Abstract
Lysozyme is a naturally occurring enzyme found in bodily secretions such as tears, saliva, and milk. It functions as an antimicrobial agent by cleaving the peptidoglycan component of bacterial cell walls, which leads to cell death. Antibiotics are also antimicrobials and have been fed at subtherapeutic levels to swine as growth promoters. These compounds benefit swine producers by minimizing production losses by increasing feed efficiency and decreasing susceptibility to bacterial infection and disease. This manuscript reviews the knowledge of the effects of lysozyme, as compared to traditional subtherapeutic antibiotics in swine feed, on pig performance and health. It is clear from decades of studies that antibiotic use in feeds increases pig performance, particularly in the nursery. Similarly, lysozyme, as a feed additive, increases growth and feed efficiency. While the mechanism by which antibiotics and lysozyme improve performance is not clearly understood, both of these feed additives improve gastrointestinal health, improve the metabolic profile, and alter the gastrointestinal bacteria ecology of swine. Therefore, lysozyme is a suitable alternative to growth-promoting subtherapeutic antibiotic use in swine feed.
Keywords: Antibiotics, Gastrointestinal, Lysozyme, Microbiota, Review, Swine
Introduction
Antimicrobials have been fed at subtherapeutic levels to swine as growth promoters for more than 60 years, and the majority of pigs produced in the U.S. receive antimicrobials in their feed at some point in their production cycle. These compounds benefit swine producers by minimizing production losses by increasing feed efficiency and decreasing susceptibility to bacterial infection and disease [1]. Wells et al. [2] observed 62 % prevalence for Salmonella in swine prior to the growing phase of production, and this number decreased to less than 15 % after 8 weeks on diets containing chlortetracycline, a broad based antimicrobial. In addition, increased Campylobacter shedding is associated with reduced performance in growing pigs [3]. Therefore, a reduction in pathogen shedding due to antibiotic use appears to be associated with increased animal performance. However, in recent years, foreign and domestic markets have been pressuring swine producers to reduce or remove antimicrobials from their diets.
Lysozyme is a 1,4-β-N-acetylmuramidase that enzymatically cleaves a glycosidic linkage in the peptidoglycan component of bacterial cell walls, which results in the loss of cellular membrane integrity and cell death [4]. In addition, hydrolysis products are capable of enhancing immunoglobulin A (IgA) secretion, macrophage activation, and rapid clearance of bacterial pathogens [5, 6]. These data indicate that lysozyme may be a viable alternative to antibiotics in diets fed to swine.
Until recently, the literature pertaining to lysozyme as a feed additive was limited to studies using transgenic vectors to deliver lysozyme. These studies have shown changes in metabolite profiles [7], intestinal microbiota [8], and intestinal morphology [9] in pigs fed milk from transgenic goats expressing human lysozyme in their mammary gland. In addition, Humphrey et al. [10], reported that diets supplemented with transgenic rice expressing lysozyme had antibiotic-like properties when fed to chicks. While these reports are encouraging, the delivery of lysozyme from transgenic goats’ milk or transgenic rice is problematic in a swine production setting. However, recent research with egg-white lysozyme showed a performance benefit when fed to young pigs [11–13].
Lysozyme sources and current use
Before discovering penicillin, Alexander Fleming discovered the enzyme lysozyme based on the ability of nasal secretions to prohibit bacterial growth [14]. Lysozyme is a naturally occurring enzyme found in bodily secretions such as tears, saliva, and milk. It functions as an antimicrobial by enzymatically cleaving a glycosidic linkage of bacterial cell walls peptidoglycan, which leads to cell death [4]. Lysozyme is found in many biological organisms from bacteria and fungi to animal bodily secretions and tissues [15, 16]. Lysozyme is an important defense mechanism and is considered a part of the innate immune system in most mammals [17], and is also an important component of human breast milk [18]. However, due to its very low concentration in sow milk (<0.065 μg/mL), lysozyme is not believed to play a major role in the prevention of infection in suckling pigs.
In vitro, lysozyme is generally considered effective against some Gram-positive bacteria, but ineffective against Gram-negative bacteria [19]. However, lysozyme, perhaps indirectly, can affect Gram-negative bacteria in vivo [11, 20]. Due to these antimicrobial properties, lysozyme has been used effectively in the food industry [21]. For example, it has been used in the cheese industry to prevent late blowing [22, 23]. Lysozyme has also been used as a preservative for other fresh foods [19], including controlling meat spoilage [24].
