0b013e3181f11c1d. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–627. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neurosci. 2012;15:816–U823. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brücke C, Doyle GL, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neuroscie. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Bartsch C, Lenartz D, Huys D, Daumann J, Woopen C, Hunsche S, Maarouf M, Klosterkotter J, Sturm V. Clinical effectiveness of unilateral deep brain stimulation in Tourette syndrome. Transl Psychiatr. 2011;1:e52. doi: 10.1038/tp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Mai JK, Huff W, Lee S-H, Koulousakis A, Klosterkoetter J, Sturm V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007;254:963–965. doi: 10.1007/s00415-006-0404-8. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Moller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TOJ, Maarouf M, Brosig A, Barnikol UB, Klosterkotter J, Sturm V. Deep brain stimulation of the NAc and its usefulness in severe opioid addiction. Mol Psychiatr. 2014;19:145–146. doi: 10.1038/mp.2012.196. [DOI] [PubMed] [Google Scholar]
- Kurata K. Activity properties and location of neurons in the motor thalamus that project to the cortical motor areas in monkeys. J Neurophysiol. 2005;94:550–566. doi: 10.1152/jn.01034.2004. [DOI] [PubMed] [Google Scholar]
- La Lumiere RT, Nawar EM, McGaugh JL. Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent DA receptor activation in both brain regions. Learn Memory. 2005;12:296–301. doi: 10.1101/lm.93205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HY, Younce JR, Albaugh DL, Kao YC, Shih YY. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage. 2013;84:11–18. doi: 10.1016/j.neuroimage.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Lak A, Stauffer WR, Schultz W. Dopamine prediction error responses integrate subjective value from different reward dimensions. PNAS. 2014;111:2343–2348. doi: 10.1073/pnas.1321596111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakie M, Arblaster LA, Roberts RC, Varma TR. Effect of stereotactic thalamic lesion on essential tremor. Lancet. 1992;340:206–207. doi: 10.1016/0140-6736(92)90470-n. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Le Jeune F, Peron J, Grandjean D, Drapier S, Haegelen C, Garin E, Millet B, Verin M. Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. Eur J Nucl Med Mol Imaging. 2010;37:1512–1520. doi: 10.1007/s00259-010-1436-y. [DOI] [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Garris PA, Mohseni P, Horne AE, Bennet KE, Agnesi F, Bledsoe JM, Lester DB, Kimble C, Min HK, Kim YB, Cho ZH. Evolution of Deep Brain Stimulation: Human Electrometer and Smart Devices Supporting the Next Generation of Therapy. Neuromodulation. 2009;12:85–103. doi: 10.1111/j.1525-1403.2009.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson’s disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kristic K, van Hoff R, Hitti FL, Blaha C, Harris B, Roberts DW, Leiter JC. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Leisman G, Braun-Benjamin O, Melilo R. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci. 2014;8:1–18. doi: 10.3389/fnsys.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Li SSY, McNally GP. The conditions that promote fear learning: Prediction error and Pavlovian fear conditioning. Neurobiology of Learning and Memory. 2014;108:14–21. doi: 10.1016/j.nlm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci. 2012;16:467–475. doi: 10.1016/j.tics.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Lang AE. The nonmotor symptoms of parkinson’s disease-an overview. Mov Disorders. 2010;25:S123–S130. doi: 10.1002/mds.22786. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Alim-Louis B. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. Neurosci Biobehav Rev. The use of pigs in neuroscience: modeling brain disorders. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre J. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned-stimulus. J Exp Psychol-Anim Behav. 1983;9:225–247. [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Roessner V, Muenchau A, Mueller-Vahl K. Tourette syndrome and other tic disorders in childhood, adolescence and adulthood. Dtsch Arztebl Int. 2012;109:821–U828. doi: 10.3238/arztebl.2012.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B Pelissolo ASTOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–34. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- Mallet L, Schuepbach M, N’Diaye K, Remy P, Bardinet E, Czernecki V, Welter M-L, Pelissolo A, Ruberg M, Agid Y, Yelnik J. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manago F, Castellano C, Oliverio A, Mele A, De Leonibus E. Role of DA receptors subtypes, D1-like and D2-like, within the nucleus accumbens subregions, core and shell, on memory consolidation in the one-trial inhibitory avoidance task. Learn Memory. 2009;16:46–52. doi: 10.1101/lm.1177509. [DOI] [PubMed] [Google Scholar]