Lysozyme is not currently used extensively as a feed additive in the animal industry. However, its effectiveness on pigs has been evaluated in different models. Until recently, the literature pertaining to lysozyme as a feed additive was limited to studies using milk from transgenic organisms or transgenic rice to produce and deliver the enzyme. Human lysozyme has been expressed in the milk of pigs [25], mice [26], and goats [8] as models for human medicine. Subsequent studies using transgenic goats’ milk suggested that lysozyme could be used as a feed antimicrobial. These studies have shown changes in metabolite profiles [7], intestinal microbiota [8], and intestinal morphology [9] in pigs fed milk from transgenic goats expressing human lysozyme in the mammary gland. Diets supplemented with transgenic rice expressing human lysozyme also improved the performance of chicks [10]. These experiments were not designed to evaluate lysozyme as a feed additive. However, results from recent experiments have shown that lysozyme sourced from chicken eggs (Neova Technologies; Abbotsford, Canada) improved growth rate and intestinal morphology and reduced Campylobacter shedding in both 10-day-old pigs consuming a milk diet [11] as well as nursery pigs [12, 13, 20]. In addition, Nyachoti et al. [27] reported the same source of lysozyme alleviated the piglet response to an oral challenge of Escherichia coli K88.
Lysozyme as a feed additive
Performance
The use of antibiotics in livestock feed is well established and can improve growth rates in several species, including swine [28–30]. The most important phenotypes for any antimicrobial feed additives are weight gain and feed efficiency. Studies using human lysozyme from transgenic goats’ milk did not show an improvement in growth of pigs consuming human lysozyme [8, 9]. This was likely due to the experimental design in these experiments as they were not conducted to evaluate the effect of lysozyme on pig performance. In these experiments, growth improvement due to lysozyme was likely masked due to the presence of antibiotics in both the control and the experimental diet [9]. Presumably, Maga et al. [8] fed diets that included antibiotics also. In addition, both Brudige et al. [9] and Maga et al. [8] fed dry, pelleted nursery diets in addition to the lysozyme-containing goats’ milk. Thus, it is unclear how much lysozyme was consumed by pigs in relation to the dry diets in these studies. Due to the changes in intestinal morphology and microflora, the pigs consumed a significant amount of lysozyme, but this amount may not have been sufficient to impact growth rate. Humphrey et al. [10] fed 152 mg human lysozyme (produced from transgenic rice) per kg feed, but did not improve the growth rate of chicks. However, the chicks had significantly improved feed efficiency over those reared on a diet containing neither the transgenic protein nor antibiotics.
Lysozyme sourced from chicken eggs improves growth performance comparable to neomycin/oxytetracycline (milk diets; [11]), carbadox/copper sulfate (nursery diets; [12]) or chlortetracycline/tiamulin hydrogen fumarate (nursery diets; [13]) compared with pigs consuming a nonmedicated diet (Fig. 1). Due to the study design, feeding group-housed pigs a milk diet, May et al. [11] did not have the statistical power to detect changes in feed efficiency. However, Oliver and Wells [12] and Oliver et al. [13] were the first examples of lysozyme improving feed efficiency in swine, where pigs consuming lysozyme had an improved feed efficiency of about 8 % compared with pigs consuming the untreated diet, which was similar to pigs consuming the antibiotic-treated feeds (Fig. 1).
Fig. 1.
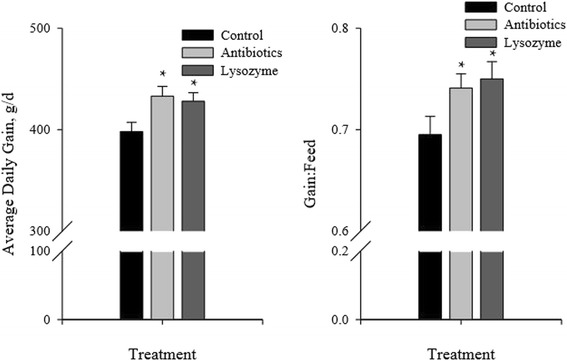
Average daily gain and feed efficiency of nursery pigs consuming control (non-mediated), control + antibiotics, or control + lysozyme diets for 28 days. Nursery pigs consuming lysozyme or antibiotics gained weight approximately 8 % faster. In addition, pigs consuming either lysozyme or antibiotics had improved feed efficiency of approximately 7 %. These data were adapted from Oliver and Wells [12] and Oliver et al. [13]. *Mean differs from control (P < 0.05)
Gastrointestinal tract
Improved villus height and crypt depth in the small intestine generally indicates improved intestinal health [31–33]. However, due to the already rapidly changing gross morphology in nursery pigs due to weaning, observed changes in intestinal morphology due to dietary subtherapeutic antibiotic are variable. Studies have shown that some antibiotics improve morphology [12, 34] whereas others do not [30, 35]. Previous work with human lysozyme from transgenic goats’ milk or transgenic rice did not show improvements in intestinal morphology in the jejunum or ileum [9, 10, 36]. Cooper et al. [36] did show a tendency for lysozyme to increase duodenal villi height and observed a decrease in lamina propria thickness. Similar to the lack of improvement in growth performance in these studies, the lack of morphology response is likely due to the concomitant presence of antibiotics in the feed, or simply a lower consumption of lysozyme.