- Martinez-Ramirez D, Morishita T, Zeilman PR, Zhongxing PC, Foote KD, Okun MS. Atrophy and other potential factors affecting long-term deep brain stimulation response: a case series. PLoS ONE. 2014;9:1–6. doi: 10.1371/journal.pone.0111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Dulla CG. Adenosine, glutamate and pH: interactions and implications. Neurological Research. 2005;27:149–152. doi: 10.1179/016164105X21850. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of DA neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:838–842. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective Deep Brain Stimulation Suppresses Low-Frequency Network Oscillations in the Basal Ganglia by Regularizing Neural Firing Patterns. J Neurosci. 2012;32:15657–15668. doi: 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38:329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, Kupsch A. Deep brain stimulation of subthalamic neurons increases striatal DA metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2002;328:105–108. doi: 10.1016/s0304-3940(02)00463-9. [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Reese R, Paul G, Reum T, Ansorge M, Kusserow H, Winter C, Morgenstern R, Kupsch A. High-frequency stimulation of the subthalamic nucleus enhances striatal DA release and metabolism in rats. J Neurochem. 2003;85:601–609. doi: 10.1046/j.1471-4159.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- Meissner W, Reum T, Paul G, Harnack D, Sohr R, Morgenstern R, Kupsch A. Striatal dopaminergic metabolism is increased by deep brain stimulation of the subthalamic nucleus in 6-hydroxydopamine lesioned rats. Neurosci Lett. 2001;303:165–168. doi: 10.1016/s0304-3940(01)01758-x. [DOI] [PubMed] [Google Scholar]
- Menalled L, Zanjani H, MacKenzie L, Koppel A, Carpenter E, Zeitlin S, Chesselet MF. Decrease in striatal enkephalin mRNA in mouse models of Huntington’s disease. Exp Neurol. 2000;162:328–342. doi: 10.1006/exnr.1999.7327. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J Pharm Biomed Anal. 1999;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey FR, Knight RT, Ojemann JG, Fetz EE. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8(9):e1002655. doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, DeLong M. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. Adv Behav Biol. 1987;32:415–427. [Google Scholar]
- Min HK, Hwang SC, Marsh MP, Kim I, Knight E, Striemer B, Felmlee JP, Welker KM, Blaha CD, Chang SY, Bennet KE, Lee KH. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage. 2012;63:1408–1420. doi: 10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H-K, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, Marsh MP, Striemer B, Felmlee JP, Luis Lujan J, Goerss S, Duffy PS, Blaha C, Chang S-Y, Bennet KE. Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: Use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul: Basic, Translational, and Clinical Research in Neuromodulation. 2014 doi: 10.1016/j.brs.2014.04.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain DA neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J. Memories and habits: Two neural systems. Guilford; New York: 1982. [Google Scholar]
- Mitchell SJ, Richardson RT, Baker FH, Delong MR. The primate globus-pallidus - neuronal-activity related to direction of movement. Exp Brain Res. 1987;68:491–505. doi: 10.1007/BF00249793. [DOI] [PubMed] [Google Scholar]
- Miwa H, Fuwa T, Nishi K, Kondo T. Subthalamo-pallido-striatal axis: a feedback system in the basal ganglia. Neuroreport. 2001;12:3795–3798. doi: 10.1097/00001756-200112040-00039. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C. Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull. 2002;58:41–47. doi: 10.1016/s0361-9230(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action - functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, Ferrer E. Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol. 2000;57:983–988. doi: 10.1001/archneur.57.7.983. [DOI] [PubMed] [Google Scholar]
- Moreno J, Garcia-Caldentey J, Regidor I, del Alamo M, de Yebenes JG. A 5-year follow-up of deep brain stimulation in Huntington’s disease. Parkinsonism Relat Disord. 2014;20:260–261. doi: 10.1016/j.parkreldis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Moro E, Lang AE, Strafella AP, Poon YYW, Arango PM, Dagher A, Hutchison WD, Lozano AM. Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol. 2004;56:290–4. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Keller CE, Gangitano M, Ly J, Thall M, Parker JA, Pascual-Leone A. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res Neuroimaging. 2002;115:1–14. doi: 10.1016/s0925-4927(02)00032-x. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247:1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Nambu A. Somatotopic organization of the primate basal ganglia. Frontiers in Neuroanatomy. 2011;5 doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Neuner I, Kupriyanova Y, Stoecker T, Huang R, Posnansky O, Schneider F, Tittgemeyer M, Shah NJ. White-matter abnormalities in Tourette syndrome extend beyond motor pathways. Neuroimage. 2010;51:1184–1193. doi: 10.1016/j.neuroimage.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Neuner I, Podoll K, Janouschek H, Michel TM, Sheldrick AJ, Schneider F. From psychosurgery to neuromodulation: Deep brain stimulation for intractable Tourette syndrome. World J Biol Psychia. 2009;10:366–376. doi: 10.1080/15622970802513317. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Sig Transd. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology. 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens DA release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10:375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels JM, Gielen F, Demeulemeester HG. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;62:966–975. doi: 10.1227/01.neu.0000333764.20575.d6. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Guridi J, Nambu A, Crossman AR. Motor manifestations and basal ganglia output activity: The paradox continues. Mov Disorders. 2013;28:416–418. doi: 10.1002/mds.25358. [DOI] [PubMed] [Google Scholar]
- Odekerken VJJ, van Laar T, Staal MJ, Mosch A, Hoffmann CFE, Nijssen PCG, Beute GN, van Vugt JPP, Lenders MWPM, Contarino MF, Mink MSJ, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RMA. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurology. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, Pahwa R, Lyons KE, Tröster AI, Vitek JL, Tagliati M SJM DBS Study Group. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. The Lancet Neurology. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- Okun MS. Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med. 2012;367:1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, Malaty IA, Goodman WK, Gilbert DM, Walker HC, Mink JW, Merritt S, Morishita T, Sanchez JC. A trial of scheduled deep brain stimulation for tourette syndrome moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70:85–94. doi: 10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishijo H, Nishino H. Functional role of the limbic system and basal ganglia in motivated behavior. J Neurol. 2000;247:23–32. doi: 10.1007/pl00007780. [DOI] [PubMed] [Google Scholar]
- Ouachikh O, Dieb W, Durif F, Hafidi A. Differential behavioral reinforcement effects of DA receptor agonists in the rat with bilateral lesion of the posterior ventral tegmental area. Behav Brain Res. 2013;252:24–31. doi: 10.1016/j.bbr.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Sgambato-Faure V, Melon C, Kachidian P, Gubellini P, Amri M, Goff LKL, Salin P. High-frequency stimulation of the subthalamic nucleus potentiates L-DOPA-induced neurochemical changes in the striatum in a rat model of Parkinson’s disease. J Neurosci. 2007;27:2377–2386. doi: 10.1523/JNEUROSCI.2949-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo A, Delgado A, Querejeta E. Differential inhibition of globus pallidus neurons by electrical or chemical stimulation of the striatum. Neuroscience Research. 2008;62:240–245. doi: 10.1016/j.neures.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pajski ML, Venton BJ. The mechanism of electrically stimulated adenosine release varies by brain region. Purinergic Signal. 2013;9:167–174. doi: 10.1007/s11302-012-9343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA, Jr, Anand A. Where in the Brain Is Depression? Curr Psychiatry Rep. 2012;14:634–642. doi: 10.1007/s11920-012-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-Amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol. 2006;5:578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Paschali A, Constantoyannis C, Angelatou F, Vassilakos P. Perfusion brain SPECT in assessing motor improvement after deep brain stimulation in Parkinson’s disease. Acta Neurochir. 2013;155:497–505. doi: 10.1007/s00701-012-1610-z. [DOI] [PubMed] [Google Scholar]