May et al. [11] and Oliver and Wells (Fig. 2; [12]) both observed increased villus heights and crypt depths, indicating improved intestinal health. However, the major morphological responses in pigs consuming lysozyme or antibiotics in liquid diets was observed in the ileum [11] compared with responses seen exclusively in the jejunum by Oliver and Wells [12]. Presumably, this is due to the different physical forms of the diets consumed. Major changes occur in the gastrointestinal tract in response to the transition from a liquid to dry diet [37], in particular to ion transport [38]. Presumably the changes in structure and function of the small intestine allowed lysozyme and antibiotics to have a greater effect on the jejunum. Oliver and Wells et al. [12] observed decreased crypt depth in pigs consuming lysozyme or antibiotics (Fig. 2), whereas they were increased in pigs consuming lysozyme in liquid diets [11]. This is likely due to the fact that cellular proliferation is very high in the crypts in the younger animal, while villi enterocytes are longer-lived in suckling animals compared with weaned animals [39]. Nyachoti et al. [27] observed increased villi height in the ileum of pigs weaned at 17 days and fed an egg white source of lysozyme, but jejunum morphology was not measured. Changes in ileal morphology were likely due to the effect of the Escherichia coli K88 challenge on the small intestine [27]. Taken together, these data indicate that this source of lysozyme improves small intestinal morphology [11, 12, 27]. Improvements in small intestinal morphology may lead to a greater absorptive capacity and be a mechanism by which lysozyme and antibiotics improve growth rates.
Fig. 2.
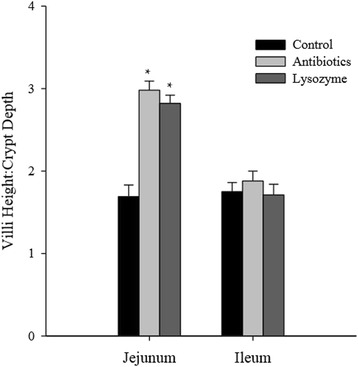
Villi height/crypt depth ratio of nursery pigs fed either a control (non-medicated), control + antibiotics, or control + lysozyme diet for 28 days. Villi height increased and crypt depth decreased exclusively in the jejunum of pigs consuming antibiotics or lysozyme, resulting in an increase of approximately 70 % in villi height to crypt depth ratio. These data were adapted from Oliver and Wells [12]. *Mean differs from control (P < 0.05)
Metabolites
Nutritional regime, health status, age, level of production, and gastrointestinal microflora are a few examples of the many factors that contribute to the metabolite profile of an animal. It is clear that both lysozyme and antibiotics alter many of these factors including growth rate, microbiota (or at least individual organisms), and gastrointestinal health. Circulating urea N is a reliable indirect measurement to show the oxidation of dietary amino acids in young pigs [40, 41]. Blood urea N (BUN) is lower in pigs consuming either lysozyme or antibiotics under a chronic immune challenge compared with control pigs [13]. This contradicts earlier work in non-challenged pigs [12]. However, considering that pigs consuming lysozyme or antibiotics accrued more protein and consumed similar amounts of feed compared with control pigs [13], the greater BUN was expected. Therefore, presumably, pigs that consumed lysozyme or antibiotics utilized more of their dietary amino acids for protein deposition than control pigs. Oliver and Wells [12] likely had too few of animals to detect a response in BUN.