- Paul G, Reum T, Meissner W, Marburger A, Sohr R, Morgenstern R, Kupsch A. High-frequency stimulation of the subthalamic nucleus influences striatal dopaminergic metabolism in the naive rat. Neuroreport. 2000;11:441–444. doi: 10.1097/00001756-200002280-00003. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. Oxford Univ Press; London: 1927. [Google Scholar]
- Percheron G, Filion M. Parallel processing in the basal ganglia: up to a point. Trends Neurosci. 1991;14:55–59. doi: 10.1016/0166-2236(91)90020-u. [DOI] [PubMed] [Google Scholar]
- Perry JC, Da Cunha C, Anselmo-Franci J, Andreatini R, Miyoshi E, Tufik S, Vital Mabf. Behavioural and neurochemical effects of phosphatidylserine in MPTP lesion of the substantia nigra of rats. Eur J Pharmacol. 2004;484:225–233. doi: 10.1016/j.ejphar.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang HP, Gatenby C, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Phillips JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: Roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, Rezai AR. Parkinson disease: Pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus - Initial experience. Radiology. 2006;239:209–216. doi: 10.1148/radiol.2391041990. [DOI] [PubMed] [Google Scholar]
- Pisa M. Motor functions of the striatum in the rat - critical role of the lateral region in tongue and forelimb reaching. Neuroscience. 1988;24:453–463. doi: 10.1016/0306-4522(88)90341-7. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Juckel G, Mavrogiorgou P, Mulert C, Folkerts M, Hauke W, Zaudig M, Möller HJ, Hegerl U. Symptom-specific EEG power correlations in patients with obsessive-compulsive disorder. International J Psychophysiol. 2006;62:87–92. doi: 10.1016/j.ijpsycho.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Prince M, Patel V, Shekhar S, Maj M, Maselko J, Phillips MR, Rahman A. Global mental health 1 - No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passinghm RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23:777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Rane P, King JA. Exploring aversion in an animal model of pre-motor stage Parkinson’s disease. Neuroscience. 2011;181:189–195. doi: 10.1016/j.neuroscience.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, Alpert NM, Haber SN, Stypulkowski PH, Rise MT, Rasmussen SA, Greenberg BD. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic DA signals? Brain Res Rev. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JNJ. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. Cognitive maps beyond the hippocampus. Hippocampus. 1997;7:15–35. doi: 10.1002/(SICI)1098-1063(1997)7:1<15::AID-HIPO3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Reijnders JSAM, Scholtissen B, Weber WEJ, Aalten P, Verhey FRJ, Leentjens AFG. Neuroanatomical correlates of apathy in parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov Disorders. 2010;25:2318–2325. doi: 10.1002/mds.23268. [DOI] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Behav Brain Res. 1998;28:235–285. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between DA D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Riedel G, Harrington NR, Hall G, Macphail EM. Nucleus accumbens lesions impair context, but not cue, conditioning in rats. Neuroreport. 1997;8:2477–2481. doi: 10.1097/00001756-199707280-00013. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurgery and Psychiatry. 2004;75:1382–1385. doi: 10.1136/jnnp.2003.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004;75:1382–1385. doi: 10.1136/jnnp.2003.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FHM, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Fumagalli M, Giannicola G, Marceglia S, Lucchiari C, Servello D, Franzini A, Pacchetti C, Romito L, Albanese A, Porta M, Pravettoni G, Priori A. Pathological gambling in Parkinson’s disease: subthalamic oscillations during economics decisions. Mov Disord. 2013;28:1644–1652. doi: 10.1002/mds.25427. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang LC, Yin HH. Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One. 2013;8:e65799. doi: 10.1371/journal.pone.0065799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, McIntyre CC, Turner RS, Wichmann T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. Eur J Neurosci. 2012;36:2213–2228. doi: 10.1111/j.1460-9568.2012.08108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Ryapolova-Webb E, Afshar P, Stanslaski S, Denison T, de Hemptinne C, Bankiewicz K, Starr PA. Chronic cortical and electromyographic recordings from a fully implantable device: preclinical experience in a nonhuman primate. J Neural Eng. 2014;11(1):016009. doi: 10.1088/1741-2560/11/1/016009. [DOI] [PubMed] [Google Scholar]
- Saikali S, Meurice P, Sauleau P, Eliat PA, Bellaud P, Randuineau G, Verin M, Malbert CH. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Methods. 2010;192:102–109. doi: 10.1016/j.jneumeth.2010.07.041. [DOI] [PubMed] [Google Scholar]
- Saleem . Atlas of the Rhesus Monkey Brain. Academic Press; London: 2007. [Google Scholar]
- Saleh C, Gonzalez V, Cif L, Coubes P. Deep brain stimulation of the globus pallidus internus and Gilles de la Tourette syndrome: Toward multiple networks modulation. Surg NeurolInternational. 2012;3:S127–142. doi: 10.4103/2152-7806.95424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E, Schwarz C, Chase K, Bhide PG, Young AB, Penney J, Vonsattel JP, Aronin N, DiFiglia M. Huntingtin localization in brains of normal and Huntington’s disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:E9–E11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Savica R, Stead M, Mack KJ, Lee KH, Klassen BT. Deep Brain Stimulation in Tourette Syndrome: A Description of 3 Patients With Excellent Outcome. Mayo Clin Proc. 2013;87:59–62. doi: 10.1016/j.mayocp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;22(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Haelbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Courbon CB, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Krueger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, Grp ES. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral DA signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurgery and Psychiatry. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: A 4-year follow-up study with rCBF SPECT. Journal of Nuclear Medicine. 2005;46:1444–1454. [PubMed] [Google Scholar]
- Shah RS, Chang SY, Min HK, Cho ZH, Blaha CD, Lee KH. Deep brain stimulation: technology at the cutting edge. Journal of Clinical Neurology. 2010;6:167–182. doi: 10.3988/jcn.2010.6.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, Springer US, Lang PJ, Greenberg BD, Haber SN, Goodman WK. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410–2. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Zetterström T, Ungerstedt U. An in vivo study of DA release and metabolism in rat brain regions using intracerebral dialysis. J Neurochem. 1986;47:113–122. doi: 10.1111/j.1471-4159.1986.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Shon YM, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Blaha CD, Lee KH. Comonitoring of adenosine and DA using the wireless instantaneous neurotransmitter concentration system: proof of principle. J Neurosurg. 2010a;112:539–548. doi: 10.3171/2009.7.JNS09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon YM, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, Bennet K, Blaha CD, Chang SY. High-frequency stimulation of the subthalamic nucleus evokes striatal DA release in a large animal model of human DBS neurosurgery. Neurosci Lett. 2010b;475:136–140. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Minzer K. Neurobiology of Tourette’s syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev-JPN. 2003;25:S70–S84. doi: 10.1016/s0387-7604(03)90012-x. [DOI] [PubMed] [Google Scholar]
- Singer HS, Reiss AL, Brown JE, Aylward EH, Shih B, Chee E, Harris EL, Reader MJ, Chase GA, Bryan RN, Denckla MB. Volumetric MRI changes in basal ganglia of children with Tourette’s syndrome. Neurology. 1993;43:950–956. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPT P-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I-U, Kim Y-D, Cho H-J, Chung S-W, Chung Y-A. An FP-CIT PET Comparison of the differences in dopaminergic neuronal loss between idiopathic Parkinson’s disease with dementia and without dementia. Alzheimer Dis Assoc Disord. 2013;27:51–55. doi: 10.1097/WAD.0b013e31824acd84. [DOI] [PubMed] [Google Scholar]