The most efficient way to measure metabolites is through metabolomic experiments. Brundige et al. [7] found 18 known serum metabolites that were changed by the consumption of lysozyme. Of these 18, most changed in a direction that was decidedly “positive” for pig health and(or) growth. Four of these (methionine, threonine, hydroxyproline, and urea) indicate a propensity for increased growth in pigs consuming lysozyme. Methionine, threonine, and hydroxyproline increased in serum indicating potential increases in protein synthesis and skeletal growth, while serum urea decreased. These findings support Oliver et al. [13], in that lysozyme consumption increased growth rate and decreased circulating urea, in addition to an increase in protein accretion compared with pigs consuming a non-medicated diet.
Cytokines and Immune Response
Immune system activation, including pro-inflammatory cytokine and acute phase protein production, prevents animals from reaching their genetic growth potential [42]. For example, poultry and swine reared in germ-free environments grow at a faster rate than animals reared in conventional production environments [43, 44]. In addition, utilizing a clean vs. a dirty environment to stimulate a chronic immune response decreases animal performance [45–47]. In pigs, an immune response does not generally result in decreased feed conversion [48–50]. However, both lysozyme [12] and antibiotics [1] improve feed efficiency in nursery swine. In addition, Nyachoti et al. [27] reported that lysozyme alleviated the piglet response to an oral challenge of Escherichia coli K88, similar to traditional antibiotics.
While cytokines primarily regulate the immune response, they have an equal effect on nutrient metabolism. During an immune response, pro-inflammatory cytokines redirect nutrients away from growth and toward the immune response [51, 52]. Although not the only mode of action, cytokines increased both muscle protein degradation and acute phase protein production [53]. Cytokines and acute phase proteins were measured in a study designed to elicit a low level immune response, to both confirm the chronic immune stimulation and to determine the effect of antibiotics and lysozyme on the immune response [13]. Interleukin-6 and pig major acute phase protein were unaffected by immune status. In contrast, circulating levels of the cytokine tumor necrosis factor-α (TNF-α) and the acute phase proteins haptoglobin and C-reactive protein (CRP) were higher in chronically immune stimulated pigs compared with pigs reared in a clean nursery. These changes in cytokines and acute phase proteins, as well as the performance changes observed, indicate that an acceptable level of immune response was generated in pigs reared in the dirty nursery to make inferences into the effect of antibiotics and lysozyme on chronically immune stimulated pigs. Pigs consuming antibiotics or lysozyme had lower TNF-α, haptoglobin, and CRP, compared with control pigs, regardless of whether pigs were under chronic immune stimulation or reared in a clean nursery. Similarly, Lee et al. [54] observed lower haptoglobin levels in antibiotic-fed pigs compared with non-medicated controls. In addition, Nyachoti et al. [27] observed lower circulating TNF-α levels post-challenge in pigs consuming lysozyme. While these later studies used a different model (acute Escherichia coli challenges), antibiotics and lysozyme fed to pigs reduced the immune response when exposed to pathogens. In addition to these studies, Cooper et al. [36] determined that RNA for transforming growth factor-β1 was increased in unchallenged pigs consuming lysozyme from transgenic goats’ milk.
Microbial ecology
It is clear that the microbiota are important to pig health and growth [26, 55]. However, Holman and Chenier [56] observed relatively minor changes to the pig’s microbiota in pigs consuming either tylosin or chlortetracycline. Unno et al. [57] showed that the use of antibiotics in swine feed inhibited potential pathogens. However, the use of chlortetracycline, sulfathiazole, and penicillin did not elicit a growth response making it impossible to determine if the change in microbiota was associated with improved performance. Clearly, more work in this area is warranted.
It is now well documented that lysozyme has antimicrobial qualities and improves pig performance and gastrointestinal health. It is likely that lysozyme alters the gastrointestinal bacterial population, either through direct bacterial elimination (Gram-positive bacteria) or changes to the ecology that favor one group of bacteria over another. However, little work has been done looking at the effect of lysozyme on pig gastrointestinal microbial populations. In a small, proof of concept experiment, Maga et al. [8] observed that lysozyme was capable of modulating the bacterial populations in the duodenum and ileum of both kid goats and piglets. In pigs, lysozyme from transgenic goats’ milk reduced both total coliforms and E. coli in the duodenum, while only total coliforms were reduced in the ileum. This small study clearly shows that lysozyme has the ability to alter microbial populations in vivo. Lysozyme was also shown to reduce enterotoxigenic E. coli (ETEC) in challenged piglets [27]. However, the observed effect of lysozyme on E. coli species seems to be variable. The prevalence of Shiga-toxigenic E. coli (STEC) is generally low in nursery pigs [20] and was not altered by lysozyme or antibiotics. The eae gene, which is an indicator gene for enteropathogenic and enterohemorrhagic E. coli (EPEC and EHEC, respectively) is observed in nursery pigs [20]. However, this gene increases over the course of the nursery phase, neither lysozyme or antibiotics seem to alter its abundance [20]. The different observations due to feeding lysozyme on E. coli may be due to the different sources of lysozyme, different species of E. coli (ETEC vs. STEC, EPEC, and EHEC), or the presence of a direct E. coli K88 challenge [27].
Maga et al. [58] studied the microbiome of pigs consuming lysozyme expressed in transgenic goats’ milk. Lysozyme decreased the levels of Firmicutes and increased the levels of Bacteroidetes in pig feces. High levels of Bacteroidetes are associated with decreased nutrient absorption [59], but the level of change in piglets consuming lysozyme is unlikely to cause decreased absorption, especially considering the changes in gut morphology and performance observed when feeding lysozyme [12, 13]. At the taxonomic Family or Order level, lysozyme decreased the abundance of bacteria associated with disease (Mycobacteriaceae, Streptococcaceae, and Campylobacterales) and increased bacteria associated with gastrointestinal health (Bifidobacteriaceae and Lactobacillaceae). These data support May et al. [11] and Wells et al. (Fig. 3, [20]), who observed a 50 % reduction of Campylobacter spp. in pigs consuming lysozyme compared with non-medicated pigs. While carbadox/copper sulfate is effective against Campylobacter spp. [3], Wells et al. [20] observed that chlortetracycline/tiamulin hydrogen fumarate did not change the Campylobacter spp. in the feces similar to lysozyme.
Fig. 3.
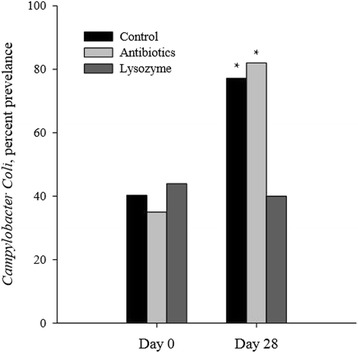
Campylobacter spp. shedding of nursery pigs fed either a control (non-medicated), control + antibiotics, or control + lysozyme diet for 28 days. Lysozyme, but not chlortetracyline/tiamulin in nursery swine feed prevented the normal increase in campylobacter shedding in the feces of nursery pigs. These data were adapted from Wells et al. [20]. *Within day, mean differs from lysozyme (P < 0.05)
Conclusions
It is clear that feeding subtherapeutic levels of antibiotics improves performance and overall health and is used extensively throughout the swine industry. However, it is also clear that swine producers are under pressure to reduce or eliminate the use of antibiotics due to concerns over antibiotic resistance. Research into possible alternatives is essential and will allow swine producers to keep the animal well-being and monetary advantages of antibiotics without the perceived negative effects of their use. Lysozyme is a natural antimicrobial already used in other facets of the food industry. In nursery pigs, lysozyme added to feed improves gastrointestinal health, reduces potential pathogen shedding, and improves growth and feed efficiency. Therefore, lysozyme is a viable alternative to traditional subtherapeutic antibiotic use in swine production.
Acknowledgments
Support for the work described in Oliver et al. (2014) was provided by the National Pork Board (#12-148, WO). Mention of trade names, proprietary products, or specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable. The USDA is an equal opportunity provider and employer.
Abbreviations
- BUN
Blood urea nitrogen
- CRP
C-reactive protein
- TNF-α
Tumor necrosis factor- α
- ETEC
Enterotoxigenic E. coli
- STEC
Shiga-toxigenic E. coli
- EPEC
Enteropathogenic E. coli
- EHEC
Enterohemorrhagic E. coli
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WO and JW contributed to the writing of this review paper. Both authors read and approved the final manuscript.