- Song IU, Park JW, Chung SW, Chung YA. Brain SPECT can differentiate between essential tremor and early-stage tremor-dominant Parkinson’s disease. J Clin Neurosci. 2014 doi: 10.1016/j.jocn.2013.11.035. in press. [DOI] [PubMed] [Google Scholar]
- Spielberger S, Hotter A, Wolf E, Eisner W, Muller J, Poewe W, Seppi K. Deep brain stimulation in Huntington’s disease: A 4-year follow-up case report. Mov Disord. 2012;27:806–807. doi: 10.1002/mds.24959. [DOI] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of stri atal cells originating the indirect pathway. Exp Neurol. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, Saint-Cyr J, Lozano A. Deep brain stimulation for Parkinson’s disease dissociates mood and motor circuits: A functional MRI case study. Mov Disorders. 2003;18:1508–1516. doi: 10.1002/mds.10593. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Janak PH. Establishing causality for DA in neural function and behavior with optogenetics. Brain Res. 2013;1511:46–64. doi: 10.1016/j.brainres.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, DA neurons and learning. Nature Neurosci. 2013;16:966–U248. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof J, Kebabian J. Two DA receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF. In vivo monitoring of DA overflow in the central nervous system by amperometric techniques combined with carbon fibre electrodes. Methods. 2004;33:322–329. doi: 10.1016/j.ymeth.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ, Wharen RE. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5:68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ. To go or not to go. Nature. 2013;494:178–179. doi: 10.1038/nature11856. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, Bargas J. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience. 2011;198:3–18. doi: 10.1016/j.neuroscience.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 DA-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of DA receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: An integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Swamy BE, Venton BJ. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal Chem. 2007;79:744–50. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- Sweet JA, Walter BL, Gunalan K, Chaturvedi A, McIntyre CC, Miller JP. Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: implications for targeting in deep brain stimulation. J Neurosurg. 2014;120:988–996. doi: 10.3171/2013.12.JNS131537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nature Neurosci. 2012;15:1281–U1152. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker RR. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49:145–153. doi: 10.1016/s0090-3019(97)00459-x. [DOI] [PubMed] [Google Scholar]
- Thill S, Cagliori D, Borghi AM, Ziemke T, Baldassarre G. Theories and computational models of affordance and mirror systems: an integrative review. Neurosci Biobehav Rev. 2013;37:491–521. doi: 10.1016/j.neubiorev.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by DA neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neuropsysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye SJ, Frye MA, Lee KH. Disrupting disordered neurocircuitry: treating refractory psychiatric illness with neuromodulation. Mayo Clin Proc. 2009;84:522–532. doi: 10.4065/84.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Van Donkelaar P, Stein JF, Passingham RE, Miall RC. Neuronal activity in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 1999;82:934–945. doi: 10.1152/jn.1999.82.2.934. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Bower MR, Worrell GA, Stead M, Chang SY, Goerss SJ, Kim I, Bennet KE, Meyer FB, Marsh WR, Blaha CD, Lee KH. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55:233–244. doi: 10.1111/epi.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: Olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- van Hartevelt TJ, Cabral J, Deco G, Møller A, Green AL, Aziz TZ, Kringelbach ML. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. PLoS One. 2014;9:e86496. doi: 10.1371/journal.pone.0086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Anal Chem. 2002;74:539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Pyschiatry. 2013;84:1029–1042. doi: 10.1136/jnnp-2011-301714. [DOI] [PubMed] [Google Scholar]