Contributor Information
W. T. Oliver, Phone: 402-762-4206, Email: William.Oliver@ars.usda.gov
J. E. Wells, Email: jim.wells@ars.usda.gov
References
- 1.Verstegen MW, Williams BA. Alternatives to the use of antibiotics as growth promoters for monogastric animals. Anim Biotechnol. 2002;13:113–27. doi: 10.1081/ABIO-120005774. [DOI] [PubMed] [Google Scholar]
- 2.Wells JE, Yen JT, Miller DN. Impact of dried skim milk in production diets on Lactobacillus and pathogenic bacterial shedding in growing-finishing swine. J Appl Microbiol. 2005;99:400–7. doi: 10.1111/j.1365-2672.2005.02629.x. [DOI] [PubMed] [Google Scholar]
- 3.Wells JE, Oliver WT, Yen JT. The effects of dietary additives on faecal levels of Lactobacillus spp., coliforms, and Escherichia coli, and faecal prevalence of Salmonella spp. and Campylobacter spp. in U.S. production nursery swine. J Appl Microbiol. 2010;108:306–14. doi: 10.1111/j.1365-2672.2009.04423.x. [DOI] [PubMed] [Google Scholar]
- 4.Ellison RT, III, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–91. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano M, Namba Y, Hanaoka M. Regulatory factors of lymphocyte-lymphocyte interaction. I. Con A-induced mitogenic factor acts on the late G1 stage of T-cell proliferation. Microbiol Immunol. 1981;25:505–15. doi: 10.1111/j.1348-0421.1981.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 6.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundige DR, Maga EA, Klasing KC, Murray JD. Consumption of pasteurized human lysozyme transgenic goats' milk alters serum metabolite profile in young pigs. Transgenic Res. 2010;19:563–74. doi: 10.1007/s11248-009-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maga EA, Walker RL, Anderson GB, Murray JD. Consumption of milk from transgenic goats expressing human lysozyme in the mammary gland results in the modulation of intestinal microflora. Transgenic Res. 2006;15:515–9. doi: 10.1007/s11248-006-0014-3. [DOI] [PubMed] [Google Scholar]
- 9.Brundige DR, Maga EA, Klasing KC, Murray JD. Lysozyme transgenic goats' milk influences gastrointestinal morphology in young pigs. J Nutr. 2008;138:921–6. doi: 10.1093/jn/138.5.921. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey BD, Huang N, Klasing KC. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J Nutr. 2002;132:1214–8. doi: 10.1093/jn/132.6.1214. [DOI] [PubMed] [Google Scholar]
- 11.May KD, Wells JE, Maxwell CV, Oliver WT. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J Anim Sci. 2012;90:1118–25. doi: 10.2527/jas.2011-4297. [DOI] [PubMed] [Google Scholar]
- 12.Oliver WT, Wells JE. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J Anim Sci. 2013;91:3129–36. doi: 10.2527/jas.2012-5782. [DOI] [PubMed] [Google Scholar]
- 13.Oliver WT, Wells JE, Maxwell CV. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J Anim Sci. 2014;92:4927–34. doi: 10.2527/jas.2014-8033. [DOI] [PubMed] [Google Scholar]
- 14.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc. 1922;93:306–17. doi: 10.1098/rspb.1922.0023. [DOI] [Google Scholar]
- 15.Tenovuo J, Lumikari M, Soukka T. Salivary lysozyme, lactoferrin and peroxidases: Antibacterial effects on cariogenic bacteria and clinical applications in preventive dentistry. Proc Finn Dent Soc. 1991;87:197–208. [PubMed] [Google Scholar]
- 16.Jolles P, Jolles J. What's new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–89. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- 17.Varahan S, Iyer VS, Moore WT, Hancock LE. Eep confers lysozyme resistance to enterococcus faecalis via the activation of the extracytoplasmic function sigma factor SigV. J Bacteriol. 2013;195:3125–34. doi: 10.1128/JB.00291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–43. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham FE, Proctor VA, Goetsch SJ. Egg-white lysozyme as a food preservative: An overview. World's Poult Sci J. 1991;47:141–63. doi: 10.1079/WPS19910015. [DOI] [Google Scholar]
- 20.Wells JE, Berry ED, Kalchayanand N, Rempel LA, Kim M, Oliver WT. Effect of lysozyme or antibiotics on faecal zoonotic pathogens in nursery pigs. J Appl Microbiol. 2015;118:1489–97. doi: 10.1111/jam.12803. [DOI] [PubMed] [Google Scholar]