- Videnovic A, Metman LV. Deep brain stimulation for Parkinson’s disease: Prevalence of adverse events and need for standardized reporting. Movement Disorders. 2008;23:343–349. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Ashe J, Delong MR, Alexander GE. Physiologic properties and somatotopic organization of the primate motor thalamus. J Neurophysiol. 1994;71:1498–1513. doi: 10.1152/jn.1994.71.4.1498. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Ashe J, DeLong MR, Kaneoke Y. Microstimulation of primate motor thalamus: Somatotopic organization and differential distribution of evoked motor responses among subnuclei. J Neurophysiol. 1996;75:2486–2495. doi: 10.1152/jn.1996.75.6.2486. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren L, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG. Conditioned avoidance response in the development of new antipsychotics. Curr Pharm Des. 2010;16:358–370. doi: 10.2174/138161210790170085. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai E, Duda J, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang G Group CS. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, Grp CSPS. Randomized trial of deep brain stimulation for Parkinson disease Thirty-six-month outcomes. Neurology. 2012;79:55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Burbaud P, Fernandez-Vidal S, Bardinet E, Coste J, Piallat B, Borg M, Besnard S, Sauleau P, Devaux B, Pidoux B, Chaynes P, du Montcel ST, Bastian A, Langbour N, Teillant A, Haynes W, Yelnik J, Karachi C, Mallet L, French Stimulation T. Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl Psychiatry. 2011;1 doi: 10.1038/tp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, Navarro S, Pidoux B, Dormont D, Bardinet E, Yelnik J, Damier P, Agid Y. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65:952–957. doi: 10.1001/archneur.65.7.952. [DOI] [PubMed] [Google Scholar]
- Wendler E, Gaspar JCC, Ferreira TL, Barbiero JK, Andreatini R, Vital M, Blaha CD, Winn P, Da Cunha C. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: Performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol Learn Memory. 2014;109:27–36. doi: 10.1016/j.nlm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- West MO, Carelli RM, Pomerantz M, Cohen SM, Gardner JP, Chapin JK, Woodward DJ. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990;64:1233–1246. doi: 10.1152/jn.1990.64.4.1233. [DOI] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Human Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPT P-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong M. Deep-brain stimulation for basal ganglia disorders. Basal Ganglia. 2011;1:65–77. doi: 10.1016/j.baga.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzikoski EC, Boschen SL, Miyoshi E, Bortolanza M, Santos LM, Frank M, Brandão ML, Winn P, Da Cunha C. Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance response. Psychopharmacology. 2012;219:159–169. doi: 10.1007/s00213-011-2384-3. [DOI] [PubMed] [Google Scholar]
- Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123:4546–4556. doi: 10.1172/JCI68341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DIG, MacLaren DAA, Winn P. Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine. Eur J Neurosci. 2009;30:504–513. doi: 10.1111/j.1460-9568.2009.06836.x. [DOI] [PubMed] [Google Scholar]
- Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005;20:2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the dorsal subiculum of the rat. Distinct neuronal zones project to different cortical and subcortical targets. Eur J Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Rajan R, Joshua M, Doupe AJ. Emergence of context-dependent variability across a basal ganglia network. Neuron. 2014;82:208–223. doi: 10.1016/j.neuron.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woopen C, Pauls KAM, Koy A, Moro E, Timmermann Lars. Early application of deep brain stimulation: Clinical and ethical aspects. Prog Neurobiol. 2013;110:74–88. doi: 10.1016/j.pneurobio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Gerardin E, Hartmann A, Valabregue R, Chupin M, Tremblay L, Vidailhet M, Colliot O, Lehericy S. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010;133:3649–3660. doi: 10.1093/brain/awq293. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Malherbe C, Hartmann A, Pelegrini-Issac M, Messe A, Vidailhet M, Lehericy S, Benali H. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond DA in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce JR, Albaugh DL, Shih YY. Deep brain stimulation with simultaneous FMRI in rodents. J Vis Exp. 2014:e51271. doi: 10.3791/51271. [DOI] [PMC free article] [PubMed] [Google Scholar]