- 21.Proctor VA, Cunningham FE. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr. 1988;26:359–95. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- 22.Scharfen EC, Mills DA, Maga EA. Use of human lysozyme transgenic goat milk in cheese making: effects on lactic acid bacteria performance. J Dairy Sci. 2007;90:4084–91. doi: 10.3168/jds.2006-808. [DOI] [PubMed] [Google Scholar]
- 23.Bottazzi V, Battistotti B, Rebecchi A, Bertuzzi S. Germination of Clostridium spores and the action of lysozyme in Grana cheese. Latte. 1996;11:80. [Google Scholar]
- 24.Nattress FM, Yost CK, Baker LP. Evaluation of the ability of lysozyme and nisin to control meat spoilage bacteria. Int J Food Microbiol. 2001;70:111–9. doi: 10.1016/S0168-1605(01)00531-1. [DOI] [PubMed] [Google Scholar]
- 25.Tong J, Wei H, Liu X, Hu W, Bi M, Wang Y, et al. Production of recombinant human lysozyme in the milk of transgenic pigs. Transgenic Res. 2011;20:417–9. doi: 10.1007/s11248-010-9409-2. [DOI] [PubMed] [Google Scholar]
- 26.Maga EA, Anderson GB, Murray JD. The effect of mammary gland expression of human lysozyme on the properties of milk from transgenic mice. J Dairy Sci. 1995;78:2645–52. doi: 10.3168/jds.S0022-0302(95)76894-1. [DOI] [PubMed] [Google Scholar]
- 27.Nyachoti CM, Kiarie E, Bhandari SK, Zhang G, Krause DO. Weaned pig responses to Escherichia coli K88 (ETEC) oral challenge when receiving a lysozyme-supplement. J Anim Sci. 2012;90:252–60. doi: 10.2527/jas.2010-3596. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz S, Kehrenberg C, Walsh TR. Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents. 2001;17:431–7. doi: 10.1016/S0924-8579(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 29.Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 30.Thymann T, Sorensen KU, Hedemann MS, Elnif J, Jensen BB, Banga-Mboko H, et al. Antimicrobial treatment reduces intestinal microflora and improves protein digestive capacity without changes in villous structure in weanling pigs. Br J Nutr. 2007;97:1128–37. doi: 10.1017/S0007114507691910. [DOI] [PubMed] [Google Scholar]
- 31.Argenzio RA, Liacos JA, Levy ML, Meuten DJ, Lecce JG, Powell DW. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-Na absorption in enteric cryptosporidiosis of pigs. Gastroenterology. 1990;98:1129–40. doi: 10.1016/0016-5085(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 32.Zijlstra RT, Whang KY, Easter RA, Odle J. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J Anim Sci. 1996;74:2948–59. doi: 10.2527/1996.74122948x. [DOI] [PubMed] [Google Scholar]
- 33.Oliver WT, Mathews SA, Phillips O, Jones EE, Odle J, Harrell RJ. Efficacy of partially hydrolyzed corn syrup solids as a replacement for lactose in manufactured liquid diets for neonatal pigs. J Anim Sci. 2002;80:143–53. doi: 10.2527/2002.801143x. [DOI] [PubMed] [Google Scholar]
- 34.Piva A, Grilli E, Fabbri L, Pizzamiglio V, Gatta PP, Galvano F, et al. Intestinal metabolism of weaned piglets fed a typical United States or European diet with or without supplementation of tributyrin and lactitol. J Anim Sci. 2008;86:2952–61. doi: 10.2527/jas.2007-0402. [DOI] [PubMed] [Google Scholar]
- 35.Shen YB, Piao XS, Kim SW, Wang L, Liu P, Yoon I, et al. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. 2009;87:2614–24. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- 36.Cooper CA, Brundige DR, Reh WA, Maga EA, Murray JD. Lysozyme transgenic goats' milk positively impacts intestinal cytokine expression and morphology. Transgenic Res. 2011;20:1235–43. doi: 10.1007/s11248-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koldovsky O, Dobiasova M, Hahn P, Kolinska J, Kraml J, Pacha J. Development of gastrointestinal functions. Physiol Res. 1995;44:341–8. [PubMed] [Google Scholar]
- 38.Pacha J. Ontogeny of Na + transport in rat colon. Comp Biochem Physiol A Physiol. 1997;118:209–10. doi: 10.1016/S0300-9629(96)00292-7. [DOI] [PubMed] [Google Scholar]
- 39.Smith MW. Postnatal development of transport function in the pig intestine. Comp Biochem Physiol A Comp Physiol. 1988;90:577–82. doi: 10.1016/0300-9629(88)90670-6. [DOI] [PubMed] [Google Scholar]
- 40.Oliver WT, Touchette KJ, Coalson JA, Whisnant CS, Brown JA, Oliver SA, et al. Pigs weaned from the sow at 10 days of age respond to dietary energy source of manufactured liquid diets and exogenous porcine somatotropin. J Anim Sci. 2005;83:1002–9. doi: 10.2527/2005.8351002x. [DOI] [PubMed] [Google Scholar]
- 41.Oliver WT, Miles JR. A low-fat liquid diet increases protein accretion and alters cellular signaling for protein synthesis in 10-day-old pigs. J Anim Sci. 2010;88:2576–84. doi: 10.2527/jas.2009-2766. [DOI] [PubMed] [Google Scholar]
- 42.Cook ME. Triennial Growth Symposium: A review of science leading to host-targeted antibody strategies for preventing growth depression due to microbial colonization. J Anim Sci. 2011;89:1981–90. doi: 10.2527/jas.2010-3375. [DOI] [PubMed] [Google Scholar]
- 43.Drew MD, Van Kessel AG, Maenz DD. Absorption of methionine and 2-hydroxy-4-methylthiobutoanic acid in conventional and germ-free chickens. Poult Sci. 2003;82:1149–53. doi: 10.1093/ps/82.7.1149. [DOI] [PubMed] [Google Scholar]
- 44.Loynachan AT, Pettigrew JE, Wiseman BS, Kunkle RA, Harris DL. Evaluation of a diet free of animal protein in germfree swine. Xenotransplantation. 2005;12:149–55. doi: 10.1111/j.1399-3089.2005.00210.x. [DOI] [PubMed] [Google Scholar]
- 45.Roura E, Homedes J, Klasing KC. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. J Nutr. 1992;122:2383–90. doi: 10.1093/jn/122.12.2383. [DOI] [PubMed] [Google Scholar]
- 46.Bassaganya-Riera J, Hontecillas-Magarzo R, Bregendahl K, Wannemuehler MJ, Zimmerman DR. Effects of dietary conjugated linoleic acid in nursery pigs of dirty and clean environments on growth, empty body composition, and immune competence. J Anim Sci. 2001;79:714–21. doi: 10.2527/2001.793714x. [DOI] [PubMed] [Google Scholar]
- 47.Renaudeau D, Gourdine JL, St-Pierre NR. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J Anim Sci. 2011;89:2220–30. doi: 10.2527/jas.2010-3329. [DOI] [PubMed] [Google Scholar]
- 48.Williams NH, Stahly TS, Zimmerman DR. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J Anim Sci. 1997;75:2481–96. doi: 10.2527/1997.7592481x. [DOI] [PubMed] [Google Scholar]
- 49.Williams NH, Stahly TS, Zimmerman DR. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J Anim Sci. 1997;75:2472–80. doi: 10.2527/1997.7592472x. [DOI] [PubMed] [Google Scholar]
- 50.Renaudeau D. Effect of housing conditions (clean vs. dirty) on growth performance and feeding behavior in growing pigs in a tropical climate. Trop Anim Health Prod. 2009;41:559–63. doi: 10.1007/s11250-008-9223-5. [DOI] [PubMed] [Google Scholar]
- 51.Johnson RW. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J Anim Sci. 1997;75:1244–55. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- 52.Spurlock ME. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J Anim Sci. 1997;75:1773–83. doi: 10.2527/1997.7571773x. [DOI] [PubMed] [Google Scholar]
- 53.Elsasser TH, Caperna TJ, Li CJ, Kahl S, Sartin JL. Critical control points in the impact of the proinflammatory immune response on growth and metabolism. J Anim Sci. 2008;86:E105–25. doi: 10.2527/jas.2007-0634. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Awji EG, Lee SJ, Tassew DD, Park YB, Park KS, et al. Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J Anim Sci. 2012;90:3709–17. doi: 10.2527/jas.2011-4434. [DOI] [PubMed] [Google Scholar]
- 55.Rist VT, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal. 2013;7:1067–78. doi: 10.1017/S1751731113000062. [DOI] [PubMed] [Google Scholar]
- 56.Holman DB, Chenier MR. Temporal changes and the effect of subtherapeutic concentrations of antibiotics in the gut microbiota of swine. Microb Ecol. 2014;90:599–608. doi: 10.1111/1574-6941.12419. [DOI] [PubMed] [Google Scholar]
- 57.Unno T, Kim J, Guevarra RB, Nguyen SG. Effects of antibiotic growth promoter and characterization of ecological succession in swine gut microbiota. J Microbiol Biotechnol. 2015;25(4):431–8. [DOI] [PubMed]
- 58.Maga EA, Desai PT, Weimer BC, Dao N, Kultz D, Murray JD. Consumption of lysozyme-rich milk can alter microbial fecal populations. Appl Environ Microbiol. 2012;78:6153–60. doi: 10.1128/AEM.00956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]


